Abstract
Plant extracts including secondary metabolites have anti-inflammatory and anti-obesity activities. This study was conducted to investigate the anti-obesity properties of fermented Artemisia annua (AW) and Salicornia herbacea (GW) in vitro and in mice. The metabolite profiling of AW and GW extracts was performed using UHPLC−LTQ−Orbitrap–MS/MS, and gene expression was analyzed using real-time PCR for adipocyte difference factors. The anti-obesity effects in mice were measured using serum AST, ALT, glucose, TG, and cholesterol levels. Metabolites of the plant extracts after fermentation showed distinct differences with increasing anti-obesity active substances. The efficacy of inhibitory differentiation adipogenesis of 3T3-L1 adipocytes was better for GW than AW in a concentration-dependent manner. RT-PCR showed that the GW extract significantly reduced the expression of genes involved in adipocyte differentiation and fat accumulation (C/EBPα, PPARγ, and Fas). In C57BL/6 mice fed the HFD, the group supplemented with AW and GW showed reduced liver weight, NAS value, and fatty liver by suppressing liver fat accumulation. The GW group significantly reduced ALT, blood glucose, TG, total cholesterol, and LDL-cholesterol. This study displayed significant metabolite changes through biotransformation in vitro and the increasing anti-obesity effects of GW and AW in mice. GW may be applicable as functional additives for the prevention and treatment of obesity.
1. Introduction
Obesity, which is considered a major public health problem, ranks as the fifth leading cause of death worldwide and has tripled in the last 40 years [1]. The World Obesity Atlas published in 2022 predicts that one billion people worldwide will live with obesity by 2030 [2]. The main cause of obesity is the increase in body fat storage efficiency because of a high-fat diet and the accumulation of fat due to adipogenesis by the differentiation of adipocytes [3]. Increased fat accumulation accompanies various diseases such as obesity, insulin resistance, type 2 diabetes, hyperglycemia, dyslipidemia, and metabolic syndrome due to changes in body weight, increased fasting blood sugar, and cholesterol [4,5]. In addition, mRNA expression of genes, such as peroxisome proliferator-activated receptor-γ (PPARγ), CCAAT/enhancer-binding protein-α (C/EBPα), and leptin, is known increase in obesity [6]. Adipose tissue plays an important role in the immune response, contributing to a chronic low-grade inflammation process linked to tumor development through adipokine secretion molecules, hormone (leptin), growth factors, and proinflammatory cytokines, proliferation, angiogenesis, and expression process [7]. Leptin participates in endocrine metabolism as well as the regulation of appetite and energy expenditure. Recent data have emphasized the brain’s ability to control the complex mechanisms of food intake and storage [8]. The highlight is the value of adipocyte-secreted hormones in obesity and cancer prevention through risk reduction and specific adapted therapies [9]. Recently, in order to prevent and treat obesity, methods such as diet and exercise, as well as next-generation anti-obesity drugs, are being developed [10]. Traditionally, various medicinal plants have been used as treatments for diseases, and various medicinal plants were known to have an effect on obesity [11]. Many studies have scientifically proven that plant extracts can be potential preventive and therapeutic agents for obesity because physiologically active compounds in plant extracts possess anti-obesity effects [12]. Medicinal plants with antioxidant and anti-obesity effects include Moringa oleifera, Solenostemma argel, and Salvia species [13]. The use of natural therapies for weight loss has increased based on their reliability, safety, and cost, compared to synthetic drugs or surgical procedures that have side effects [14]. Among many medicinal plants, annual wormwood (AW: Artemisia annua L.) is an annual plant belonging to the Asteraceae family that is known for its anti-inflammatory, anti-cancer, antibacterial, and anti-obesity effects and can be found in some parts of Asia, including Korea and China [15]. Compounds, such as ketone, camphor, and 1,8-cineole, exist in AW essential oil [16], and extracts of AW are reported to have excellent antioxidant capacity [17]. In addition, AW has been reported to contribute to the prevention of obesity, including compounds with various anti-obesity activities, such as artemisinic acid, rhamnetin, myricetin, and sabinene [18,19,20]. AW is used as livestock feed and raw material for medicine and cosmetics [21,22].
Glasswort (GW: Salicornia europaea) is a halophyte belonging to the pemphigus family Chenopodiaceae, which grows on the coasts of temperate and subtropical regions [23]. GW is known to have various functionalities such as antioxidant, anti-cancer, and anti-obesity [24,25]. Chemical constituents such as sterols, quinic acid derivatives, flavonoid derivatives, triterpenoid saponins, and pentadecyl ferulate are present in GW, and some of them have antioxidant activity [26]. In addition, there are various anti-obesity compounds such as trans-ferulic acid (TFA) and isorhamnetin 3-O-β-D-glucopyranoside in GW, which are known to regulate adipogenesis and differentiation inhibition [27,28]. GW is applied as a sodium substitute to various foods such as in baking and sausage making [29,30]. Some of the medicinal herbs used in traditional medicine were biologically activated through the biotransformation of bacteria through fermentation [31]. It is known that fermentation induces the structural destruction of plant cell walls in plant foods and induces the release or synthesis of various antioxidant compounds, especially increasing the amount of phenols and flavonoids due to microbial hydrolysis [32]. As such, AW and GW have been studied for their various physiological activities, but most of them focused on antioxidant and anti-cancer effects. In particular, there are fewer studies on the anti-obesity effects of fermented natural products, and studies comparing anti-obesity effects, physiological activities, and metabolomes before and after fermentation have not been reported.
This study was conducted to elucidate the prospective substances for anti-obesity and to investigate the effects of these medicinal plants as functional additives, as well as natural medicaments. Therefore, the metabolome changes in the medicinal plants AW and GW before and after fermentation, and the differentiation inhibitory ability using 3T3-L1 adipocytes in vitro, were confirmed. In addition, to evaluate the possibility of obesity prevention, male C57BL/6 mice were fed the HFD supplemented with AW and GW.
2. Materials and Methods
2.1. Preparation of Medicinal Plants
Annual wormwood (AW) and glasswort (GW) were purchased in powder form from a Korean food company and stored in a refrigerator at 4 °C before use (AW: Ingreen Co., Ltd., Gangwha-do, Republic of Korea; GW: Suncheon Bay Hamcho Agricultural Cooperative Corporation, Suncheon-si, Jeollanam-do, Republic of Korea).
2.2. Fermentation of Medicinal Plants
The medicinal plant powder, 5% (w/v) of AW and GW was added separately to one-fifth diluted Man Rogosa Sharpe (MRS) broth and Bacillus minimal medium (BMM) for the fermentation. Then, pH was adjusted to 7.0 ± 0.5 and sterilized at 121 °C for 15 min. For fermentation, 1% (v/v) of Lactobacillus plantarum SK3494 and Enterococcus faecium SK4369, which was isolated from natural extract of each plant [33,34], was inoculated into the respective medium. The AW and GW were fermented for 16 h at 37 °C using a shaking incubator (BF-60SIRL, Biofree, Seoul, Republic of Korea) under shaking of 100 rpm.
2.3. Extraction of Fermented Plants
Before and after fermentation, solution was collected at each time point (0 and 16 h) and stored at −20 °C. After fermentation, ultra-pure water was added to each solution at a rate of 70% and then boiled at 100 °C for 15 min using a water bath (Wise Bath, Seoul, Republic of Korea). Extracted solutions were cooled to room temperature and centrifuged at 14,500 rpm for 15 min (Mega 17R; Hanil, Seoul, Republic of Korea). The collected supernatants were freeze-dried using a freeze dryer (UniFreeze FD-8, Daihan Scientific Ltd., Seoul, Republic of Korea) and stored in a deep freezer at −80 °C for future experiments.
2.4. Chemicals and Reagents
The HPLC-grade water and methanol were purchased from Fisher Scientific (Pittsburgh, PA, USA). Formic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.5. Preparation of Metabolic Extracts
The freeze-dried supernatants of non-fermented and fermented samples from AW and GW were extracted using 75% methanol (50 mg/mL). The mixtures were sonicated for 30 min and incubated for 24 h under deep-freezing (−20 °C) conditions. Each of the samples was centrifuged at 11,000 rpm for 10 min, and the collected supernatant was filtered through a 0.22 μm polytetrafluoroethylene filter and dried using a speed vacuum concentrator (Biotron, Seoul, Republic of Korea). The dried samples were reconstituted with 75% methanol to a final concentration of 20 mg/mL, to be used for instrument analysis.
2.6. UHPLC–LTQ–Orbitrap–MS Profiling
Metabolite profiling of non-fermented and fermented extracts of AW and GW was preformed using UHPLC–LTQ–Orbitrap–MS/MS. The 2-chloro-phenylalanine (1.5 µg/mL) was used as an internal standard (IS). A sample of 5 μL was injected into a UHPLC system equipped with a Vanquish binary pump H system (Thermo Fisher Scientific, Waltham, MA, USA) coupled with auto-sampler and column compartment at the flow rate of 3 mL/min. Chromatographic separation was performed on a Phenomenex KINETEX® C18 column (100 mm × 2.1 mm, 1.7 μm particle size: Torrance, CA, USA). The mobile phase, 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), was used in ESI negative mode. The gradient parameters were set as follows: 5% solvent B was maintained initially for 1 min, followed by a linear increase to 100% solvent B over 9 min and then sustained at 100% solvent B for 1 min, with a gradual decrease to 5% solvent B over 3 min. The total run time was 14 min. The column temperature was set to 40 °C, the flow rate was 0.3 mL/min, and the injection volume was 5 μL. The MS data were collected in the range of 100–1000 m/z (under negative- and positive-ion modes) using an Orbitrap Velos ProTM system, which was combined with an ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled with an HESI-II probe. The probe heater and capillary temperatures were set to 300 °C and 350 °C, respectively. The capillary voltage was set to 2.5 kV in negative mode (positive mode: 3.7 Kv).
2.7. Cell Viability Assay
Cell viability of the plant extracts in 3T3-L1 preadipocytes was measured using cell count kit-8 (WST-8/CCK8, Abcam, ab228554), according to the manufacturer’s instructions. The 3T3-L1 preadipocytes were seeded at 1 × 104 cells in a 96-well plate (SPL, SPL30096) and cultured overnight in a CO2 incubator at 37 °C. After incubation, the cells were treated with various concentrations (0.2~50 µg/mL) of plant extracts dissolved in 10 mg/mL DMSO solution and cultured for 72 h. Then, 10 μL of cell count kit-8 (WST-8/CCK8, Abcam, ab228554) reagent was added, and after 30 min, absorbance was measured at 460 nm using a microplate reader. The cell viability was calculated using the following equation:
Cell viability (%) = (OD Sample − OD Media)/(OD DMSO solution − OD Media) × 100
2.8. Oil Red O Staining
Oil Red O [35] staining was performed to confirm intracellular lipid droplet production. Differentiated 3T3-L1 cells were washed with PBS and fixed with 10% formalin for 30 min. After washing, 60% isopropanol and 0.5% ORO solution (Sigma-Aldrich, St. Louis, MO, USA) were added to the fixed cells and stained for 20 min at room temperature. Then, the cell was washed with distilled water, dried at 37 °C, and added to 200 μL of isopropanol to dissolve intracellular ORO solution; then, 100 μL of each was transferred to a 96-well plate, and absorbance was measured at 540 nm using a microplate reader (Synergy 2, BioTek Instruments Inc., Winooski, VT, USA). The ability to inhibit adipocyte differentiation of fermented AW and GW was confirmed for each concentration of 3.1~50 μg/mL.
2.9. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Reverse transcription-polymerase chain reaction (RT-PCR) was performed to confirm the expression of regulators in fat metabolism using test substances in differentiated 3T3-L1 cells. Changes in mRNA levels of CCAAT/enhancer-binding protein-alpha (C/EBPα), peroxisome proliferator-activated receptor-γ (PPARγ), leptin, and Fas, regulators involved in fat metabolism, were analyzed using RT-PCR and shown in Table 1. Total RNA was isolated using TRI reagent (Sigma, St. Louis, MO, USA), cDNA was synthesized using Power cDNA synthesis kit (iNtRON, Seoul, Republic of Korea), according to the manufacturer’s instructions, and real-time PCR was performed using SYBR green and each primer. PCR was performed using a real-time PCR machine (Corbett, Mortlake, Australia), and gene expression was quantified and analyzed using Rotor-Gene Q Series Software 2.3.1. (Qiagen, Hilden, Germany).

Table 1.
Primer sequences of target genes used in the PCR.
2.10. Animals and Diet
The animal experimentation was approved by the NDIC Co., Ltd. Institutional Animal Care and Use Committee (IACUC), Gyeonggi-do, Republic of Korea, in accordance with the guidelines of IACUC (N2021001). Six-week-old male C57BL/6 mice (20~21 g) were obtained from ORIENTBIO Inc., Republic of Korea. The animals were housed in a polycarbonate breeding box under a controlled environment (temperature: 21 ± 2 °C; humidity: 50 ± 20%; lighting time: 12 h/day) and were freely fed food and water. Mice were placed into 6 groups of 8 mice each: normal diet (ND, TEKLAD Certified Global 18% Protein Rodent DIET 2918C, Harlan TEKLAD, Madison, WI, USA); high-fat diet (HFD, D12492; 5.24 kcal/g with 60% of fat-, 20% of protein-, and 20% of carbohydrate-derived calories, Research DIETS Inc., New Brunswick, NJ, USA); HFD supplemented with fermented 100 mg/kg AW extract (AW 100); HFD supplemented with fermented 300 mg/kg AW extract (AW 300); HFD supplemented with fermented 100 mg/kg GW extract (GW 100); and HFD supplemented with fermented 300 mg/kg GW extract (GW 300). Oral administration was performed once a day, and the body weight of the experimental animals was measured once a week for 12 weeks. Feed intake was measured once a week after the test substance was administered. The food efficiency ratio was calculated as FER = total body weight gain (g)/total food intake (g).
2.11. Sample Preparation and Treatment
For autopsy, after the inhalational anesthesia with isoflurane, blood was collected through the abdominal vena cava and the liver was removed. The extracted liver was washed with physiological saline, dried with a filter paper, and then the absolute weight was measured using an electronic balance. After calculating the absolute weights, a portion of liver was fixed in 10% neutral buffered formalin for histological examination. The remaining liver was rapidly frozen for hepatic triglyceride (Hepatic TG) analysis and placed in deep freezer (Model 706, Thermo Fisher Scientific Inc., Clevelang, OH, USA) below −70 °C. Blood collected from the abdominal vena cava was placed in a 0.6 mL SST tube (Microtainer, BD, USA), completely solidified, centrifuged at 4 °C at 5000 rpm for 15 min, put into a 1.5 mL tube, and stored in deep freezer (Model 706, Thermo Fisher Scientific Inc., USA) below −70 °C.
2.12. Histological Analysis
For histological analysis, liver was fixed in 10% neutral buffered formalin. The fixed tissue was prepared as a paraffin block through a general tissue-processing process and sectioned. The remaining tissues were stored in 10% neutral buffered formalin solution. Hematoxylin and Eosin (H&E) staining was performed on thin slices of liver tissue. The accumulation of lipid droplets in the liver tissue and the degree of adipose inflammation were analyzed using the evaluation method used in the non-alcoholic steatosis model. Non-alcoholic fatty liver disease activity score [13] evaluation, which is a synthesis of five broad categories (steatosis, inflammation, hepatocellular injury, fibrosis, and miscellaneous features), was evaluated on H&E-stained liver tissue slides using the method described in a previous study [36].
2.13. Serum and Hepatic Triglyceride Analysis
At the time of autopsy, serum was collected from the abdominal vena cava. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose [24], total cholesterol (T-Chol), triglycerides (TGs), HDL-cholesterol (HDL-C), and LDL-cholesterol (LDL-C) were analyzed using a blood chemistry analyzer (AU480, Beckman Coulter, Germany) using serum stored in deep freezer. Fasting blood insulin levels were measured using the Rat/Mouse Insulin ELISA Kit (EZRMI-13K, Millipore, MA, USA), according to the manufacturer’s instructions, with the separated serum collected from the abdominal vena cava during autopsy and placed in a 0.6 mL SST tube (Microtainer, BD, USA). The remaining serum was stored in deep freezer. At necropsy, 200 mg of liver tissue collected was weighed and washed with normal physiological saline. After adding 1 mL of PBS containing 1% Triton X-100 per 200 mg of liver tissue, homogenization was performed. The liver homogenate was centrifuged at 10,000 rpm at 4 °C for 10 min to separate the supernatant, and then the TG content in the liver tissue was measured with a microplate reader (570 nm) using a TG quantification kit (Cell Biolabs, STA-396, San Diego, CA, USA).
2.14. Data Processing and Statistical Analysis
UHPLC–LTQ–Orbitrap–MS raw data files were converted to NetCDF (*.cdf) using Thermo X caliber software (version 2.1, Thermo Fisher Scientific). Retention time correction, peak detection, and alignment were processed using the Metalign software package (http://www.metalign.nl, accessed on 15 November 2021). Metabolites were tentatively identified based on various data comparing mass fragment patterns, retention time, and MS analysis data with standard compounds under identical conditions and in commercial databases, such as the National Institutes of Standards and Technology Library (version 2.0, 2011, FairCom, Gaithersburg, MD, USA), pubchem (https://pubchem.ncbi.nlm.nih.gov/accessed on 1 December 2021), chemspider (http://www.chemspider.com/ on 1 December 2021), and the Human Metabolome Database (HMDB; https://hmdb.ca/ accessed on 1 December 2021). Statistical analysis was performed using SIMCA-P+ software (version 12.0, Umetrics, Umea, Sweden) based on partial least squares discriminant analysis (PLS-DA) modeling to determine metabolite differences between different incubation times. The peaks were selected based on variable importance in the projection (VIP) values and significant differences were determined using analysis of variance (ANOVA). In cell, experiment results were expressed as the mean values ± standard deviations, and significance was tested using the paired-comparison t-test of IBM SPSS Statistics 25 for Windows (IBM, New York, NY, USA) (p < 0.05). For multiple comparison, Duncan’s multiple range test was used after comparison test with one-way ANOVA, and significance was verified at p < 0.05 level. All results obtained in the mouse experiment were expressed as mean values ± SD, and body weight and weight gain were tested using one-way ANOVA using IBM SPSS Statistics 25 for Windows (IBM, New York, NY, USA), and Fisher’s Least Significant Difference (LSD) test was performed (p ≤ 0.05; p ≤ 0.01).
3. Results
3.1. Multivariate Analysis in Annual Wormwood and Glasswort with LAB-Mediated Fermentation
Non-fermented and fermented extracts of AW and GW were analyzed with UHPLC–MS/MS combined with multivariate analysis on the ingredients to investigate the metabolic change using LAB-mediated fermentation. As a result of principal component analysis (PCA), PC1 (43.4%) and PC2 (17.4%) showed a clear separation of four kinds of extracts, reflecting a metabolic difference between plant species and fermentation (Figure 1A). Similar cluster patterns were also observed in the partial least squares discriminant analysis (PLS-DA) model with a high predictive ability (Q2 = 0.976) as well as a considerable significance metric (p = 1.87 × 10−5) (Figure 1B).
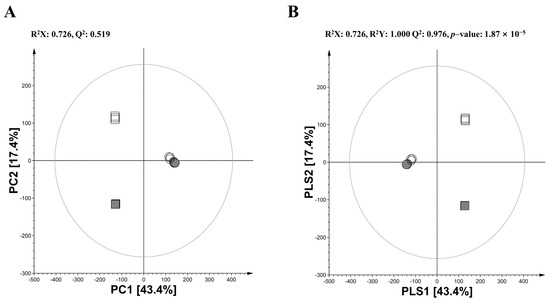
Figure 1.
(A) PCA score plot and (B) PLS-DA score plot derived from UHPLC–LTQ–Orbitrap–MS/MS datasets for non-fermented and fermented extracts of annual wormwood (AW) and glasswort (GW). (■: AN, non-fermentation of AW; □: AF, fermentation of AW with L. plantarum; ●: GN, non-fermentation of GW; and ○: GF, fermentation of GW with Ec. Faecium).
Based on the PLS-DA model for profiling the dataset, we selected the significantly discriminant metabolites using variable importance in projection (VIP) values > 1.0 and p > 0.05. A total of forty-two significantly discriminant metabolites were selected, and thirty-nine of them were tentatively identified including three peptides, two organic acids, six fatty acids, eight phenolic acids and derivatives, seven quinic acids and derivatives, seven benzoic acids and derivatives, and four flavonoids, among two others (Table 2). Plant extracts are rich sources of volatile terpenoids and phenolic compounds with complex mixtures of organic framework-added functional groups including alcohol, aldehydes, esters, ethers, ketones, and phenols. It is used as an analgesic, antibacterial, antidepressant, antimicrobial, and antioxidant agent [22,26]. Polyphenolic compounds are one of the most critical ingredients related to free radical scavenging activity in medicinal plants. They exhibit various biological properties, such as antioxidant, cardioprotective, anti-mutagenic, antibacterial, and anti-inflammatory activities [24].

Table 2.
Tentatively identified metabolites in non-fermented and fermented extracts of AW and GW based on the UHPLC–LTQ–Orbitrap–MS/MS analyses.
3.2. Relative Metabolite Abundance in Annual Wormwood and Glasswort with LAB-Mediated Fermentation
The relative abundance of the significantly discriminant metabolites are shown in the box-and-whisker plots (Figure 2). We observed distinct metabolic change from LAB-mediated fermentation in AW and GW. Several metabolites showed increased or decreased patterns from fermentation in both AF and GF.
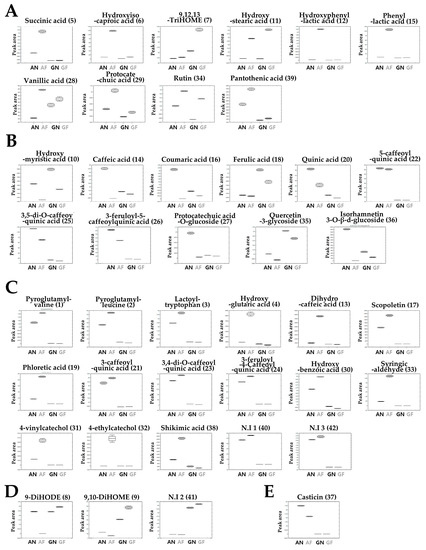
Figure 2.
Box-and-whisker plots illustrating the relative abundance of metabolite levels from the fermentation of AW and GW. (A) Increased and (B) decreased metabolites in both AF and GF. (C) Increased and (E) decreased metabolites only in the AF group. (D) Increased metabolites only in the GF group. (Line: mean; box: standard error; whisker: standard deviation.)
The relative contents of several metabolites in both AF and SF groups were higher than each non-fermented group, including succinic acid (5), hydroxyisocaproic acid (6), 9,12,13-TriHOME (7), hydroxystearic acid (11), hydroxyphenyllactic acid (12), phenyllactic acid (15), vanillic acid (27), protocatechuic acid (28), rutin (34), and pantothenic acid (39) (Figure 2A). On the other hand, some metabolites showed lower concentrations from fermentation, that is hydroxymyristic acid (10), caffeic acid (14), coumaric acid (16), ferulic acid (18), quinic acid (20), 5-caffeoylquinic acid (22), 3-5-dicaffeoylquinic acid (25), 3,5-feruloyl-5-caffeoylquinic acid (26), protocatechuic acid-O-glucoside (27), quercetin-3-glycoside (35), and isorhamnetin-3-glucoside (36) (Figure 2B). Some metabolites showed different patterns for each fermented group. Unlike the GF group, the AF group showed a decrease in casticin (37) and an increase in pyroglutamyl-valine (1), pyroglutamyl-leucine (2), lactotyl-tryptophan (3), hydroxyglutaric acid (5), dihydrocaffeic acid (13), scopoletin (17), phloretic acid (19), 3-caffeoylquinic acid (21), 3,4-dicaffeoylquinic acid (23), 3-feruloyl-4-caffeoylquinic acid (24), hydroxybenzoic acid (30), syringic aldehyde (33), 4-ethylcatechol (32), 4-vinylcatechol (31), shikimic acid (38), and two non-identified metabolites (40, 42) (Figure 2C). Relatedly, 9-DiHODE (8), 9,10-DiHOME (9), and N.I 2 (41) increased only in SF (Figure 2D).
3.3. Phenolic Acid Degradation Pathways by Biotransformation of Plant Substrates Using LAB
To investigate the biotransformation of plant substrates, we focused on the phenolic acid degradation pathway in annual wormwood and glasswort. Among the significantly discriminant metabolites, thirteen compounds related to phenolic acid degradation from LAB were selected to construct a metabolic pathway. Chlorogenic-acid-derived caffeic acid, quinic acid, p-coumaric acid, and ferulic acid showed a common decrease in both fermentation groups. The contents of metabolites produced by phenolic acid degradation in the AF group were all increased, including shikimic acid, dihydrocaffeic acid, vinyl catechol, ethyl catechol, phloretic acid, hydroxybenzoic acid, protocatechuic acid, and vanillic acid. The SF group also showed an increase in vanillic acid and protocatechuic acid in the phenolic acid degradation pathway (Figure 3).
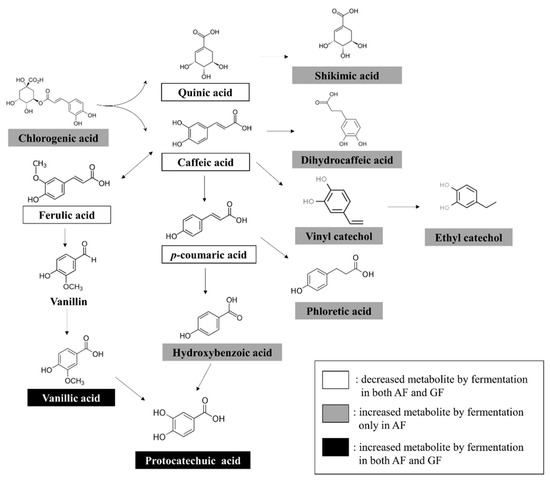
Figure 3.
Schematic representation of the degradation pathways of phenolic acids from fermentation of AW and GW.
3.4. Inhibition of 3T3-L1 Adipocyte Differentiation
To evaluate the anti-obesity effects of these plants, the adipogenesis and fat accumulation were investigated in vitro. Figure 4A shows the adipocyte differentiation inhibitory activity of fermented AW and GW at various concentrations (3.1~50 μg/mL). If the fat accumulation of the control group was 100%, the fermented AW extract showed concentration-dependent adipocyte differentiation inhibitory activity, and at 50 μg/mL, fat accumulation was suppressed as much as the undifferentiated adipocyte control. The GW extract had lower fat accumulation than the control at all concentrations except 3.1 μg/mL and showed great anti-differentiation activity even at low concentrations. Figure 4B shows the results of observing differentiated 3T3-L1 adipocytes treated with fermented AW and GW at various concentrations of 3.1~50 μg/mL under a 100× optical microscope. Both extracts inhibited the differentiation of adipocytes in a concentration-dependent manner, and in the morphology it was observed that the size and number of lipid droplets decreased. Oil Red O staining was performed in vitro, after which the physiological data and effects on adipogenesis were observed based on the histology. In the micrographs, it can be seen that the area stained with Oil Red O was significantly reduced when 3T3-L1 adipocytes were treated with the highest concentration of 50 μg/mL of the AW extract. On the other hand, the GW extract was observed to inhibit the formation of lipid droplets even at low concentrations.
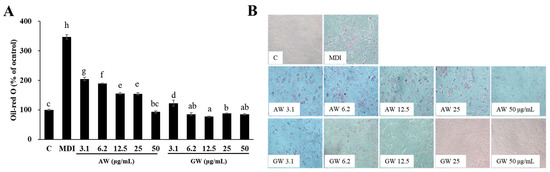
Figure 4.
Inhibitory ability of 3T3-L1 adipocyte differentiation from fermented AW and GW extracts. (A) Test with oil red O staining method. (B) Using an optical microscope. All data are expressed as mean ± SD. Significant differences were determined by Duncan’s test after one-way ANOVA and expressed in letters (a–h) (p < 0.05). C: Control; MDI: differentiated control; AW: annual wormwood; GW: glasswort.
Cells were treated at concentrations ranging from 0.2 to 50 μg/mL to the maximal concentration, showing high survival rates in all treatment groups. AW and GW before and after fermentation at concentrations of 50 μg/mL or less in 3T3-L1 cells did not show cytotoxicity.
3.5. Gene Expression Analyzed Using Real-Time PCR (RT-PCR)
To test the anti-obesity effect of plant extracts on lipogenesis in adipose tissue, the real-time PCR was analyzed. The effect of the AW and GW extracts on the expression of adipocyte differentiation-related factors (C/EBPα, PPARγ, leptin, and Fas) in 3T3-L1 cells was experimented (Figure 5). PPARγ is a nuclear hormone receptor that plays a major role in regulating the expression of proteins necessary for the development of functional mature adipocytes. C/EBPα plays a synergistic role in terminal adipocyte differentiation. C/EBPα and PPARγ are integrated in the control of lipid and carbohydrate metabolism. In the real-time PCR results, the gene expression of C/EBPα and PPARγ for adipogenesis decreased compared to the MDI group. After adipocyte differentiation (MDI), the expression of all genes, except leptin, increased significantly compared to before differentiation (control). There was no significant difference in gene expression of adipocytes treated with AW extract compared to MDI. On the other hand, when the GW extract was treated at a concentration of 6.2 μg/mL or more, the expression of differentiation-related genes, except for leptin, was significantly reduced compared to MDI. In addition, C/EBPα and PPARγ, which are closely related to each other, showed a similar decrease pattern, and Fas involved in adipogenesis was reduced. In the case of leptin, GW tended to be less produced than AW, but there was no significant difference. As a result, since the GW extract of 6.2 μg/mL or more reduces the expression of adipocyte genes to the same level as the control, it can be assumed to have an inhibitory activity on the differentiation of 3T3-L1 preadipocytes. The effects of the GW extract on the inhibition of adipogenesis were observed more apparently.
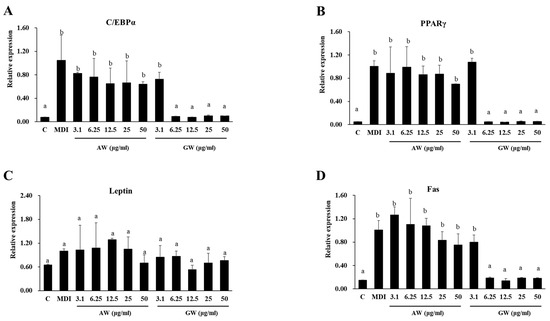
Figure 5.
Effect of fermented AW and GW extracts on the mRNA expressions of (A) C/EBPα, (B) PPARγ, (C) leptin, and (D) Fas, regulatory factors involved in 3T3-L1 preadipocytes. All data are expressed as mean ± SD. Significant differences were determined by Duncan’s test after one-way ANOVA and expressed in letters (p < 0.05). C: Control; MDI: differentiated control; AW: annual wormwood; GW: glasswort.
3.6. Changes in Body and Organ Weight in C57BL/6 Mice
Figure 6A shows the body weight and organ weight changes for 12 weeks of C57BL/6 mice fed a high-fat diet containing fermented AW and GW extracts at 100 and 300 mg/kg. The body weight of the GW 300 significantly decreased at 10 and 12 weeks compared to the HFD (p < 0.05). The final body weight of the ND was 29.73 ± 3.59 g, an increase of 8.86 ± 2.25 g from the initial body weight, whereas the final body weight of the HFD was 45.50 ± 2.15 g, an increase of 24.43 ± 2.26 g from the initial weight. The HFD significantly increased body weight compared to the ND from the second week of the experiment, and a significant weight increase was observed continuously until the end of the test (p < 0.01), indicating that obesity was induced in mice due to the high-fat diet. The final body weight of AW 100 among the AW extract treatment groups was 42.26 ± 3.80 g, and there was no significant difference compared to the HFD, but the weight gain was significantly reduced to 21.13 ± 2.26 g (p < 0.05). Relatedly, among the GW extract treatment groups, GW 300 had a final body weight of 41.71 ± 4.27 g and a body weight gain of 20.71 ± 3.68 g, which was significantly reduced compared to the HFD (p < 0.05). In addition, the body weight of the GW 300 significantly decreased at 10 and 12 weeks compared to the HFD (p < 0.05), and the body weight gain significantly decreased at 7, 10, 11, and 12 weeks (p < 0.05). The weights of the initial mice were similarly set in the range of 20~21 g. The FER was lowest in the ND and highest in the HFD. Compared to the HFD, the FER of the AW 300 and GW 300 groups was significantly lower (p < 0.05). In particular, AW 300 had a small FER even though its weight gain was the second highest after the HFD, suggesting that weight gain was small even though it consumed a lot of food. Therefore, it was considered that the AW and GW extracts affected the weight loss and FERs of C57BL/6 mice. Figure 6B shows the liver weights of C57BL/6 mice fed a high-fat diet containing fermented AW and GW extracts of 100 and 300 mg/kg. Liver weights in the ND were significantly decreased compared to the HFD (p < 0.01). The AW 100 and AW 300 had no significant differences. Compared to the HFD, the liver weights of the GW 100 and GW 300 decreased significantly (p < 0.05). In conclusion, GW inhibited the accumulation of triglycerides in the liver.
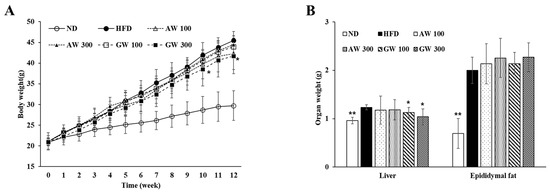
Figure 6.
Changes in (A) body weight and (B) organ weight of C57BL/6 mice during the experiment period. Data are presented as mean ± SD (n = 8). Significant difference from HFD using one-way ANOVA followed by the LSD test: * p < 0.05, ** p < 0.01. ND: Normal diet; HFD: 60% high-fat diet; AW 100: HFD + fermented annual wormwood (AW) 100 mg/kg; AW 300: HFD + fermented AW 300 mg/kg; GW 100: HFD + fermented glasswort (GW) 100 mg/kg; GW 300: HFD + fermented GW 300 mg/kg.
3.7. Histological Analysis
In order to analyze non-alcoholic steatosis, using image J program photographed the lipid droplet and inflammatory symptoms in liver tissue. Figure 7A showed the histological feature of the liver based on hematoxylin and cosin staining in rats fed control and HFD. Figure 7A displayed liver tissue images of C57BL/6 mice fed a high-fat diet containing fermented AW and GW extracts (100 and 300 mg/kg). Compared to the ND, it was confirmed that the number of lipid droplets produced in the liver tissue of obese HFD increased. Lipid droplets were less in the liver tissue of C57BL/6 mice fed with the GW extract than with the AW extract. Therefore, it was considered that GW inhibits lipid accumulation in the liver more than AW. The most common cause of chronic disease by excessive fat accumulation in the liver is non-alcoholic fatty liver disease (NAFLD). NAFLD caused that high carbohydrate diet induced serum free fatty acid, thereafter increased the absorption of FFA biosynthesis of TG in liver. NAFLD includes a wide spectrum of liver damage ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), advanced fibrosis, and hepatocellular carcinoma [37]. Figure 7B shows the NAFLD activity score measuring the histological characteristics of non-alcoholic fatty liver disease (NAFLD) in C57BL/6 mice fed a high-fat diet containing fermented AW and GW extracts (100 and 300 mg/kg). The NAS of the C57BL/6 mice fed normal diet (ND) in which obesity was not induced was 0, and NAFLD did not appear, and it was significantly lower than that of the HFD (3.00 ± 0.19) (p < 0.01). AW 100 (2.5 ± 0.38) and AW 300 (2.25 ± 0.49) decreased concentration dependently compared to the HFD, but no significant difference occurred. GW 100 (1.75 ± 0.41) and GW 300 (1.13 ± 0.13) also decreased in a concentration-dependent manner with significant differences compared to the HFD (p < 0.05, p < 0.01).
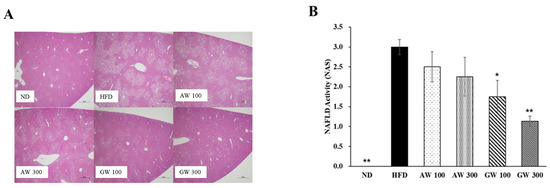
Figure 7.
Effect of fermented AW and GW extracts on (A) histological features of the liver and (B) non-alcoholic fatty liver disease activity score in liver of C57BL/6 mice. Original magnifications: ×40; scale bar: 500 μm. Data are presented as mean ± SD (n = 8). Significant difference from HFD using t-test: * p < 0.05, ** p < 0.01. ND: Normal diet; HFD: 60% high-fat diet; AW 100: HFD + fermented annual wormwood (AW) 100 mg/kg; AW 300: HFD + fermented AW 300 mg/kg; GW 100: HFD + fermented glasswort (GW) 100 mg/kg; GW 300: HFD + fermented GW 300 mg/kg.
3.8. Serum and Hepatic Triglycerie Analysis
Serum analysis was performed in C57BL/6 mice fed a high-fat diet containing fermented AW and GW extracts at 100 and 300 mg/kg (Table 3). Serum AST and ALT levels are indicators of the liver dysfunction condition, caused by the HFD. AST (163.38 ± 20.60) and ALT (122.50 ± 31.01) values of AW 100 were significantly increased, compared to the HFD (p < 0.01, p < 0.05). The level of AST in the normal group was 94 U/L, and the HFD G2 group significantly increased; in contrast, in the supplemented AW 300 groups was significantly decreased. The ALT values of the supplemented GW groups were significantly decreased; in particular, the G6HFD 300 (46.63 ± 3.30) groups showed the lowest value of all the other HFD groups (p < 0.05). In the case of blood Glu, those of the ND (118.00 ± 10.49), GW 100 (212.63 ± 12.40), and GW 300 (210.50 ± 17.02) were decreased when compared to the HFD at 226.00 ± 4.76 mg/dL (p < 0.01). Blood T-Chol significantly decreased in all groups compared to the HFD at 210.75 ± 8.39 mg/dL. Blood TGs significantly decreased at the GW 300 at 80.00 ± 3.33 mg/dL, compared to the HFD at 97.75 ± 5.31 mg/dL (p < 0.01). HDL-C was significantly reduced, compared to the HFD at 97.13 ± 2.73 mg/dL, in the AW 300 and GW 300 at 89.25 ± 2.94 and 91.50 ± 2.17 mg/dL, respectively, treated with high-concentration extract (p < 0.05). The GW extract treatment groups, GW 100 14.75 ± 0.70 and GW 300 13.63 ± 0.91 mg/dL, showed reduced LDL-C, compared to the HFD at 18.88 ± 1.33 mg/dL (p < 0.05). As a result of the blood test, when obesity was induced, the values of all blood analysis indicators increased. On the other hand, the AW extract reduced T-Chol and HDL-C in C57BL/6 mice, and the fermented GW extract reduced blood ALT, Glu, T-Chol, TGs, HDL-C, and LDL-C. As a result, since AW and GW reduce the levels of ALT, Glu, T-Chol, TGs, HDL-C, and LDL-C in the blood, it is thought that they have the possibility of preventing obesity. Insulin levels in the HFD, in which obesity was induced by eating a high-fat diet, were increased, compared to the ND. Among the plant extract treatment groups, only GW 300 at 2.70 ± 0.66 ng/mL significantly reduced blood insulin levels (p < 0.01), compared to the HFD at 5.55 ± 0.96 ng/mL. When obesity was induced, hepatic TGs increased, and the AW 300 at 103.31 ± 89.76 µg/mg tissue decreased, compared to the HFD at 145.66 ± 56.7 µg/mg tissue, but there was no significant difference. Hepatic TG levels tended to decrease in a dose-dependent manner in the GW extract treatment group, and GW 300 decreased significantly, compared to the HFD (p < 0.05). Therefore, it can be assumed that GW 300 suppressed the accumulation of triglycerides in the liver.

Table 3.
Blood chemistry in control and HFD-fed C57BL/6 rats treated with AW and GW.
4. Discussion
Obesity is a major public health problem with high prevalence worldwide and is accompanied by various diseases such as diabetes, hyperglycemia, dyslipidemia, and metabolic syndrome [5]. Treatments are being developed to prevent and treat obesity, but side effects such as nausea and diarrhea exist [9]. In order to develop plant-derived functional materials with fewer side effects and superior anti-obesity effects than drugs, this study aimed to reveal the anti-obesity activity of fermented annual wormwood (AW) and glasswort (GW) at both the in vitro and in vivo levels. In addition, metabolome changes were verified for the difference before and after fermentation. AW (Artemisia annua L.) is a common type of wormwood native to temperate Asia but naturalized worldwide and belonging to the family Asteraceae [15]. Its bioactive derivatives control ROS, DNA damage, DNA repair, cell cycle arrest, apoptosis, inflammatory response, angiogenesis, and multiple signaling pathways, showing an anti-cancer effect [38]. In addition, it has an anti-hyperglycemic effect and reduces insulin resistance by increasing adiponectin secretion in adipocytes [35].
GW (Salicornia herbacea L.) belongs to Chenopodiaceae and has been used as a food ingredient or as a folk remedy for improving intestinal function, indigestion, gastrointestinal disease, kidney disease, and diabetes [39]. GW is seaweed that contains physiologically active substances, such as dietary fiber, and minerals such as potassium, magnesium, and calcium that stimulate intestinal movement. GW is known to have beneficial effects on obesity by reducing body weight and fat levels and improving blood lipids [40].
The bacteria used for AW fermentation are Lactobacillus plantarum, a lactic acid bacterium [41]. It is known that pH decreases by producing lactic acid as a fermentation metabolite [42]. At this time, as the fermentation progresses, the pH decreases and the acidity increases, which is considered to reduce the activity of Lactobacillus plantarum. GW showed a small change in pH during fermentation, an increase in viable cell count, and then a tendency to die after a certain period of time, which was similar to previous studies [33]. Since Enterococcus faecium, the strain used for GW fermentation, mainly uses carbohydrates as an energy source, it is thought that the death occurred due to a lack of carbohydrates as fermentation proceeded [43].
AW and GW contain phenolic compounds, and there is no difference in physiological activity before and after fermentation. It is true that fermentation is known as one of the ways to improve the physiological activity of natural products, but it is difficult to see that some phenols are unconditionally improved due to small changes [44].
Untargeted metabolomics were employed to investigate metabolic change using LAB-mediated fermentation in AW and GW. Among the discriminant metabolites, we observed the relative abundance of several metabolites related to bacterial biotransformation of plant-derived compounds such as flavonoids and phenolic acids. Flavonoids in plants are major secondary metabolites, mainly in the form of flavonoid glycosides [45]. Flavonoid glycosides can be decomposed into aglycone and phenolic acid by microorganisms. Some LAB have enzymes that convert flavonoid glycosides such as α-l-rhamnosidase and β-d-glucosidase, but these abilities vary among specific species and strains. In this study, the reduction of quecetin-3-glycoside and isorhamnetin-3-glycoside using fermentation was observed, but the types of aglycone and flavonol produced from bioconversion could not be identified. On the other hand, the content of rutin tended to increase through fermentation. It has been reported that there is a difference in the conversion ability of rutin for each strain. In the study of buckwheat fermented by 14 LAB strains, most of them decreased the content of the rutin, but some strains increased [46]. Rutin is known to have various pharmacological activities such as antimicrobial, anti-inflammatory, and anti-cancer effects [47,48]. In addition, rutin administration in high-fat diet mice was affected, affecting the intestinal bacteria and anti-obesity effect [49].
Bacterial degradation of phenolic compounds has also been reported along with metabolic changes. The reaction of phenolic acid decarboxylase (PAD) and reductase in LAB decreased phenolic acid as a substrate and increased some benzoic acid as a product [50,51,52]. We also observed the fermentation patterns of metabolites in the degradation pathways of quinic acid, caffeic acid, p-coumaric acid, and ferulic acid. As a clear reduction of phenolic acid, we identified the products of eight benzoic acid related to four kinds of phenolic acid in the AF group and only two compounds (vanillic acid and protocatechuic acid) related to ferulic acid in the GF group. The converted metabolites derived from phenolic acid showed high bioavailability in human and health benefits [53,54]. Dihydrocaffeic acid and the catechol group converted from caffeic acid were reported as potent antioxidant properties caused by the scavenging of intracellular reactive oxygen species [41,55,56]. Relatedly, phloretic acid converted from p-coumaric acid was one of the substances that recently attracted attention as physiological activities, such as protecting the host from influenza infection by regulating the immune balance, that have been reported [57,58]. Hydroxybenzoic acid and its derivatives are known to exhibit various physiological activities such as anti-obesity, antibacterial, anti-mutagenic, anti-algae, anti-inflammatory, and antioxidant activities [59,60,61]. In addition, protocatechuic acid was also reported to have various effects such as anti-obesity, anti-inflammatory, and antioxidant effects through in vivo and in vitro tests [62,63]. Vanillic acid is a substance used pharmacologically with various physiological activities such as anti-obesity, antioxidant, anti-cancer, antifungal, and liver protection [64,65].
Additionally, we observed the production of several metabolites related to amino acid and fatty acid metabolism of LAB. These metabolites were presumed to be produced using nutrients in the medium rather than compounds derived from plants. Phenyllactic acid, hydroxyphenyllactic acid, and hydroxyisocaproic acid were produced by amino acid catabolism, which was phenylalanine, tyrosine, and leucine, respectively [66,67]. In fatty acid metabolism, oleic acid and linoleic acid were metabolized to hydroxystearic acid, azelaic acid, and hydroxyoctadecanoic acid [68,69]. Among these increased metabolites, hydroxy fatty acids and phenylpropanoids produced by LAB have been reported to have antifungal activity that can be useful to biopreservation [70,71,72]. On the other hand, some dipeptides (pyroglutamyl-valine, pyroglutamyl-leucine, and lactoyl-tryptophan) increased only in the AF group. Those compounds included the taste active peptide derivatives in food fermentation of LAB [73]. An anti-inflammatory effect and attenuating dysbiosis in vivo has been reported for pyroglutamyl-leucine [74,75]. Similarly, some oxylipins (9-DiHODE, 9,10-DiHOME, and 9,12,13-TriHOME) dramatically increased in the GF group, which are well-known as bioactive lipids for their anti-inflammatory and antioxidant effects [76,77].
Relatedly, the anti-obesity effect of fermented AW and GW was confirmed in vitro using 3T3-L1 cells, which can differentiate between fibroblasts and adipocytes and are widely used in adipogenesis and biochemical studies of adipocytes [78]. There was no cytotoxicity because both AW and GW showed a high survival rate up to 50 μg/mL. Lipid droplet generation can be confirmed by staining triglyceride and cholesterol ester accumulated due to lipolysis using Oil Red O staining reagent, and the degree of fat differentiation can be quantified by dissolving it and measuring absorbance [79,80]. Both the fermented AW and GW extracts inhibited the differentiation of adipocytes in a concentration-dependent manner, and it was morphologically observed that the size and number of lipid droplets produced when adipocytes differentiated and accumulated fat decreased. Excessive lipid accumulation and formation of lipid droplets cause obesity [81], and it was confirmed that GW suppressed the formation of lipid droplets even at low concentrations when compared to AW. Therefore, it can be said that fermented GW has the potential for preventing obesity.
During adipogenesis, several types of genes and transcription factors were regulated. In this experiment, the effects of fermented AW and GW extracts on the expression of adipocyte differentiation-related factors C/EBPα, PPARγ, leptin, and Fas in 3T3-L1 cells were investigated. C/EBPα and PPARγ are specific transcription factors expressed in adipocytes, and the activities of the two regulators were enhanced in the early stages of adipogenesis and promote adipogenesis [82]. When adipocytes were compared before and after differentiation, C/EBPα and PPARγ significantly increased after differentiation, and transcription factors were suppressed in the GW group, similarly to before differentiation. Leptin, a protein produced and secreted by adipocytes, increased as fat accumulation increased and is a hormone that induces appetite suppression to control obesity. Leptin levels increased after differentiation, but there was no significant difference. The level of leptin in AW was similar to that after differentiation, but in the case of GW, it slightly decreased. Since leptin secretion increased with adipocyte size and fat accumulation, it was thought that the level of leptin was low in GW where fat accumulation was suppressed [83]. When the expression of Fas, which is involved in lipid synthesis, transport, and storage, was inhibited, adipogenesis and triglyceride accumulation were reduced [84]. The expression of Fas increased after differentiation, and GW suppressed the expression to the same level as the control group. Demineralized glasswort and annual wormwood leaves, which have been shown to have an inhibitory effect on the differentiation of adipocytes, and fermented lemons showed similar gene expression inhibitory activity [28,85,86]. Therefore, 3T3-L1 cells increase the expression of all genes as fat accumulates after differentiation, but GW extract reduces the expression of adipocyte-expressed genes C/EBPα, PPARγ, and Fas, suggesting that the differentiation of adipocytes is inhibited.
The anti-obesity activity was investigated through weight change, histological change, and serum analysis. C57BL/6 mice were used as experimental animals, and they are often used as obesity-prevention models because they lack the leptin gene and cannot control appetite, causing obesity [87]. In this experiment, it was also confirmed that the weight of the high-fat diet (HFD) group continued to increase, and obesity was caused by the high-fat diet. After 12 weeks, the final weight and weight gain (g) of GW 300 decreased compared to the HFD, and the weight gain (g) of AW 100 decreased (p < 0.05). The food efficiency ratios (FERs) of the AW 300 group and GW 300 group significantly decreased compared to the HFD (p < 0.05). If the FER was small, it can be predicted that obesity was prevented in the high-concentration treatment group of natural products [88].
Obesity refers to an increase in body fat caused by the accumulation of triglycerides in adipose tissue, and it has been reported that the risk of metabolic diseases increases as the abdominal fat content increases [89]. In this experiment, the weight of the liver was measured to confirm the formation of abdominal fat. Similar to the previous study [86], in which C57BL/6 mice fed the HFD gained weight as cholesterol and triglycerides accumulated in the liver, it was confirmed that the liver weight increased in this experiment. The liver weight of mice fed the GW extract significantly decreased compared to the HFD (p < 0.05). The GW extract inhibited liver fat accumulation. In addition, when lipids accumulated in the liver as obesity progressed, lipid droplets also accumulated [90]. An obesity-induced HFD increased the number of lipid droplets in liver tissue compared to the ND, and it was confirmed that the number of lipid droplets in GW was smaller than that in AW. Non-alcoholic fatty liver diseases (NAFLDs) are known to be liver damage diseases caused mainly by insulin resistance, accompanied by obesity and dyslipidemia due to the accumulation of triglycerides in hepatocytes [91]. The non-alcoholic fatty liver disease activity score [13] is a method of expressing NAFLD as a score for histological characteristics. Non-alcoholic steatohepatitis (NASH) can be diagnosed if the NAS is five or higher, and “not NASH” if the NAS is less than three [36]. The HFD group has a high risk of NASH with a score of three, and all groups except the HFD group can be diagnosed as not NASH, so it can be said that there is no liver damage. In addition, the content of useful substances having various activities increased by this decomposition. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), biomarkers that can estimate liver health, increased in obesity. Similarly, AST and ALT of an obesity-induced HFD increased significantly compared to the ND. The ALT of the GW 300 group significantly decreased compared to the HFD (p < 0.01). On the other hand, AW 100 increased both the AST (p < 0.01) and ALT (p < 0.05), compared to the HFD, which was contrary to previous studies in which the AST and ALT of the AW extract were lower than those fed a high-fat diet [15,25]. When AST and ALT increased, it can be said that liver damage had occurred. In the present study, two of the 100 AW groups had higher AST and ALT than other animals, suggesting that liver damage may have occurred. Blood glucose [24] was the highest in an obesity-induced HFD and significantly decreased in the GW-treated group, compared to the HFD group (p < 0.01). The GLU-lowering effect of GW was similar to a previous study that revealed a hyperglycemia-preventive effect [92]. The total cholesterol (T-Chol) and high-density lipoprotein-cholesterol (HDL-C) of mice fed fermented AW extract significantly decreased when compared to the HFD (p < 0.05). The fermented GW extract significantly reduced GUL, T-Chol, triglycerides (TGs) (p < 0.01), HDL-C, and LDL-C (p < 0.05). GW lowered the lipid concentration in the blood than AW. This result was similar to a previous experiment in which T-Chol, low-density lipoprotein-cholesterol (LDL-C), and TG levels were reduced in mice fed high-fat diets and seaweed [93]. The occurrence of cardiovascular disease can be confirmed by the TC/HDL-C ratio, and it is known that the occurrence of the disease is low when the ratio is low. The TC/HDL-C ratios were ND (1.38), HFD (2.17), AW 100 (2.03), AW 300 (1.99), GW 100 (1.90), and GW 300 (1.78), and ND and GW 300 were low. Therefore, the fermented GW extract can reduce the risk of obesity and cardiovascular disease by reducing blood ALT, GUL, T-Chol, TGs, LDL-C, blood fat, and cholesterol.
Obesity increases insulin resistance, which does not normalize blood glucose levels, and increases the risk of developing type 2 diabetes [94]. In the case of the HFD, insulin resistance was high, but blood sugar increased, so it can be judged that insulin resistance occurred. Among the experimental groups, GW 300 showed a decrease in insulin level (p < 0.01), and even though they ate a high-fat diet, the rate of insulin secretion was regulated, and it is thought that the GW extract lowered insulin resistance. When obesity was induced, hepatic triglycerides (hepatic TGs) increased, and among them, the GW 300 group significantly decreased compared to the HFD (p < 0.05), and it was found that obesity was suppressed. Glasswort supplementation showed similar results to a previous study [95] that significantly reduced hepatic triglycerides compared to mice fed a high-fat diet.
5. Conclusions
In this study, in vitro experiments using 3T3-L1 adipocyte cells and in vivo experiments using C57BL/6 mice were conducted to investigate the anti-obesity effects of fermented annual wormwood (AW) and glasswort (GW). In addition, an analysis of compound changes was investigated comparing before and after fermentation. In particular, phenolic compounds were abundant in AW, and antioxidant activity was also high. In both AW and GW plants, significant metabolite changes from fermentation were observed. The metabolites increased from fermentation were metabolites produced by decomposing the plant substrate and substances produced by strain biosynthesis. Specifically, several substances with reported physiological activity were identified from the degradation of phenolic acid derived from plants using lactic acid bacteria. It is suggested that the compounds involved in the anti-obesity activity of GW can be activated by the biotransformation of fermentation, but detailed research is needed for the exact mechanism.
The AW and GW extracts were not toxic to 3T3-L1 adipocytes up to the maximum concentration of 50 µg/mL, and the plants after fermentation had better ability to inhibit adipocyte differentiation. The fermented AW and GW extracts inhibited the differentiation of adipocytes in a concentration-dependent manner and also reduced the size and number of lipid droplets. In particular, fermented GW inhibited lipid droplet accumulation even at low concentrations and significantly reduced the expression of C/EBPα, PPARγ, and Fas, transcription factors and genes involved in adipogenesis that were expressed during adipocyte differentiation. When C57BL/6 mice were supplemented with fermented AW extract, the body weight, food efficiency ratio (FER), and total blood cholesterol (T-Chol) reduced compared to obese mice. The supplementation of GW extract to C57BL/6 mice prevented liver lipid accumulation and fatty liver as well as weight loss. GW extract also prevented liver disease, reduced blood lipids, and regulated insulin.
Through this study, the anti-obesity activity of glasswort was revealed in vitro and in vivo experiments such as weight loss and lipid accumulation inhibition. Compounds with anti-obesity activity were activated through biotransformation using fermentation. As seen in the results, it is suggested that glasswort and annual wormwood be used for companion animals as well as human health as supplementary food additives for the control of obesity and metabolic disorders. The metabolic extracts from the fermented plants have shown promising anti-obesity effects which need to be substantiated further with large-scale animal studies and cross-sectional population trials with physiological studies.
Author Contributions
S.-K.K. designed this research and acquired funds and supervision; C.-H.L. and S.-H.K. performed the experiments, data interpretation, and data analyzer; J.-Y.O. investigated; J.-M.K. reviewed and edited; S.P. supervised and acquired funds; K.-H.K. found resources. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ 01689101), Rural Development Administration, Republic of Korea.
Institutional Review Board Statement
The animal experiment protocol approval No.: N 2021001 was obtained from the NDIC animal care and use committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this research are available in this article.
Acknowledgments
The authors are thankful to the NDIC Corporation for their continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loos, R.J.; Yeo, G.S. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Lee, S.Y.; hye Jang, D.; Lee, S.J.; Cho, J.-Y.; Kim, S.-H. Inhibitory effects of Porphyra dentata extract on 3T3-L1 adipocyte differentiation. J. Anim. Sci. Technol. 2020, 62, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Kajikawa, M.; Higashi, Y. Obesity and Endothelial Function. Biomedicines 2022, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Krempler, F.; Breban, D.; Oberkofler, H.; Esterbauer, H.; Hell, E.; Paulweber, B.; Patsch, W. Leptin, peroxisome proliferator-activated receptor-γ, and CCAAT/enhancer binding protein-α mRNA expression in adipose tissue of humans and their relation to cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 443–449. [Google Scholar] [CrossRef]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Ferroni, P.; Gaudagni, F. Obesity and colorectal cancer role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Duquenne, M.; Folgueira, C.; Bourouh, C.; Millet, M.; Dam, J.; Prevot, V. Leptin brain entry via a tanycyclic LepR : EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 2021, 3, 1071–1090. [Google Scholar] [CrossRef]
- Ryan, D.H. Next generation antiobesity medications: Setmelanotide, semaglutide, tirzepatide and bimagrumab: What do they mean for clinical practice? J. Obes. Metab. Syndr. 2021, 30, 196–208. [Google Scholar] [CrossRef]
- Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; de Lourdes Pereira, M. The role of medicinal and aromatic plants against obesity and arthritis: A review. Nutrients 2022, 14, 985. [Google Scholar] [CrossRef]
- Wang, H.-N.; Xiang, J.-Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- El-Shiekh, R.; Al-Mahdy, D.; Hifnawy, M.; Abdel-Sattar, E. In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. S. Afr. J. Bot. 2019, 123, 43–50. [Google Scholar] [CrossRef]
- Redha, A.A.; Perna, S.; Riva, A.; Petrangolini, G.; Peroni, G.; Nichetti, M.; Iannello, G.; Naso, M.; Faliva, M.A.; Rondanelli, M. Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 2021, 84, 104600. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Radzi, C.W.J.W.M.; Cordell, G.A.; Yaze, I. Potential of traditional medicinal plants for treating obesity: A review. arXiv 2012, arXiv:1208.1923. [Google Scholar]
- Choi, E.-Y.; Park, C.Y.; Ho, S.H.; Park, S.-J.; Kim, D.; Han, B.; Kim, S.-H. Anti-Obesity Effects of Artemisia annua Extract in Zucker Fatty Rats and High-Fat Diet Sprague Dawley Rats through Upregulation of Uncoupling Protein 1. J. Obes. Metab. Syndr. 2021, 30, 32–43. [Google Scholar] [CrossRef]
- Şenkal, B.C.; Kiralan, M.; Yaman, C. The effect of different harvest stages on chemical composition and antioxidant capacity of essential oil from Artemisia annua L. J. Agric. Sci. 2015, 21, 71–77. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential oil and acetone extract composition and antioxidant capacity. Ind. Crops Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Baek, H.K.; Shim, H.; Lim, H.; Shim, M.; Kim, C.-K.; Park, S.-K.; Lee, Y.S.; Song, K.-D.; Kim, S.-J.; Yi, S.S. Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model. J. Vet. Sci. 2015, 16, 389–396. [Google Scholar] [CrossRef]
- Darusman, L.; Batubara, I.; Utami, M. Fractionation of active components from Piper cf. fragile essential oil as aromatherapy for anti-obesity. In Proceedings of the International Symposium on Medicinal and Aromatic Plants 1023, Chiang Mai, Thailand, 15 December 2011; pp. 23–28. [Google Scholar]
- Kim, M.J.; Jeon, D.; Kwak, C.; Ryoo, S.; Kim, Y. Rhamnetin Exhibits Anti-Tuberculosis Activity and Protects against Lung Inflammation. Bull. Korean Chem. Soc. 2016, 37, 1703–1709. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M. Potential of wormwood (Artemisia absinthium Linn.) herb for use as additive in livestock feeding: A review. Pharma Innov. 2017, 6, 176–187. [Google Scholar]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia species with high biological values as a potential source of medicinal and cosmetic raw materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Ozawa, T.; Wu, J.; Fujii, S. Effect of inoculation with a strain of Pseudomonas pseudoalcaligenes isolated from the endorhizosphere of Salicornia europea on salt tolerance of the glasswort. Soil Sci. Plant Nutr. 2007, 53, 12–16. [Google Scholar] [CrossRef]
- Altay, A.; Celep, G.S.; Yaprak, A.E.; Baskose, I.; Bozoglu, F. Glassworts as possible anticancer agents against human colorectal adenocarcinoma cells with their nutritive, antioxidant and phytochemical profiles. Chem. Biodivers. 2017, 14, e1600290. [Google Scholar] [CrossRef]
- Park, Y.-H.; Lee, J.-J.; Son, H.-K.; Kim, B.-H.; Byun, J.; Ha, J.-H. Antiobesity effects of extract from Spergularia marina Griseb in adipocytes and high-fat diet-induced obese rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, J.Y.; Lee, Y.G.; Lee, H.J.; Shim, H.J.; Lee, J.H.; Kim, S.-J.; Ham, K.-S.; Moon, J.-H. Four new dicaffeoylquinic acid derivatives from glasswort (Salicornia herbacea L.) and their antioxidative activity. Molecules 2016, 21, 1097. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea. Immunopharm. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, M.-J.; Kim, J.-H.; Kim, S.-H.; Go, H.-K.; Kweon, M.-H.; Kim, D.-H. Desalted Salicornia europaea powder and its active constituent, trans-ferulic acid, exert anti-obesity effects by suppressing adipogenic-related factors. Pharm. Biol. 2018, 56, 183–191. [Google Scholar] [CrossRef]
- Kim, H.-W.; Hwang, K.-E.; Song, D.-H.; Kim, Y.-J.; Lim, Y.-B.; Ham, Y.-K.; Yeo, E.-J.; Chang, S.-J.; Choi, Y.-S.; Kim, C.-J. Effect of glasswort (Salicornia herbacea L.) on the texture of frankfurters. Meat Sci. 2014, 97, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Cavaleiro, C.; Ramos, F. Sodium reduction in bread: A role for glasswort (Salicornia ramosissima J. Woods). Compr. Rev. Food Sci. Food Saf. 2017, 16, 1056–1071. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-H.; Jin, J.-S. Fermentation of traditional medicine: Present and future. Orient. Pharm. Exp. Med. 2012, 12, 163–165. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kothari, D.; Niu, K.-M.; Han, S.G.; Yoon, J.E.; Lee, H.-G.; Kim, S.-K. Effect of fermented medicinal plants as dietary additives on food preference and fecal microbial quality in dogs. Animals 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-R.; Niu, K.-M.; Kang, S.-K.; Han, S.-G.; Lee, B.-J.; Kim, S.-K. Antioxidant and antibacterial activities of Lactobacillus-fermented Artemisia annua L. as a potential fish feed additive. J. Life Sci. 2017, 27, 652–660. [Google Scholar]
- Ghanbari, M.; Lamuki, M.S.; Habibi, E.; Sadeghimahalli, F. Artemisia annua L. Extracts Improved Insulin Resistance via Changing Adiponectin, Leptin and Resistin Production in HFD/STZ Diabetic Mice. J. Pharmacopunct. 2022, 25, 130–138. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Younossi, Z.N.; Konenig, A.B.; Abdeltif, D.; Wymer, M. Global epidemiology of NAFLD meta analytic assessment of prevalence, incidence, and outcome. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Jung, E.J.; Paramanantham, A.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.-M.; Ryu, C.H.; Hong, S.C.; Chung, K.H.; Kim, C.W. Artemisia annua L. Polyphenol-Induced Cell Death Is ROS-Independently Enhanced by Inhibition of JNK in HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1366. [Google Scholar] [CrossRef]
- Kang, S.; Choi, Y.; Hong, J. Modulation of arachidonic acid metabolism and inflammatory process in macrophages by different solvent fractions of Glasswort (Salicornia herbacea L.) extract. Korean J. Food Sci. Technol. 2018, 50, 671–679. [Google Scholar]
- Na, E.-J.; Kim, D.-J.; Kim, J.-H.; Kim, G.-R. Recent trends in anti-obesity and anti-inflammatory studies in modern health care. Technol. Health Care 2019, 27, 519–530. [Google Scholar] [CrossRef]
- De La Cruz, J.; Ruiz-Moreno, M.; Guerrero, A.; López-Villodres, J.; Reyes, J.; Espartero, J.; Labajos, M.; González-Correa, J. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J. Nutr. Biochem. 2015, 26, 549–555. [Google Scholar] [CrossRef]
- da Silva Sabo, S.; Vitolo, M.; González, J.M.D.; de Souza Oliveira, R.P. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Audisio, M.C.; Oliver, G.; Apella, M.C. Effect of different complex carbon sources on growth and bacteriocin synthesis of Enterococcus faecium. Int. J. Food Microbiol. 2001, 63, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, G.-M.; Cha, G.-S. Biotransformation of flavonoids by newly isolated and characterized Lactobacillus pentosus NGI01 strain from kimchi. Microorganisms 2021, 9, 1075. [Google Scholar] [CrossRef]
- Zieliński, H.; Wiczkowski, W.; Honke, J.; Piskuła, M.K. In vitro expanded bioaccessibility of quercetin-3-rutinoside and quercetin aglycone from buckwheat biscuits formulated from flours fermented by lactic acid bacteria. Antioxidants 2021, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Gullon, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Yan, X.; Zhai, Y.; Zhou, W.; Qiao, Y.; Guan, L.; Liu, H.; Jiang, J.; Peng, L. Intestinal Flora Mediates Antiobesity Effect of Rutin in High-Fat-Diet Mice. Mol. Nutr. Food Res. 2022, 66, 2100948. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Rogozinska, M.; Korsak, D.; Mroczek, J.; Biesaga, M. Catabolism of hydroxycinnamic acids in contact with probiotic Lactobacillus. J. Appl. Microbiol. 2021, 131, 1464–1473. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes over the fermentation period in phenolic compounds and antioxidant and anticancer activities of blueberries fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human EA. hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef]
- Moon, J.-H.; Terao, J. Antioxidant activity of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Cai, Y.; Luo, Y.; Shi, X. Safety assessment of desaminotyrosine: Acute, subchronic oral toxicity, and its effects on intestinal microbiota in rats. Toxicol. Appl. Pharmacol. 2021, 417, 115464. [Google Scholar] [CrossRef] [PubMed]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Son, J.H.; Kim, S.-Y.; Jang, H.H.; Lee, S.N.; Ahn, K.J. Protective effect of protocatechuic acid against inflammatory stress induced in human dermal fibroblasts. Biomed. Dermatol. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Nour, O.A.; Ghoniem, H.A.; Nader, M.A.; Suddek, G.M. Impact of protocatechuic acid on high fat diet-induced metabolic syndrome sequelae in rats. Eur. J. Pharmacol. 2021, 907, 174257. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Fernandez, M.; Zuniga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef]
- Averesch, N.J.; Krömer, J.O. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds—Present and future strain construction strategies. Front. Bioeng. Biotechnol. 2018, 6, 32–51. [Google Scholar] [CrossRef]
- Serra, S.; De Simeis, D.; Castagna, A.; Valentino, M. The fatty-acid hydratase activity of the most common probiotic microorganisms. Catalysts 2020, 10, 154. [Google Scholar] [CrossRef]
- Hirata, A.; Kishino, S.; Park, S.-B.; Takeuchi, M.; Kitamura, N.; Ogawa, J. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J. Lipid Res. 2015, 56, 1340–1350. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Yoo, J.A.; Lee, C.-J.; Kim, Y.-G.; Lee, B.-E.; Yoon, M.-H. Antifungal Effect of Phenyllactic Acid Produced by Lactobacillus casei Isolated from Button Mushroom. J. Mushroom 2016, 14, 162–167. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. Genomic features of Lactobacillus species. Front. Biosci. -Landmark 2009, 14, 1362–1386. [Google Scholar] [CrossRef]
- Hirai, S.; Horii, S.; Matsuzaki, Y.; Ono, S.; Shimmura, Y.; Sato, K.; Egashira, Y. Anti-inflammatory effect of pyroglutamyl-leucine on lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2014, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shirako, S.; Kojima, Y.; Tomari, N.; Nakamura, Y.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Sato, K. Pyroglutamyl leucine, a peptide in fermented foods, attenuates dysbiosis by increasing host antimicrobial peptide. npj Sci. Food 2019, 3, 18–27. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Gavrish, G.E.; Goriainov, S.V.; Chistyakov, V.V.; Astakhova, A.A.; Azbukina, N.V.; Sergeeva, M.G. Oxylipin profiles as functional characteristics of acute inflammatory responses in astrocytes pre-treated with IL-4, IL-10, or LPS. Int. J. Mol. Sci. 2020, 21950, 1780. [Google Scholar] [CrossRef] [PubMed]
- Gosset, V.; Goëbel, C.; Laine, G.; Delaplace, P.; Du Jardin, P.; Feussner, I.; Fauconnier, M.-L. The role of oxylipins and antioxidants on off-flavor precursor formation during potato flake processing. J. Agric. Food Chem. 2008, 56, 11285–11292. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jun, H.Y.; Kim, J.H. Antiadipogenic effect of Korean glasswort (Salicornia herbacea L.) water extract on 3T3-L1 adipocytes. J. Korean Soc. Food Sci. Nutr. 2014, 43, 814–821. [Google Scholar] [CrossRef]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: Size and optical density distribution. Eur. J. Histochem. EJH 2013, 57, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Prusty, D.; Park, B.-H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Poeggeler, B.; Schulz, C.; Pappolla, M.A.; Bodó, E.; Tiede, S.; Lehnert, H.; Paus, R. Leptin and the skin: A new frontier. Exp. Dermatol. 2010, 19, 12–18. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, J.H.; Kim, M.-J. Ethanol extract of Hippophae rhamnoides L. leaves inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Korean J. Plant Resour. 2015, 28, 582–590. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-J.; Jang, S.-H.; Kim, T.H.; Kim, H.-D.; Kim, S.-W.; Won, C.-K.; Cho, J.-H. Annual wormwood leaf inhibits the adipogenesis of 3T3-L1 and obesity in high-fat diet-induced obese rats. Nutrients 2017, 9, 554. [Google Scholar] [CrossRef]
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.J.; Le, C.T.; Beerthuijzen, J.M.; Murphy, W.J. Obesity as an immune-modifying factor in cancer immunotherapy. J. Leukoc. Biol. 2018, 104, 487–497. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.-J.; Kim, Y.-H.; Kim, D.-B.; Shin, G.-H.; Cho, J.-H.; Kim, B.K.; Lee, B.-Y.; Lee, O.-H. Codonopsis lanceolata extract prevents diet-induced obesity in C57BL/6 mice. Nutrients 2014, 6, 4663–4677. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Choi, J.-H.; Kim, Y.-G.; Lee, C.-H. Effect of dietary intake of Salicornia herbacea L. hot water extract on anti-obesity in diet-induced obese rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 950–956. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, S.; Kim, J.; Kim, B.; Wu, C.; Lee, J.H.; Jun, H.j.; Kim, N.; Lee, D.; Lee, S.J. Ursolic acid improves lipid and glucose metabolism in high-fat-fed C57BL/6J mice by activating peroxisome proliferator-activated receptor alpha and hepatic autophagy. Mol. Nutr. Food Res. 2015, 59, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2007, 65, S57–S63. [Google Scholar] [CrossRef]
- Seo, H.-B.; Kwak, Y.-Y.; Nam, J.-O.; Song, Y.-J.; Kim, B.-O.; Ryu, S.-P. Glasswort powder diet activates lipid metabolism in rat. J. Life Sci. 2012, 22, 478–485. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, S.K.; Choi, J.G.; Chung, S.H. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR mice. Arch. Pharmacal Res. 2006, 29, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Pichiah, P.T.; Cha, Y.S. Salicornia herbacea prevents weight gain and hepatic lipid accumulation in obese ICR mice fed a high-fat diet. J. Sci. Food Agric. 2015, 95, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).