Aligning the Epidemiology of Malnutrition with Food Fortification: Grasp Versus Reach
Abstract
1. Introduction
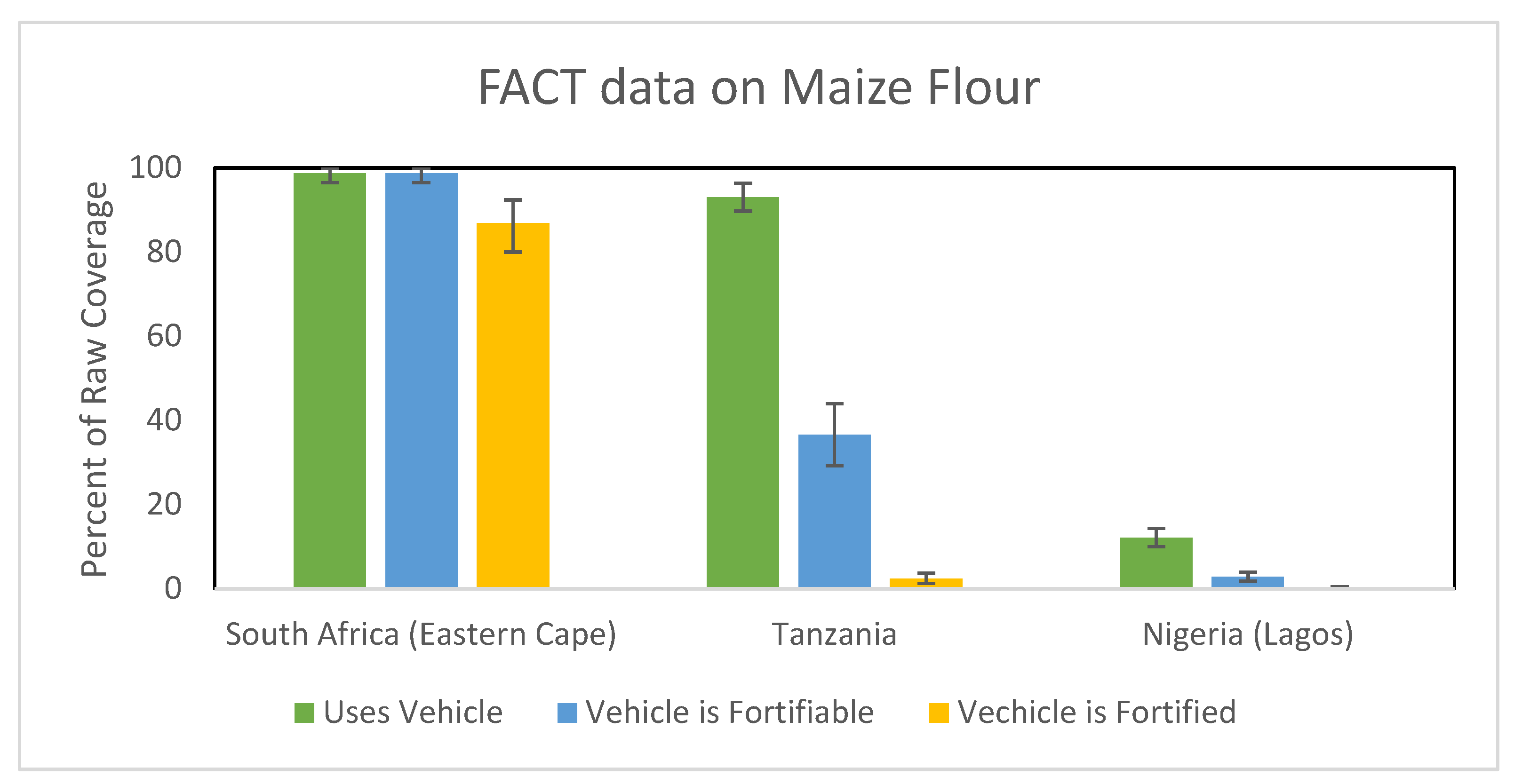
2. Measuring LSFF Program Coverage
3. LSFF Program Equity
4. Monitoring and Evaluation of LSFF Programs
5. Program Co-Coverage and Risk of Excess Intake
6. The Role of Modeling
7. Data Networks/Support
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; IFAD; WFP; WHO. The State of Food Security and Nutrition in the World 2021. FAO: Rome, Italy, 2021. [Google Scholar]
- Black, R. Micronutrient Deficiency-an Underlying Cause of Morbidity and Mortality. Bull. World Health Organ. 2003, 81, 79. [Google Scholar]
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.N. Micronutrient Deficiencies among Preschool-Aged Children and Women of Reproductive Age Worldwide: A Pooled Analysis of Individual-Level Data from Population-Representative Surveys. Lancet. Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Osendarp, S.J.M.; Adu-Afarwuah, S.; Ahmed, S.; Ajello, C.; Bergeron, G.; Black, R.; Christian, P.; Cousens, S.; de Pee, S.; et al. Review of the Evidence Regarding the Use of Antenatal Multiple Micronutrient Supplementation in Low- and Middle-Income Countries. Ann. N. Y. Acad. Sci. 2019, 1444, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.; Verburg, G.; Bhutta, Z.; Black, R.E.; de Pee, S.; Fabrizio, C.; Headey, D.; Heidkamp, R.; Laborde, D.; Ruel, M.T. Act Now before Ukraine War Plunges Millions into Malnutrition. Nature 2022, 604, 620–624. [Google Scholar] [CrossRef]
- Horton, S.; Mannar, V.; Wesley, A. Micronutrient Fortification (Iron and Salt Iodization); Copenhagen Consensus Center: Copenhagn, Denmark, 2008. [Google Scholar]
- Global Fortification Data Exchange. Available online: https://www.fortificationdata.org (accessed on 7 August 2022).
- Keats, E.C.; Neufeld, L.M.; Garrett, G.S.; Mbuya, M.N.N.; Bhutta, Z.A. Improved Micronutrient Status and Health Outcomes in Low-and Middle-Income Countries Following Large-Scale Fortification: Evidence from a Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 109, 1696–1708. [Google Scholar] [CrossRef]
- Castillo-Lancellotti, C.; Tur, J.A.; Uauy, R. Impact of Folic Acid Fortification of Flour on Neural Tube Defects: A Systematic Review. Public Health Nutr. 2013, 16, 901–911. [Google Scholar] [CrossRef]
- Mills, J.L.; Signore, C. Neural Tube Defect Rates before and after Food Fortification with Folic Acid. Birth. Defects Res. A Clin. Mol. Teratol. 2004, 70, 844–845. [Google Scholar] [CrossRef]
- Iodine Global Network. Iodine Global Network Annual Report. 2021. Available online: https://www.ign.org/cm_data/2021-IGN-Annual-Report-11560-1.pdf (accessed on 25 January 2023).
- Micronutrient Initiative. Fortification Rapid Assessment Tool; Micronutrient Initiative: Ottawa, ON, Canada, 2003. [Google Scholar]
- Coates, J.; Colaiezzi, B.; Fiedler, J.; Wirth, J.; Lividini, K.; Rogers, B. Applying Dietary Assessment Methods for Food Fortification and Other Nutrition Programs; Global Alliance for Improved Nutrition: Geneva, Switzerland, 2012. [Google Scholar]
- Friesen, V.M.; Mbuya, M.N.N.; Neufeld, L.M. The Fortification Assessment Coverage Toolkit (FACT); Global Alliance for Improved Nutrition: Geneva, Switzerland, 2019. [Google Scholar]
- Baye, K. Maximising Benefits and Minimising Adverse Effects of Micronutrient Interventions in Low-and Middle-Income Countries. Proc. Nutr. Soc. 2019, 78, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Dary, O.; Imhoff-Kunsch, B. Measurement of Food Consumption to Inform Food Fortification and Other Nutrition Programs: An Introduction to Methods and Their Application. Food Nutr. Bull. 2012, 33, S141–S145. [Google Scholar] [CrossRef]
- Luthringer, C.L.; Rowe, L.A.; Vossenaar, M.; Garrett, G.S. Regulatory Monitoring of Fortified Foods: Identifying Barriers and Good Practices. Glob. Health Sci. Pract. 2015, 3, 446–461. [Google Scholar] [CrossRef]
- Friesen, V.M.; Aaron, G.J.; Myatt, M.; Neufeld, L.M. Assessing Coverage of Population-Based and Targeted Fortification Programs with the Use of the Fortification Assessment Coverage Toolkit (FACT): Background, Toolkit Development, and Supplement Overview. J. Nutr. 2017, 147, 981S–983S. [Google Scholar] [CrossRef]
- Aaron, G.J.; Friesen, V.M.; Jungjohann, S.; Garrett, G.S.; Neufeld, L.M.; Myatt, M. Coverage of Large-Scale Food Fortification of Edible Oil, Wheat Flour, and Maize Flour Varies Greatly by Vehicle and Country but Is Consistently Lower among Themost Vulnerable: Results from Coverage Surveys in 8 Countries. J. Nutr. 2017, 147, 984S–994S. [Google Scholar] [CrossRef]
- Zamora, G.; De-Regil, L.M. Equity in Access to Fortified Maize Flour and Corn Meal. Ann. N. Y. Acad. Sci. 2014, 1312, 40–53. [Google Scholar] [CrossRef]
- Whitfield, K.C.; Smith, T.J.; Rohner, F.; Wieringa, F.T.; Green, T.J. Thiamine Fortification Strategies in Low- and Middle-Income Settings: A Review. Ann. N. Y. Acad. Sci. 2021, 1498, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Aaron, G.J.; Nahar, B.; Knowles, J.; Neufeld, L.M.; Rahman, S.; Mondal, P.; Ahmed, T. Household Coverage of Vitamin A Fortification of Edible Oil in Bangladesh. PLoS ONE 2019, 14, e0212257. [Google Scholar] [CrossRef]
- Knowles, J.M.; Garrett, G.S.; Gorstein, J.; Kupka, R.; Situma, R.; Yadav, K.; Yusufali, R.; Pandav, C.; Aaron, G.J.; Acuin, C.S.; et al. Household Coverage with Adequately Iodized Salt Varies Greatly between Countries and by Residence Type and Socioeconomic Status within Countries: Results from 10 National Coverage Surveys. J. Nutr. 2017, 147, 1004S–1014S. [Google Scholar] [CrossRef]
- Wirth, J.P.; Leyvraz, M.; Sodani, P.R.; Aaron, G.J.; Sharma, N.D.; Woodruff, B.A. Coverage of Adequately Iodized Salt Is Suboptimal and Rice Fortification Using Public Distribution Channels Could Reach Low-Income Households: Findings from a Cross-Sectional Survey of Anganwadi Center Catchment Areas in Telangana, India. PLoS ONE 2016, 11, e0158554. [Google Scholar]
- Ba, D.M.; Ssentongo, P.; Liao, D.; Du, P.; Kjerulff, K.H. Non-Iodized Salt Consumption among Women of Reproductive Age in Sub-Saharan Africa: A Population-Based Study. Public Health Nutr. 2020, 23, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Mkambula, P.; Mbuya, M.N.N.; Rowe, L.A.; Sablah, M.; Friesen, V.M.; Chadha, M.; Osei, A.K.; Ringholz, C.; Vasta, F.C.; Gorstein, J. The Unfinished Agenda for Food Fortification in Low- and Middle-Income Countries: Quantifying Progress, Gaps and Potential Opportunities. Nutrients 2020, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.; Dewey, K.G. Perspective: Putting the Youngest among Us into the Nutrition “Call for Action” for Food Fortification Strategies. Am. J. Clin. Nutr. 2021, 114, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Codling, K.; Laillou, A.; Rudert, C.; Borath, M.; Gorstein, J. Universal Salt Iodisation: Lessons Learned from Cambodia for Ensuring Programme Sustainability. Matern. Child. Nutr. 2020, 16, e12827. [Google Scholar] [CrossRef]
- Vasta, F. Digital QA/QC Systems for Food Fortification Project. Available online: https://www.gainhealth.org/digital-qaqc-systems-food-fortification-project (accessed on 22 March 2023).
- Mejia, L.A.; Kuo, W.Y.; Beltran-Velazquez, F. Provision of Micronutrients in Coexisting Public Health Programs and Risk of Excessive Intake: Regulatory Considerations. Ann. N. Y. Acad. Sci. 2019, 1446, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, S.; Gannon, B.M.; Davis, C.R.; Chileshe, J.; Kaliwile, C.; Masi, C.; Rios-Avila, L.; Gregory, J.F.; Tanumihardjo, S.A. High Provitamin A Carotenoid Serum Concentrations, Elevated Retinyl Esters, and Saturated Retinol-Binding Protein in Zambian Preschool Children Are Consistent with the Presence of High Liver Vitamin A Stores. Am. J. Clin. Nutr. 2015, 102, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Tanumihardjo, S.A.; Rhodes, E.C.; Mapango, C.; Kazembe, B.; Phiri, F.; Kang’ombe, D.D.; Sheftel, J.; Orchardson, V.; Tripp, K.; et al. Vitamin A Deficiency Has Declined in Malawi, but with Evidence of Elevated Vitamin A in Children. Am. J. Clin. Nutr. 2021, 113, 854–864. [Google Scholar] [CrossRef] [PubMed]
- van Stuijvenberg, M.E.; Dhansay, M.A.; Nel, J.; Suri, D.; Grahn, M.; Davis, C.R.; Tanumihardjo, S.A. South African Preschool Children Habitually Consuming Sheep Liver and Exposed to Vitamin A Supplementation and Fortification Have Hypervitaminotic A Liver Stores: A Cohort Study. Am. J. Clin. Nutr. 2019, 110, 91–101. [Google Scholar] [CrossRef]
- Mazariegos, M.; Martínez, C.; Mazariegos, D.I.; Méndez, H.; Román, A.V.; Palmieri, M.; Tomás, V. Análisis de La Situación y Tendencias de Los Micronutrientes Clave En Guatemala, Con Un Llamado a La Acción Desde Las Políticas Públicas; FHI360: Washington, DC, USA, 2016. [Google Scholar]
- Palmieri, M.; Flores-Ayala, R.; Mesarina, K.; Inés Mazariegos, D.; Martínez, C.; López, B.; Claudia Santizo, M.; Whitehead, R.D.; Yaw Addo, O.; Aponte, J.; et al. Intervention Program Methods and Outcomes Experiences and Lessons Learned in Developing and Implementing a Population-Based Nutrition and Health Surveillance System in Guatemala 2011–2021. Curr. Dev. Nutr. 2011, 18, nzac027. [Google Scholar]
- Abu, B.A.Z.; Oldewage-Theron, W.; Aryeetey, R.N.O. Risks of Excess Iodine Intake in Ghana: Current Situation, Challenges, and Lessons for the Future. Ann. N. Y. Acad. Sci. 2019, 1446, 117–138. [Google Scholar] [CrossRef]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess Iodine Intake: Sources, Assessment, and Effects on Thyroid Function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Vosti, S.A.; Kagin, J.; Engle-Stone, R.; Luo, H.; Tarini, A.; Clermont, A.; Assiene, J.G.; Nankap, M.; Brown, K.H. Strategies to Achieve Adequate Vitamin A Intake for Young Children: Options for Cameroon. Ann. N. Y. Acad. Sci. 2020, 1465, 161–180. [Google Scholar] [CrossRef]
- Kagin, J.; Vosti, S.A.; Engle-Stone, R.; Rettig, E.; Brown, K.H.; Nankap, M.; Ndjebayi, A. Measuring the Costs of Vitamin A Interventions: Institutional, Spatial, and Temporal Issues in the Context of Cameroon. Food Nutr. Bull. 2015, 36, S172–S192. [Google Scholar] [CrossRef] [PubMed]
- Making the Case for Micronutrient Data–Webinar. Available online: https://datafornutrition.org/webinars/ (accessed on 29 March 2023).
- Adams, K.P.; Luo, H.; Vosti, S.A.; Kagin, J.; Ngnie-Teta, I.; Ndjebayi, A.; Assiene, J.G.; Engle-Stone, R. Comparing Estimated Cost-Effectiveness of Micronutrient Intervention Programs Using Primary and Secondary Data: Evidence from Cameroon. Ann. N. Y. Acad. Sci. 2022, 1510, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Knight, F.; Woldt, M.; Sethuraman, K.; Bergeron, G.; Ferguson, E. Household-Level Consumption Data Can Be Redistributed for Individual-Level Optifood Diet Modeling: Analysis from Four Countries. Ann. N. Y. Acad. Sci. 2022, 1509, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Adams, K.P.; Ferguson, E.L.; Woldt, M.; Kalimbira, A.A.; Likoswe, B.; Yourkavitch, J.; Chrisinger, B.; Pedersen, S.; Segovia De La Revilla, L.; et al. Modeling Food Fortification Contributions to Micronutrient Requirements in Malawi Using Household Consumption and Expenditure Surveys. Ann. N. Y. Acad. Sci. 2022, 1508, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Knight, F.; Bourassa, M.W.; Ferguson, E.; Walls, H.; de Pee, S.; Vosti, S.; Martinez, H.; Levin, C.; Woldt, M.; Sethurman, K.; et al. Nutrition Modeling Tools: A Qualitative Study of Influence on Policy Decision Making and Determining Factors. Ann. N. Y. Acad. Sci. 2022, 1513, 170–191. [Google Scholar] [CrossRef]
- Smarter Futures. FORTIMAS an Approach for Tracking the Population Coverage and Impact of a Flour Fortification Program 2014. Available online: https://www.ifglobal.org/publications/fortimas-an-approach-for-tracking-the-population-coverage-and-impact-of-a-flour-fortification-program/ (accessed on 25 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourassa, M.W.; Atkin, R.; Gorstein, J.; Osendarp, S. Aligning the Epidemiology of Malnutrition with Food Fortification: Grasp Versus Reach. Nutrients 2023, 15, 2021. https://doi.org/10.3390/nu15092021
Bourassa MW, Atkin R, Gorstein J, Osendarp S. Aligning the Epidemiology of Malnutrition with Food Fortification: Grasp Versus Reach. Nutrients. 2023; 15(9):2021. https://doi.org/10.3390/nu15092021
Chicago/Turabian StyleBourassa, Megan W., Reed Atkin, Jonathan Gorstein, and Saskia Osendarp. 2023. "Aligning the Epidemiology of Malnutrition with Food Fortification: Grasp Versus Reach" Nutrients 15, no. 9: 2021. https://doi.org/10.3390/nu15092021
APA StyleBourassa, M. W., Atkin, R., Gorstein, J., & Osendarp, S. (2023). Aligning the Epidemiology of Malnutrition with Food Fortification: Grasp Versus Reach. Nutrients, 15(9), 2021. https://doi.org/10.3390/nu15092021





