Abstract
In recent years, people have tended to consume phytonutrients and nutrients in their daily diets. Isorhamnetin glycosides (IGs) are an essential class of flavonoids derived from dietary and medicinal plants such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba. This review summarizes the structures, sources, quantitative and qualitative analysis technologies, health benefits, bioaccessibility, and marketed products of IGs. Routine and innovative assay methods, such as IR, TLC, NMR, UV, MS, HPLC, UPLC, and HSCCC, have been widely used for the characterization and quantification of IGs. All of the therapeutic effects of IGs discovered to date are collected and discussed in this study, with an emphasis on the relevant mechanisms of their health-promoting effects. IGs exhibit diverse biological activities against cancer, diabetes, hepatic diseases, obesity, and thrombosis. They exert therapeutic effects through multiple networks of underlying molecular signaling pathways. Owing to these benefits, IGs could be utilized to make foods and functional foods. IGs exhibit higher bioaccessibility and plasma concentrations and longer average residence time in blood than aglycones. Overall, IGs as phytonutrients are very promising and have excellent application potential.
1. Introduction
Phytonutrients are chemical compounds that are only present in natural plants and are beneficial to the human body [1]. They are widely used in food and nutraceuticals due to their health-promoting benefits [2]. Flavonoids are a class of polyphenolic compound distributed in many fruits, vegetables, and plants [3]. The six major subclasses of flavonoids, which include flavones (e.g., luteolin), flavonols (quercetin), flavanones (hesperidin), catechins or flavanols (epicatechin), anthocyanidins (cyanidin), and isoflavones (daidzein), have been reported to represent various families of phytonutrients [4]. Accumulating evidence based on observational and clinical studies shows that a plant-based dietary pattern rich in fruits, vegetables, and whole grains has a clear effect on the prevention of various chronic diseases [5], and people also tend to consume dietary flavonoids from fruits and vegetables. Flavonoids are widely found in food, and most of them exist in their glycosidic forms [6,7].
Isorhamnetin glycosides (IGs), as natural flavonol compounds, are primarily extracted from various plant-based foods or medicinal plants such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba [8,9,10]. IGs are biologically important flavonols with proven beneficial properties that give them medicinal value [11,12]. They possess diverse biological and pharmacological properties, such as antioxidant, anti-inflammatory, anti-cancer, antidiabetic, anti-obesity, and hepatoprotective properties [13,14,15,16,17]. Due to their beneficial biological activities, IGs have been considered a significant potential class of phytonutrients, and an increasing number of products containing IGs are circulating on the market in many countries, including the United States, Canada, Mexico, China, India, and some European countries [18,19].
Here, for the first time, a review of all studies that describe the biological activity of IGs is presented, with particular emphasis on molecular signaling pathways and mechanistic explanations for their health-promoting potential. This review also introduces the structure of IGs and the primary sources of IGs. Moreover, current methods for the analysis and quantification of IGs are summarized. Furthermore, this paper also focuses on the main bioaccessibility of IGs. Overall, this article strongly supports the use of IGs as phytonutrients.
2. Structure of IGs
IGs are a type of glycosylated flavonol composed of an isorhamnetin skeleton and sugar groups. Their aglycone isorhamnetin, i.e., 3,4′,5,7-tetrahydroxy-3′-methoxyflavone, is an O-methylated flavonol (Figure 1). Generally, d-glucose, d-galactose, l-rhamnose, d-xylose, l-arabinose, sophorose, and rutinose are the most common sugar groups of IGs. They are linked to the aglycone by an O-glycosidic bond. According to the number of sugar groups, IGs are classified as mono-, di-, tri-, or tetra-glycosides. Position substitutions mostly happen at C-3 and C-7, for example, isorhamnentin-3-O-β-d-glucoside (4) and isorhamnetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside (20) from Hippophae rhamnoids [20]; isorhamnetin-3-O-α-l-rhamnoside (3) from Laportea bulbifera Wedd. [21]; and isorhamnetin-7-O-β-d-glucoside (1) and isorhamnetin-7-O-α-l-rhamnoside (2) from Nitraria tangutorum Bolor [22]. Of course, sometimes, substitution occurs at C-4′, for instance, isorhamnetin-4′-O-β-d glucoside (9) from Allium cepa L. [23]; isorhamnetin-3,4′-O-β-d-diglucoside (17) from Allium ascalonicum [24]; isorhamnetin-3-O-β-d -glucoside-4′-O-β-d-xyloside (21) [25]; and isorhammetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-4′-O-β-d-glucoside (35) [26]. In addition, some sugar group derivatives, such as isorhamnetin-3-O-[2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] (10) [27] and isorhamnetin-3-O-β-d (6-acetyl-glucoside) (7) [28], have also been obtained.
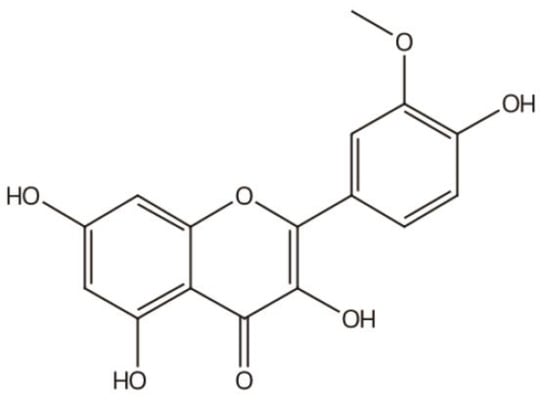
Figure 1.
Basic parent nucleus of isorhamnetin glycosides (IGs).
In the present review, we systematically summarize the 49 compounds of IGs reported thus far (Table 1 and Figure 2).

Table 1.
Isorhamnetin glycoside (IG) compounds (1–49). According to the number of sugar groups, IGs are divided into monoglycosides (1–9), diglycosides (10–34), triglycosides (35–48), and tetraglycosides (49).

Figure 2.
Chemical structures of IGs (compounds 1–49). Monoglycosides (1–9), diglycosides (10–34), triglycosides (35–48), and tetraglycosides (49). Abbreviations: Glc: D-glucose, Rha: L-rhamnose, Glccur: D-glucuronic, Gal: D-galactose, Xyl: D-xylose, Ara: L-arabinose. Abbreviations: Glc: d-glucose, Rha: l-rhamnose, Glccur: d-glucuronic, Gal: d-galactose, Xyl: d-xylose, Ara: l-arabinose.
3. Sources of IGs
IGs as nutritional supplements can be obtained from some foods and medicinal plants. Commonly consumed foods containing IGs include Hippophae rhamnoides, Opuntia ficus-indica, Vaccinium corymbosum, Vaccinium myrtillus, Brassica juncea, rice, and onions. The main medicinal sources of Igs are Ginkgo biloba, pollen Typhae, Microctis folium, Sambucus nigra, and Calendula officinalis (Figure 3).

Figure 3.
Plants with IG content.
3.1. Opuntia ficus-indica
Opuntia ficus-indica, otherwise known as the prickly pear or nopal cactus, is a multipurpose crop that grows wild in the arid and semi-arid regions of the world [70]. It is used not only in the diet to provide food and feed, but also for healthcare due to its antioxidant, anti-inflammatory, and anxiolytic properties [71,72].
IGs have already been described to be the most abundant flavonoid in Opuntia ficus-indica [8, 73–74] and in different Opuntia species [73]. Variable amounts of IG distributed in the cladode, pulp, and peel of the Tunisian Opuntia ficus-indica have been investigated [74]. Isorhamnetin-3-O-rutinoside (24) was found at very high and significant levels in the cladodes (703.33 ± 28.45 mg/100 g, DW (dry weight)), pulps (271.39 ± 25.59 mg/100 g, DW), and peels (254.51 ± 31.03 mg/100 g, DW). Moreover, isorhamnetin-3-O-glucoside (4) was also found in the cladodes (149.71 ± 10.13 mg/100 g, DW), pulps (184.14 ± 14.91 mg/100 g, DW) and peels (223.66 ± 14.44 mg/100 g, DW).
3.2. Hippophae rhamnoides
Hippophae rhamnoides (also named sea buckthorn) [20] constitutes a rich source of IGs [10]. Its berries have been categorized as a “medicine food homology” fruit by China’s National Health Commission for both nutritional and medicinal purposes [19]. Hippophae rhamnoides has a wide range of positive biological, physiological, and medicinal effects, such as antioxidative, anti-inflammatory, antidiabetic, anticarcinogenic, hepatoprotective, and dermatological effects [75].
IGs have been found in all parts of the sea buckthorn plant, including the berries, leaves, and seeds [76]. An investigation of six cultivated Hippophae rhamnoides varieties revealed that the berries contained an average of 917 mg/100 g DW of flavonol glycosides [77], whereas the content of flavonol glycosides in leaves was higher than that in berries, with an average of 1118 mg/100 g DW. Isorhamnetin-3-hexoside (75.0~406.1 mg/100 g, DW), isorhamnetin-3-rhamnosylglucoside (24) (52.5~190.0 mg/100 g DW), isorhamnetin-3-neohesperidoside (15) (110.1~323.8 mg/100 g, DW), and free isorhamnetin were predominant in the berries. Isorhamnetin-3-rhamnoside (3) (41.8~159.1 mg/100 g, DW), isorhamnetin-3-glucoside-7-rhamnoside (20) (67.6~129.3 mg/100 g, DW), isorhamnetin-3-rhamnosylglucoside (24) (66.7~253.0 mg/100 g, DW), isorhamnetin-3-neohesperidoside (15) (60.6~172.1 mg/100 g, DW), and isorhamnetin-3-rutinoside-7-glucoside (47) (36.0~117.3 mg/100 g, DW) were predominant in the leaves. Another study determined the content of IG from the berries of different cultivars of sea buckthorn. It was found that isorhamnetin derivatives represented over 65% of the total flavonols in sea buckthorn berries [78]. Isorhamnetin-3-O-rutinoside (24) had the highest content, in the range of 96.4~228 mg/100 g dry matter (DW). The study also confirmed that high concentrations of isorhamnetin-3-O-glucoside (4) (62.0~217.0 mg/100 g, DW) and isorhamnetin-3-O-glucoside-7-O-rhamnoside (20) (37.8~90.8 mg/100 g, DW) were detected in sea buckthorn berries.
3.3. Ginkgo biloba
Ginkgo biloba is one of the most commonly used herbal supplements in the world [79], and is also a crucial source of IGs [80]. It has been demonstrated that Ginkgo biloba has various remarkable biological properties, including neuroprotective, anticancer, cardioprotective, and stress-alleviating properties, and could affect tinnitus, geriatric conditions, and psychiatric disorders [81]. The major compounds of Ginkgo biloba are terpene lactones and flavone glycosides [82]. Flavonol glycosides are most prevalent in Ginkgo biloba leaves, and have been identified as derivatives of the aglycones quercetin, kaempferol, and isorhamnetin, which are, by themselves, present in only small amounts in the leaves. The dominant flavonol glycosides of Ginkgo biloba leaves were found to be kaempferol-3-O-rutinoside and isorhamnetin-3-O-rutinoside (24), and content of the latter ranged from 30 to 80 mg/100 g [9].
3.4. Pollen Typhae
Pollen Typhae, also known as Pu huang in Chinese, is the dried pollen of Typha angustifolia, Typha orientalis Presl, or plants of the same genus [83]. Pu huang was acknowledged as a functional food by the National Health Commission of the People’s Republic of China in 2002 [84]. Pollen Typhae has been used as a traditional remedy for analgesia, hemostasis, stranguria, hematuria, and injuries in China. Isorhamnetin-3-O-neohesperidoside (15) and typhaneoside (45), together with other minor flavonoid glycoside congeners, are the main active constituents of pollen Typhae [85]. Isorhamnetin-3-O-rhamnosylglucoside (24), isorhamnetin-3-O-neohesperidoside (15) (0.2546~0.3674%), and typhaneoside (45) (0.3361~0.5229%) were identified in different pollen Typhae sources [86,87,88].
3.5. Calendula officinalis
Calendula officinalis is an ornamental, culinary, and valuable herbaceous medicinal plant used medicinally worldwide [89]. It has been widely used as an anti-inflammatory, anticancer, sedative, and antipyretic drug [90]. Calendula officinalis is rich in nutrients and contains many terpenes, flavonoids, carotenoids, and lipids [91]. Typhaneoside (45) (2.22~5.01 mg/g, DW), narcissin (24) (2.10~8.52 mg/g, DW), isorhamnetin-3-O-glycoside (4) (0.42 ± 0.98 mg/g, DW), and isorhamnetin-3-O-(6″-acetyl)-glycoside (7) (0.69 ± 3.27 mg/g, DW) were identified in the florets of different varieties of Calendula officinalis [42,92]. Isorhamnetin glycosides are considered one of the anti-inflammatory material bases of Calendula officinalis [93].
3.6. Other Sources
IGs are found in many vegetables, fruits, and medicinal plants. Isorhamnetin-3-O-glucoside (4) is one of the most abundant flavonoids and is widely distributed in rice varieties [94]. Isorhamnetin-3,7-diglucoside (18) is a major flavonoid compound in Brassica juncea leaves [95]. IGs have also been detected in Vaccinium corymbosum and Vaccinium myrtillus [96,97]. Narcissin (24) (1.72–5.17 mg/g, DW) was extracted from Microctis folium, which is a commonly used herbal tea material [98,99]. IGs have also been found in different varieties of onion [100,101]. Isorhamnetin-4’-glucoside (9) has been reported as a minor flavonoid in onion [23]. Sambucus nigra, known as the “elderberry”, has a long history as a medicinal plant [102]. Its extract contains narcissin (24) and isorhamnetin-3-O-glucoside (4), which are capable of regulating glucose and lipid metabolism [103].
4. IG Identification and Quantification Methods
Different techniques have been used for the characterization, identification, and quantification of IGs, including spectral techniques and chromatographic techniques. The following review addresses the applicability of the ultraviolet–visible spectrum (UV), infrared spectroscopy (IR), nuclear magnetic resonance (NMR), mass spectrometry (MS), thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), ultra-performance liquid chromatography (UPLC), and high-speed counter-current chromatography (HSCCC) methods developed for the determination of IGs.
4.1. Spectral Techniques and Mass Spectrometry
Various spectral methods have been employed for the identification and quantification of IGs. UV, IR, MS, and NMR have been used to determine the structure of IGs.
4.1.1. UV
The UV absorption spectra of flavonoids mainly have two absorption bands in MeOH, i.e., band Ⅰ, which is caused by the electron transition of the cinnamoyl group, and band Ⅱ, which is caused by the electron transition of the benzoyl group. Regarding UV in flavonols, band Ⅱ absorption usually occurs in the region of 240–280 nm, and is relatively affected by increased hydroxylation of the A-ring; meanwhile, band Ⅰ absorption occurs in the region of 328–385 nm and is relatively affected by increased hydroxylation of the B-ring and C-ring. The addition of diagnostic reagents (NaOMe, NaOAc, NaOAc/H3BO3, AlCl3, and AlCl3/HCl) has a certain impact on the UV spectrum [104]. For example, the UV spectrum of isorhamnetin-3-O-β-d-galactoside-(1→4)-α-l-rhamnoside-(1→6)-β-d-galactoside (38) showed two absorption maxima: 359 nm for band I, and 258 nm for band II. A large bathochromic shift (up to 56 nm) in band I with NaOMe was observed, and was attributed to the presence of free 4′-OH. A free 7-OH group occurred with small bathochromic shift (16 nm) in band II upon the addition of a NaOAc reagent. Additionally, a 5, 7-dihydroxy A-ring was expected to result from the AlCl3 and AlCl3/HCl UV spectra (λmax nm: 359, 258 (MeOH); 415 (+56), 271 (NaOCH3); 403 (+46), 270 (A1Cl3); 403 (+46), 268 (AlCl3/HCl); 402, 274 (+16) (NaOAc); 364 (+5), 255 (NaOAc/H3BO4)) [55,105].
4.1.2. IR
IR can be used to determine the characteristic functional groups of IGs. For example, the characteristic functional groups of isorhamnetin-3-O-α-l-arabinoside-7-O-β-d-glucoside (26) isolated from the Callianthemum genus were determined using IR. Its spectrum showed the characteristic absorption bands of a hydroxyl (3444.87 and 3429.43 cm−1), a carbonyl (1653.00 cm−1), and a phenyl group (1600.92 and 1490.97 cm−1) [57]. If the IR spectrum contained a band of 1725 cm−1 for ester carbonyl, it indicated that a hydroxyl was acylated [92]. For example, the IR spectrum of isorhamnetin-3-O-(6-acetyl-glucoside) (7) showed a band at 1725 cm−1, which indicated the presence of an ester carbonyl [106].
4.1.3. NMR
NMR is a widely used spectroscopic technique for structure identification. The 1H NMR and 13C NMR spectra were used to determine chemical shifts in the functional groups and carbon skeleton of IGs.
Strong regularity in the 1H NMR spectrum of IGs can be found. The chemistry shifts of H-6 and H-8 of the A-ring are in the ranges 6.00~6.20 and 6.30~6.50 ppm, respectively, and appear as doublets, with a coupling constant of 2.5 Hz, because of two aromatic protons in the meta position. In the B-ring, H-2′, in the range of 7.20~7.90 ppm, appears as a doublet with a coupling constant of 2.5 Hz; H-5′, in the range of 6.70~7.10 ppm, appears as a doublet with a coupling constant of 8.5 Hz; H-6′, in the range of 7.20~7.90 ppm, appears as a doublet of doublets, with coupling constants of 2.5 and 8.5 Hz; and a singlet at 3.80 ppm belongs to 3′-OMe [23,57,107].
Some information on sugar linkage can also be obtained from the 1H NMR spectrum. The chemical shift in the H-1 (anomeric) proton varies according to the glycosylation pattern, e.g., 7-O-glucosides occurred at 4.8~5.2 ppm, while 7-O-rhamnosides occurred at 5.1~5.3 ppm; moreover, 3-O-glucosides occurred at 5.7~6.0 ppm, while 3-O-rhamnosidesoccurred at 5.0~5.1 ppm [105].
The A 13C NMR spectra of IGs can determine the number and environment of each carbon [57]. Moreover, the 1H and 13C-NMR signals and the linkages of each saccharide can easily be assigned using 2D-NMR, including COSY, HSQC, and HMBC technology. For example, an analysis of the HMQC spectrum of isorhamnetin-3-O-α-l-arabinopyranose-7-β-d-glucopyranoside (26) can enable all the protons and corresponding carbons in the structure to be assigned. In the HMBC spectrum, correlations between H-1” of arabinose and C-3, and between H-1‴ of glucose and C-7, indicated that arabinose was attached to the C-3 of the aglycone, and glucose was attached to the C-7 of the aglycone, respectively. Thus, they were combined to form isorhamnetin-3-O-α-l-arabinopyranose-7-β-d-glucopyranoside (26) [107].
4.1.4. MS
MS analysis is based on the mass-to-nucleus ratio and is used to determine molecular structure and weight. The loss of some ion fragments from a molecular or pseudomolecular ion is very characteristic of the mass spectra of IGs.
Electrospray ionization (ESI), an ionization technique, is often used for the MS analysis of IGs. The collision-induced dissociation of a pseudomolecular ion caused a characteristic fragment ion of isorhamnetin glycoside at m/z 315, which was assigned to isorhamnetin [108]. MS is also used in the determination of the attachment of sugars in IGs. In the mass spectrometry of isorhamnetin-glucoside-di-rhamnoside, a precursor ion at m/z 769 originated from the product ion at m/z 315, which is the characteristic ion of isorhamnetin aglycone, and the loss of 454 Da corresponded exactly to two rhamnose units (2 × 146 Da) and one hexose unit (162 Da) [109].
Atmospheric pressure chemical ionization (APCI) is another choice of method for detecting the molecular structure and weight of IGs. The regularities of the characteristic ions of isorhamnetin 3-O-glucoside (4) obtained in APCI-MS were analyzed; a pseudo molecular ion of m/z 477 and a second fragment of m/z 315 were provided, a characteristic fragment ion of m/z 315 was assigned to isorhamnetin, and the loss of 162 Da corresponded to one glucose unit [108].
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a powerful new technique that can rapidly identify and quantify IGs [110].
4.2. Chromatographic Techniques
IGs can be distinguished from each other on the basis of chromatographic techniques. Therefore, the analysis, characterization, and quantification of IGs are usually performed using the following chromatographic techniques: TLC, HPLC, UPLC, and HSCCC.
4.2.1. TLC
TLC is a method that can be used to detect IGs, and has the advantages of rapidity, simplicity, and economy. TLC is usually carried out in ascending mode on standard silica gel plates or microcrystalline cellulose. IGs can be eluted on thin-layer chromatography plates along with the standard compounds and distinguished by their retardation factor (Rf). TLC on silica gel layers for flavonol glycosides is often eluted with an EtOAc-Pyr-H2O-MeOH system, an n-BuOH–HOAc–H2O system, an EtOAc–methyl ethyl ketone–HOAc–H2O system, anEtOAc–HOAc–H2O system [111], a buthanol–EtOH–H2O system [23], or another developing solvent system [107]. Generally, the spots with IGs on a TLC plate can be observed directly under UV light, and the spots are dark. They will appear yellow or green under UV light after the addition of NH3 (gas) or a 1:1 mixture of 2% diphenyl-boric acid-ethanolamine complex in EtOH and 10% polethylenglycol 4000 in MeOH stain [112]. Moreover, a 1% ethanolic solution of ferric chloride or aluminum chloride is often used as a TLC dipping solution.
Isorhamnetin-3-O-glucoside (4) and isorhamnetin-3-O-rutinoside (24) were detected in the aerial parts of Peucedanum tauricum Bieb. TLC separation of the compounds was performed on silica gel plates with two different mobile phases (ethyl acetate–methyl ethyl ketone–formic acid–water, 5:3:1:1, or ethyl acetate–formic acid–water, 9:1:1). The abovementioned compounds were identified by comparing the hRf (100 × Rf) values with those of standard compounds [111].
4.2.2. HPLC and UPLC
HPLC is suitable for analyzing active components in natural extracts due to its simplicity, sensitivity, precision, and selectivity. In order to identify and quantify IGs, the chromatographic conditions of HPLC mainly include the use of a reverse-phase C18 column, acidic water, and MeOH or MeCN as a mobile phase [23,92,113].
HPLC–diode array detection (DAD) coupled with mass spectrometry can be also developed for the analysis of IGs. Narcissin (24) (4.9%) and isorhamnetin-3-sophoroside-7-rhamnoside (43) (3.7%) were found to be the major flavonoid glycosides in Hippophae rhamnoides, and were analyzed ia HPLC-DAD-ESI-MS/MS [114]. The HPLC-DAD-ESI-MS/MS analysis of the Hippophae rhamnoides berries of two subspecies provided information on the structure and composition of IGs [10].
Usually coupled with UV, ultraviolet photodiode array, or MS detectors, UPLC is an advanced liquid chromatography technique with the advantages of high resolution, high speed, and high sensitivity [115]. It has become a popular analytical tool for the analysis of many natural compounds, including IGs. Phenolic compounds in sea buckthorn were identified based on UPLC-MS analyses, and it was found that the major compounds contained isorhamnetin-3-O-rutinoside (24), isorhamnetin-3-O-sophoroside-7-O-rhamnoside (43), isorhamnetin-3-O-glucoside (4), and isorhamnetin-3-O-rhamnoside-glucoside-7-O-rhamnoside (40) [116]. The berries of Hippophae rhamnoides were analyzed via UPLC/PDA/ESI-MS, and it was revealed that their chemical constituents were composed of isorhamnetin-3-neohesperidin (15), isorhamnetin-3-glucoside (4), isorhamnetin-3-rhamnoside (3), isorhamnetin-3-sophoroside-7-rhamnoside (43), and free IG in different proportions [77].
4.2.3. HSCCC
High-speed counter-current chromatography (HSCCC), a new, continuous, and efficient liquid–liquid partition chromatography, eliminates the irreversible adsorptive loss of samples onto solid support matrix columns, and has excellent sample recovery compared with certain conventional methods [117,118]. IGs can be separated and purified efficiently through multiple distribution processes using HSCCC. Isorhamnetin-3-O-glucoside (4) (13 mg) was obtained via one-step HSCCC separation from a 240 mg sample of the medicinal herb lotus plumule [119]. HSCCC was also successfully applied to the preparative isolation of IGs [120].
5. The Health-Promoting Effects of IGs
IGs possess a variety of biological properties, including antioxidant, anti-inflammatory, and anti-cancer properties. Research has recently been undertaken to investigate their pharmacological benefits for the treatment of various diseases, such as diabetes, obesity, hepatic diseases, and thrombosis. Their health-promoting effects are summarized below.
5.1. Antioxidant Activity
Oxidative damage induced by free radicals results in detrimental outcomes, such as a loss of cellular function and the dysfunction of organic systems [121]. It is worth mentioning that numerous in vitro and in vivo studies have demonstrated the strong antioxidant and radical-scavenging properties of IGs (Table 2).
β-carotene-linoleic acid, 2,2-diphenyl-1-picrylhydrazil (DPPH) scavenging, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), oxygen radical absorbance capacity (ORAC), peroxyl radical-scavenging capacity (PSC), superoxide scavenging, peroxynitrite (ONOO(-)) assays, and CUPric reducing antioxidant capacity (CUPRAC) are commonly used indirect assays for identifying antioxidant activity. IGs isolated from the stamens of Nelumbo nucifera showed significant antioxidant activity, as determined via DPPH and ONOO(-) assays [11]. Brassicin (1) exhibited stronger free radical-scavenging ability than vitamin C [13] and exhibited DPPH radical- and ONOO(-)-scavenging activity [122]. Isorhamnetin 3-O-robinobioside (22), isorhamnetin 3-O-(2″,6″-O-α-dirhamnosyl)-β-galactoside (37) [123], typhaneoside (45), and isorhamnetin 3-O-neohesperidoside (15) [124] have been demonstrated to exhibit antioxidant activity using a DPPH radical-scavenging activity assay. Astragaloside (13) and narcissin (24) possessed antioxidant capacity, which was evaluated using ABST [118]. Narcissin (24) and isorhamnetin 3-O-rutinoside-7-O-glucoside (47) exhibited obvious antioxidant activity, which was detected using DPPH, β-carotene-linoleic acid, and ABST [65,125]. Isorhamnetin 3-O-neohesperidoside (15) was a potent inhibitor of xanthine oxidase and superoxide anion scavengers [126]. Furthermore, researchers have revealed the antioxidant properties of isorhamnetin 3-O-glucoside (4) and isorhamnetin 3-O-galactoside (8) in all the antioxidant activity tests employed [127,128,129,130].
Evaluation of the antioxidant properties of IGs were also carried out using various cell type experiments and animal models. The oral administration of isorhamnetin-3,7-diglucoside (18) to streptozotocin-induced diabetic rats significantly reduced their levels of 5-(hydroxymethyl) furfural (5-HMF), which is an indicator of the glycosylation of hemoglobin, and of stress [95]. Similarly, isorhamnetin 3-O-robinobioside (22) exhibited significant antioxidant effects on the human chronic myelogenous leukemia cell line K562 [131]. IGs had the ability to inhibit the formation of H2O2-induced radicals in the surrounding environment of intestinal epithelial cells [132]. Moreover, the transcriptional genes of the antioxidant system and the DNA repair pathway were upregulated after incubation with isorhamnetin 3-O-neohesperidoside (15) in pKS plasmid DNA [133]. Narcissin (24) and isorhamnetin 3-O-glucoside (4) demonstrated strong inhibition of reactive oxygen species (ROS) production in the oxidative burst activity of whole blood, neutrophils, and mononuclear cells [134]. Plant extracts rich in IGs also exhibited antioxidant activity. IG-rich concentrate from Opuntia ficus-indica juice had the ability to inhibit the formation of H2O2-induced radicals in the surrounding environment of intestinal epithelial cells [135]. The total antioxidant activity of Hippophae rhamnoides berry extracts, evaluated via ORAC and PSC, was significantly associated with total phenolics, including isorhamnetin-3-rutinoside (24) and isorhamnetin-3-glucoside (4) [136].

Table 2.
Antioxidant activity of IGs.
Table 2.
Antioxidant activity of IGs.
| Isorhamnetin Glycosides | Study Model | Method/Assay | Conclusion | Ref. |
|---|---|---|---|---|
| Isorhamnetin-3-O-glucoside (4), Narcissin (24) | / | DPPH, ONOO- | Showed potent antioxidant activity, with IC50 values of 11.76 and 9.01 μM in DPPH assay, and 3.34 and 2.56 μM in the ONOO- assay. | [11] |
| Brassicin (1) | / | DPPH, ABTS | Showed radical-scavenging activity of DPPH radical and peroxynitrite, with IC50 values of 13.3 and 2.07 μM. | [13] |
| Brassicin (1) | / | DPPH, peroxynitrite | Showed radical-scavenging activity of DPPH radical and peroxynitrite, with IC50 values of 13.3 and 2.07μM. | [122] |
| Narcissin (24); isorhamnetin, 3,4′-diglucoside (17) | LPS-induced Raw264.7 mouse macrophage cells | NO | Had an inhibitory effect on the production of NO induced by LPS. | [137] |
| Isorhamnetin-3-O-glucoside (4), 3-O-galactoside (8) | β-carotene- linoleic acid | DPPH, ABTS, CUPRAC | Act as free radical scavengers and chain-breaking antioxidants of DPPH, with IC50 values of 4.84 and 4.51 μM. | [127] |
| Isorhamnetin 3-O-galactoside (8) | DPPH | Showed high antioxidant activity compared to Trolox (standard antioxidant compound). | [128] | |
| Typhaneoside (45); isorhamnetin-3-O-neohesperidoside (15) | HUVECs treated with LPS | NO, MDA, SOD | Reduced levels of MDA, increased SOD activity and NO bioactivity. | [124] |
| Isorhamnetin 3-O-robinobioside (22) | K562 cell line induced by H2O2 | CAA | Inhibited oxidation (IC50 = 0.225 mg/mL) and genotoxicity (by 80.55% at 1000 μg/mL). | [131] |
| Isorhamnetin 3-O-robinoside (22); isorhamnetin 3-O-(2″,6″-O-α- dirhamnosyl)-β-galactoside (37) | / | DPPH | Effectively scavenged DPPH radicals, with IC50 values of 3.8 and 4.3 μM. | [123] |
| Isorhamnetin-3-O-glucoside (4) | / | DPPH, ABTS, FRAP | Highly correlated with DPPH, ABTS, and FRAP (r = 0.672, r = 0.660, r = 0.943, respectively). | [130] |
| Astragaloside (13), narcissin (24) | / | ABTS | Possessed antioxidant capacity, with IC50 values of 33.43 and 40.97 μg/mL. | [118] |
| Narcissin (24); isorhamnetin 3-O-glucoside (4) | / | DPPH | Showed pronounced antioxidant activity, with IC50 values of 165.62 and 177.91 μg/mL. | [65] |
| Narcissin (24); isorhamnetin-3-O-rutinoside-7-O-glucoside (47) | / | DPPH, ABTS | Showed obvious antioxidant activity. | [125] |
| Narcissin (24) | HepG2 cells | CAA | Showed significant in vitro antioxidant activity, with CAA value significantly correlated with narcissin (24) (R2 = 0.998). | [136] |
| IGs | H2O2-induced intestinal epithelial cells | ORAC | Able to counteract protein oxidation. | [132] |
| Isorhamnetin 3-O-neohesperidoside (15) | Hydroxyl radical-induced DNA damage pKS plasmid | MDA, DNA-strand scission assay | Transcriptions of several genes related to the antioxidant system (HMOX2 and TXNL) were upregulated. | [133] |
| Isorhamnetin 3-O-neohesperidoside (15) | / | ABTS, xanthine/xanthine oxidase | Was a potent inhibitor of xanthine oxidase (IC50 = 48.75 μg/mL) and superoxide anion scavengers (IC50 = 30 μg/mL). | [126] |
| Isorhamnetin 3-O-galactoside (8) | / | ABTS | Showed ABTS radical-scavenging activity (IC50 = 6 ± 0 μM). | [129] |
| Narcissin (24); isorhamnetin 3-O-glucoside (4) | Whole blood, neutrophils, or monocytes | ROS | Demonstrated potent inhibition of ROS production. | [134] |
5.2. Anti-Inflammatory Activity
IGs have anti-inflammatory properties due to different mechanisms. As an important inflammatory mediator, high-mobility-group protein 1 (HMGB1) contributes to organ damage and inflammation [138]. Isorhamnetin 3-O-galactoside (8) (5 μM) has been demonstrated to significantly inhibit the release of HMGB1 and reduce HMGB1-dependent inflammatory responses in human endothelial cells. It was found that 8 (4.8 mg/mouse) could also inhibit HMGB1 receptor expression, the HMGB1-mediated activation of NF-kB, and the production of tumor necrosis factor (TNF-α) in mice [139].
Mitogen-activated protein kinase (MAPK) signaling pathways, including p38, c-Jun N-terminal kinase (JNK), and extracellular regulated kinases (ERK), play crucial roles in inflammatory responses [140]. Isorhamnetin 3-O-galactoside (8) (50 μM) reduced cecal ligation and endothelin C receptor perforation-mediated shedding and down-regulated the phosphorylation of p38 MAPK, ERK 1/2, and JNK [14]. Similarly, isorhamnetin 3-O-glucuronide (5) exhibited anti-inflammatory activity by increasing heme oxygenase-1 (HO-1) expression and suppressing the JNK and p38 signaling pathways in LPS-induced RAW264.7 macrophage cells [141]. Moreover, isorhamnetin 3-O-glucuronide (5) inhibited the production of ROS (10 μM), as well as the release of elastase, in a human neutrophil model (1 μM) and suppressed the upregulation of inducible nitric oxide synthase (iNOS) expression (5 μM), and could be considered to display anti-inflammatory activity [46,142].
Many studies have shown the anti-inflammatory properties of IGs by inhibiting inflammatory cytokines. The inflammatory activity of narcissin (24) (100 μM) and isorhamnetin 3-O-glucoside (4) (100 μM) was mediated via the inhibition of nuclear factor kappa-B (NFκB) and inflammatory mediators such as TNF-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6) in phytohaemagglutinin-stimulated human peripheral blood mononuclear cells (PBMC) [132]. Likewise, narcissin (24) (40 μM) achieved the inhibition of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in advanced glycation end product (AGE)-induced RAW264.7 cells [143]. Isorhamnetin-3-O-[2,3-O-isopropylidene-α-l-rhamnopyranosyl]-(1→6)-O-β-d-glucopyranoside (11) (25 μM) showed a significant inhibitory effect on NO release and the secretion of the cytokines IL-6 and TNF-α [48]. Isorhamnetin-3,4′-diglucoside (17) (100 μg/mL) and isorhamnetin 3-O-glucoside (4) (100 μg/mL) have shown the inhibitory effect of IL-6 production on TNF-α-stimulated human osteosarcoma MG-63 cells [144]. Isorhamnetin 3-O-glucoside (4) (100 μg/mL) showed distinct anti-inflammatory activity with no toxicity on RAW 264.7 macrophage cells as compared to dexamethasone [145]. Seddik Ameur et al. studied the anti-inflammatory activity of IGs extracted from Opuntia ficus-indica flowers, and their results showed that isorhamnetin-3-O-robinobioside (22) is the product responsible for the anti-inflammatory activity [146]. Both Opuntia ficus-indica extract (OFI-E) and isorhamnetin-3-O-rhamnosylglucoside (24) (125 ng/mL) significantly inhibited cyclooxygenase-2 (COX-2), TNF-α, and IL-6 production, of which 24 compounds have been suggested to be suitable natural compounds for the development of a new anti-inflammatory ingredient [147]. The total flavonoid-rich IGs from sea buckthorn exhibited a protective effect against LPS/CS-induced airway inflammation by inhibiting the ERK, PI3K/Akt, and PKCα pathways and diminishing the expression of IL-1β, IL-6, and COX2 in mice [148].
5.3. Anti-Cancer Activity
Flavonoids have great potential for anticancer prevention [149]. IGs have also been proven to possess anticancer effects. Brassicin (1) (22.8 µg/mL) showed in vitro cytotoxicity against human colon cancer cells in the HCT116 cell line [150]. Isorhamnetin 3-O-neohesperidoside (15) (2.47 μg/mL) showed potent cytotoxicity against breast ductal carcinoma and colorectal adenocarcinoma (Caco-2) cells [151]. Narcissin (24) showed cytotoxic effects in Hela cells and the hormone dependent prostate carcinoma LNCaP cell line (IC50 = 20.5 μg/mL) [152,153].
Mechanically, IGs have been involved in the induction of apoptosis and the inhibition of cancer cell proliferation (Figure 4A). Apoptosis, the most vital cell death mechanism, ultimately contributes to tumor progression [154]. Mitochondria play an essential role in cell death signaling and ROS generation [155]. The production of ROS above a threshold level can trigger apoptosis in cancer cells, thereby limiting further cancer progression [156]. After the excessive production of ROS, the expression of genes related to the mitochondrial apoptosis pathway (Bax, Caspase9, and Caspase3) was aggravated, and the expression of the anti-apoptotic gene Bcl-2 was reduced [157]. Emerging evidence suggests that IGs promote ROS generation and the activation of mitochondria-dependent apoptosis in cancer cells (Figure 4B). Isorhamnetin-3-O-β-d-glucuronide (5) (25–100 μΜ) dose-dependently exhibited a strong cytotoxic effect through the ROS-dependent apoptosis pathway in the human breast cancer cell line MCF-7 [158]. In xenografted immunosuppressed mice, Opuntia ficus-indica extract (OFI-E) and isorhamnetin-3-O-glucosyl-rhamnoside (28) reduced tumor growth through the overexpression of cleaved Caspase-9, Hdac11, and Bai1 proteins. Moreover, OFI-E reduced the expression of Bcl-2 [159]. IGs from opuntia ficus-indica pads were cytotoxic against HT-29 cells (IC50 = 4.9 ± 0.5 μg/mL) and Caco-2 cells (IC50 = 8.2 ± 0.3 μg/mL) as they induced apoptosis [160]. Isorhamnetin-3-O-rhamnosylglucoside (24) induced cell death in the human colon cancer cell line HT-29 (10 μg/mL) through an increase in the Bax/Bcl-2 ratio, indicating that 24 induced apoptosis through mitochondrial damage [15]. Isorhamnetin 3-O-robinobioside (22) enhanced the apoptosis effects in tested human lymphoblastoid TK6 cells, which were confirmed via DNA fragmentation and PARP cleavage, indicating the release of caspase-3 [161]. Numerous studies show the beneficial effects of IGs and their capability for suppressing proliferation in cancer cells. Ana et al. extracted natural extracts from Opuntia ficus-indica and Opuntia robusta (ED50 value < 0.5 mg GAE/mL) residues, and evaluated their anti-proliferative effects in human colon cancer HT29 cells. Their results verified that IGs inhibited cell growth and induced cell cycle arrest at different checkpoints (G1, G2/M, and S) [162]. Isorhamnetin-3-O-rhamnosylglucoside (24) (394.68 ± 25.12 μM) inhibited the proliferation of chronic myelogenous leukemia cells [163,164]. Isorhamnetin 3-O-2′′′′-O-acetyl−β-d-xylopyranosyl-(1→6)-[β-d-apiofuranosyl-(1→2)]-β-d-glucopyranoside (36) (IC50 = 57/42/59 μM) and isorhamnetin 3-O-2‴-O-acetyl−β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside (10) (IC50 = 71/60/67 μM) were investigated for their potential cytotoxic activity in three cancer cell lines (Jurkat cells, cervical carcinoma cells, and MCF7 cells) and showed moderate antiproliferative activity [27].
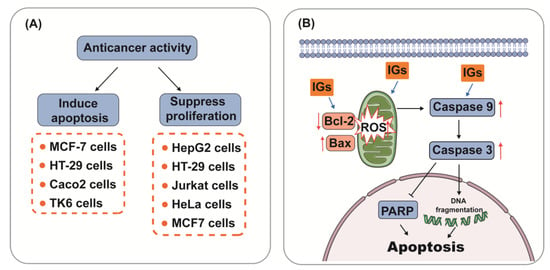
Figure 4.
Anticancer activity (A) and mechanism of regulating the apoptotic pathway (B) of IGs.
Furthermore, isorhamnetin 3-O-glucoside (4) (10 μM) exerted its inhibitory effects on matrix metalloproteinase-9 and -2 in HT1080 human fibrosarcoma cells by interfering with activator protein-1 transcription factor binding [165]. Isorhamnetin-3,7-diglucoside (18) (50–100 μg/mL) induced a 20% decrease in cancer intestinal cell survival through glycogen synthase kinase 3-beta regulation in intestinal cells [166].
5.4. Hepatoprotective Ability
The liver is the most essential and functional organ in the body, and it is where primary detox and metabolic events occur [167]. Liver injury can be caused by various factors, including alcohol, microbial infection, drugs, biological toxins, and chemical agents [168]. Flavonoids in many different foods and medicinal plants have therapeutic potential in liver disease [169].
Studies have confirmed that IGs play an important role in liver injury by modulating multiple pathways (Figure 5). The hepatoprotective effects of IGs are closely linked with their antioxidant and anti-inflammatory effects. Isorhamnetin 3-O-galactoside (8) (100 mg/kg) reduced serum TNF-α levels, aminotransferase activities, and the hepatic level of malondialdehyde (MDA); attenuated increases in iNOS and COX-2 protein and mRNA expression levels; attenuated increases in nuclear factor kappa-B (NF-κB) and c-Jun nuclear translocation; and augmented the levels of HO-1 and mRNA expression and the nuclear level of nuclear factor E2-related factor 2 (Nrf2) in a carbon tetrachloride (CCl4)-induced hepatic damage model (Figure 5A). This suggests that IGs exhibit hepatoprotective effects by enhancing the antioxidative defense system and reducing the inflammatory signaling pathways [16]. A similar result was obtained for the hepatoprotective effects of isorhamnetin 3-O-glucoside (4) (20 μg/mL/mouse). It suppressed the increase in plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in CCl4-induced liver injury mice [170]. Opuntia ficus-indica fruit juice (3 mL/rat) administration exerted protective and curative effects against the CCl4-induced degenerative process in rat liver [171]. The oral administration of a phenolic-rich fraction of sea buckthorn leaves (25–75 mg/kg) significantly protected against CCl4-induced elevation in AST, ALT, c-glutamyl transpeptidase, and bilirubin in the serum, and also protected against histopathological changes produced by CCl4, such as hepatocytic necrosis, fatty changes, and vacuolation [172]. In another study, typhaneoside (45) exhibited hepatoprotective effects on D-GalN-induced cytotoxicity in primary cultured mouse hepatocytes [173]. The phytochemical constituents of cactus branch extract (92 mg/kg), which were found to possess excellent antioxidant properties, had protective effects against lithium-induced hepatotoxicity and oxidative stress in rats [174].
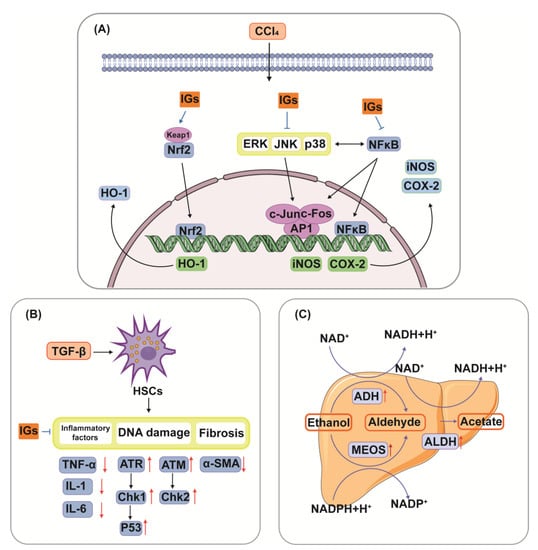
Figure 5.
Hepatoprotective mechanism of IGs. Networks of molecular signaling underlying anti-oxidative stress and anti-inflammatory effects of IGs in CCl4-induced hepatic damage(A). IGs inhibit TGF-β-induced activation of HSCs through the DNA damage pathway (B). Hepatic metabolic pathways through which IGs alleviate the adverse effects of ethanol (C).
IGs also had an improvement effect on hepatic lipid accumulation. In high-fat diet-fed mice, OFI-E (0.3%, 0.6%) reduced fatty acid synthesis and increased fatty acid oxidation and caused a decrease in hepatic fat accumulation, thereby preventing hepatic steatosis [70]. Isorhamnetin-3-O-glucoside (4), isorhamnetin, 3,4′-diglucoside (17), and isorhamnetin 3-O-β-d-glucopyranosyl-7-O-β-d-gentiobioside (47) (30 µM) had significant inhibitory effects on sodium oleate-induced triglyceride overloading in HepG2 cells [53]. Furthermore, biochemical and histopathological studies showed that sea buckthorn flavonoids (200 mg/kg, po) significantly improved biomarkers in the serum and liver of tetracycline-induced nonalcoholic fatty liver mice [175].
Zhang G et al. observed that isorhamnetin-3-O-β-d-glucopyranoside-7-O-α-l-rhamnoside (20) (40 μM) exhibited a profound inhibitory effect on the activation of hepatic stellate cells (HSCs) induced by transforming growth factor-β (TGF-β), and decreased the levels of inflammatory factors. It over-regulated the proteins of the DNA damage signaling pathway, including the ataxia telangiectasia mutated gene (ATM), Rad3-related gene (ATR), checkpoint kinase1 (Chk1), checkpoint kinase2 (Chk2), p53, and alpha-smooth muscle actin (α-SMA) (Figure 5B) [176]. In addition, the active components of sea buckthorn berry (20 and 40 mg/kg) had inhibitory effects on the development of fibrosis in rats after bile duct ligation, and they attenuated liver injury and inflammation by downregulating the expression of αSMA, while over-regulating the DNA damage signaling pathways and their related genes.
Isorhamnetin 3-O-β-d-glucopyranoside (4) alleviated the adverse effect of ethanol ingestion by enhancing the activities of alcohol dehydrogenase (ADH), the microsomal ethanol oxidizing system (MEOS), and aldehyde dehydrogenase (ALDH) in a hepatic alcohol-metabolizing enzyme system in rats (Figure 5C) [177]. In addition, sea buckthorn fermentation liquid (1.75, 2.675, 5.35 g/kg) protected against alcoholic liver disease and modulated the composition of the gut microbiota. It lowered ALT, AST, TNF-α, MDA, and IL-6, while modulating the gut microbiota composition [178].
5.5. Antidiabetic Activity
The antidiabetic properties of IGs may appear through different functions. IGs inhibit various pathways associated with the progression of diabetes, including the regulation of glucose metabolism and enhancing insulin secretion [179].
IGs exert inhibitory activity on several enzymes involved in diabetes management. In the small intestine, IGs inhibit the activity of α-amylase and α-glucosidase, thereby reducing the conversion of dietary saccharides into easily absorbed monosaccharide, and thus, reducing the postprandial enhancement of blood glucose levels (Figure 6). Isorhamnetin-3-O-glucoside (4) showed a strong ability to bind to α-amylase and α-glucosidase (the IC50 values were 0.16 ± 0.06 and 0.09 ± 0.01 µM) [180]. Narcissin (24) (IC50 = 0.129 mM) could be useful in lowering postprandial blood glucose by inhibiting α-amylase activity [181]. Meanwhile, 24 was a good 15-lipoxygenase (IC50 = 45 ± 2 µM) inhibitor [182,183]. Isorhamnetin glucosyl-rhamnosyl-pentoside (50 μg/mL) was reported to exhibit antihyperglycemic activity by inhibiting α-amylase activity [184]. Sea buckthorn aqueous extracts were correlated with lipase/α-amylase inhibitory activity in all phases of a digestion model in vitro, with gastric and intestinal fractions largely inhibiting enzyme activity [185].
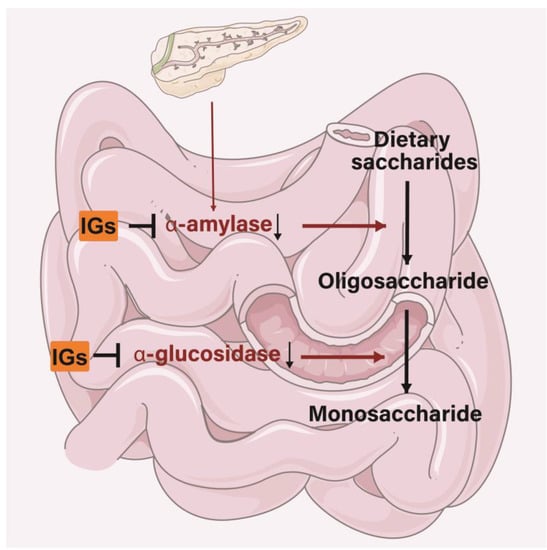
Figure 6.
Mechanism of IGs inhibiting α-amylase and α-glucosidase.
Dipeptidyl peptidase-IV (DPP-IV) inhibitors promote insulin secretion by prolonging the activities of incretin glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide [186]. In vitro experiments showed that isorhamnetin 3-O-glucoside (4) (IC50, 6.53 ± 0.280 μM) and isorhamnetin 3-O-rutinoside (24) (IC50, 8.57 ± 0.422 μM) had strong inhibitory effects on DPP-IV, which may provide new insights into isorhamnetin glucosides as DPP-IV inhibitors for controlling blood glucose [187]. The inhibition of protein tyrosine phosphatase 1B (PTP1B) activity increased insulin sensitivity and reduced blood glucose levels [17]. In vitro, 4 (IC50, 1.16 ± 0.03 μM) and 24 (IC50, 1.20 ± 0.05 μM) exhibited potent inhibitory activity against PTP1B, revealing that they could be potential anti-diabetic drugs [188].
Moreover, IGs improved the secondary complications of diabetes. In diabetes, the overexpression of aldose reductase induces the conversion of glucose to sorbitol via the polyol pathway, thereby inducing complications of diabetes, such as neuropathy, nephropathy, and retinopathy [189]. Isorhamnetin-7-O-β-neohesperidoside (12) (IC50 = 5.45 ± 0.26 µg/mL) and isorhamnetin 3-O-glucoside (4) (IC50 = 21.55 ± 1.52 µg/mL) exhibited remarkable aldose reductase inhibition activity [12]. It was also found that 4 (25 mg/kg) inhibited rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues [190]. Isorhamnetin 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside (24) (IC50 = 9 μM) was determined to exhibit a high degree of rat lens aldose reductase inhibitory activity in vitro [191].
5.6. Anti-Obesity Activity
Flavonoids could protect against obesity-related pathology by inhibiting adipogenesis and exerting anti-inflammatory activity [192]. Sea buckthorn leaf extract contains a high content of flavonoid glycosides, especially isorhamnine-3-glucoside (4) and quercetin-3-glucoside [78]. Flavonoid glycosides extracted from sea buckthorn leaves (SLGs) could suppress diet-induced obesity in C57BL/6J mice [98]. In this study, the authors mentioned that 12 weeks of oral administration with a high-fat diet (HFD, 60 kcal% fat) + 0.04% (w/w) SLGs significantly prevented adiposity and dyslipidemia by suppressing lipogenesis and the absorption of dietary fat. This anti-obesity effect was explained by the improvement of inflammation and a decrease in gluconeogenesis. Narcissin (24) and 4 (30 μM) showed moderate inhibitory effects on triglyceride and glycerol-3-phosphate dehydrogenase activity in a 3T3-L1 preadipocyte [193]. Furthermore, it was demonstrated by Chang-Suk Kong et al. that 4 (20 μM) potently suppressed adipogenic differentiation by downregulating peroxisome proliferator-activated receptor-γ, CCAAT/enhancer-binding proteins, sterol regulatory element-binding protein 1, and the adipocyte-specific proteins in 3T3-L1 preadipocytes. Furthermore, the specific mechanism mediating its action occurred through the activation of AMPK [194].
IG-rich plant extracts also have obvious anti-obesity effects. César Rodríguez-Rodríguez et al. have demonstrated that oral treatments of HFD, with a low (0.3%) or high (0.6%) dose of OFI-E rich in isorhamnetin glycosides, to C57BL/6 mice for 12 w ameliorated the development of HFD-induced obesity-related metabolic abnormalities by reducing weight gain, increasing insulin secretion, and enhancing energy expenditure in mice [70]. Further mechanistic studies verified that OFI-E and IGs could reduce fatty acid synthesis and increase fatty acid oxidation, leading to reduced fat accumulation in adipose tissue, thereby preventing adipocyte hypertrophy. OFI-cladode infusions (1%, administered daily in the drinking water) reduced proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 in the colon, adipose tissue, and spleen in Swiss male mice fed an HFD, as well as IL-6 and TNF-α in the plasma. These results suggested that OFI-cladode ameliorated HFD-induced obesity-related inflammation [195]. The results showed that intragastric administration of the extract from Hippophae rhamnoides seeds with concentrations of 100 and 300 mg/kg led to anti-obesity, triglyceride-lowering, and hypoglycemic effects in obese mice. It markedly inhibited macrophage infiltration into adipose tissue by regulating PPARγ and PPARα gene expression and inhibiting adipose tissue inflammation [196]. Oral sea buckthorn flavonoid administration (0.06% and 0.31% w/w, mixed in the diet) was able to alleviate body weight gain and insulin resistance in high-fat- and high-fructose-diet-induced C57BL/6J mice [197]. An extract of black soybean leaves (EBL), which mainly contains quercetin glycosides and isorhamnetin glycosides, inhibited HFD-induced obesity. Dietary supplements with 1% (wt/wt diet) EBL significantly reduced weight gain, improved glucose homeostasis, and decreased the glucose, insulin, HbA1c, and HOMA-IR index levels in HFD-fed mice. Mechanistic studies revealed that EBL inhibited hyperglycemia and hepatic steatosis through the adiponectin and AMPK signaling pathways, while isorhamnetin 3-O-α-l-rhamnopyranosyl (1→2)]-β-d-galactopyranosid (33) (50 μM) directly reduced lipid accumulation in HepG2 cells by enhancing AMPK activity [62].
5.7. Antithrombotic Activity
Thrombosis is a critical event in diseases correlated with atherosclerosis, myocardial infarction, and stroke [198]. The aggregation of platelets at the site of injury, as well as thrombin generation and fibrin formation triggered by the activation of tissue factors, are involved in thrombosis formation [199]. Therefore, the therapeutic mechanism includes the inhibition of platelet activation, adhesion, and aggregation, the improvement of fibrinolytic system function, and the regulation of coagulation system function [200].
Sae-Kwang Ku et al. assessed the antithrombotic activity of isorhamnetin 3-O-galactoside (8) from Oenanthe javanica. Studies have confirmed that it (10 μM) could significantly prolong the activated partial thromboplastin time and prothrombin time, inhibit the activity of thrombin and factor X, and inhibit the thrombin in human umbilical vein endothelial cells activated by TNF-α and the generation of factor X. In addition, isorhamnetin 3-O-galactoside (2.5 mg/kg) also elicited consistent anticoagulant effects in mice [201]. IGs isolated from sea buckthorn fruits showed marked anticoagulant and antiplatelet activity [202]. A thrombus-formation analysis system indicated that isorhamnetin 3-O-β-glucoside-7-O-α-rhamnoside (20) (50 µg/mL) and isorhamnetin 3-O-β-glucoside-7-O-α-(3‴-isovaleryl)-rhamnoside (34) (50 µg/mL) demonstrated anti-coagulant potential in whole blood. BartoszSkalski et al. came to the consistent conclusion that isorhamnetin 3-O-β-glucoside-7-O-α-(3‴-isovaleryl)-rhamnoside (34) (5, 10 µg/mL) possessed anti-platelet and anticoagulant properties, which extended the thrombin time and inhibited aggregation induced by thrombin [69]. Isorhamnetin 3-O-rhamnosylglucoside (24) (0.4 mg/mL) can stimulate the endothelial cell to produce tissue plasminogen activators and prostaglandins and possesses antithrombotic properties [87]. Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-glucoside (15) isolated from pollen Typhae can also stimulate porcine aortic endothelial cells to produce tPA, and it was revealed that it has antithrombotic effects. Sae-Kwang Ku et al. demonstrated that isorhamnetin-3-O-galactoside (8) (10 μM) inhibited the TNF-α-induced production of plasminogen activator inhibitor type 1 (PAI-1) and reduced the ratio of PAI-1 to tissue-type plasminogen activator (tPA) [201].
5.8. Toxic Effects
Flavonoids are natural components of fruits, vegetables, tea, wine, traditional medicines (such as ginkgo biloba), and a considerable number of herbal dietary supplements. With growing interest in alternative medicine, the general population is consuming more flavonoids [203]. Since flavonoids are common edible ingredients in our daily diets, research on their potential cytotoxicity is warranted.
Currently, there are no systematic toxicological studies on IGs, and further studies are needed. Bee bread (BB) is a fermented mixture of plant pollen, honey, and bee saliva, and is rich in flavonoid glycoside derivatives [204]. Filipa Sobral et al. collected a variety of BB samples, and the most abundant compounds in BB1 (>400 µg/mL) were isrohamnetin-O-hexosyl-O-rutinoside and isorhamnetin-O-pentosyl-hexoside. They found that the BB1 sample showed no toxicity to non-tumor porcine liver primary cells [205]. Isorhamnetin-3-rutinoside-4′-glucoside (35), isolated from P. lanceolata inflorescences, showed significantly less cytotoxicity towards the nontumorigenic cell line MCF-12A at a concentration of 400 µM [206]. Isorhamnetin-3-O-β-d-galactopyranoside (8) and isorhamnetin-3-O-β-d-glucopyranoside (4) (100 µg/mL) isolated from Salsola imbricata Forssk. exhibited no cytotoxicity in RAW 264.7 macrophage cells [158]. Furthermore, it was demonstrated that the viability of PBMCs was slightly decreased after 48 h of incubation with isoretin-3-O-rutin (24) (0–180 µM) from Cyrtosperma johnstonii. However, the decrease in cell viability was no greater than 30% [207]. A brine shrimp toxicity assay of extracts and isolated compounds from Terminalia macroptera leaves showed that narcissin (24) was not toxic against brine shrimp larvae at the tested concentrations (200 µM) [182].
6. Bioaccessibility of IGs
The bioaccessibility of bioactive compounds refers to the maximum fraction of the compound released from the food matrix into the lumen of the gastrointestinal tract to be absorbed [208]. Most flavonoids exist in nature as glycosides, in which sugar residues modify the absorption mechanism and their ability to enter cells or interact with transporters and cellular lipoproteins [209,210]. Flavonoid glycosides exhibit better bioavailability both in vitro and in vivo, which is probably due to their higher aqueous solubility and stability during digestion [8]. At the same time, the gut microbiota plays an important role in improving the bioavailability and enhancing the absorption of flavonoids [211]. The deglycosylation of flavonoid glycosides by the gut microbiota enhances the bioavailability of flavonoids [212].
Compared with isorhamnetin aglycone, IGs have higher accessibility. Antunes-Ricardo et al. found that glycosylation protected isorhamnetin from degradation during simulated digestion, and IGs were better retained in the circulatory system than aglycone [8]. Isorhamnetin-3-O-rutinoside (24) (93.2 ± 0.2%) and isorhamnetin 3-O-glucoside (4) (66.8 ± 1.7%) from almond skins showed higher bioaccessibility than isorhamnetin (25.1 ± 7.0%) after simulated digestion [213]. Isorhamnetin glucosyl-rhamnosyl-rhamnoside, isorhamnetin glucosyl-rhamnosyl-pentoside, isorhamnetin hexosyl-hexosyl-pentoside, and isorhamnetin glucosyl-pentoside showed high bioaccessibility in the peels of four prickly pear varieties during in vitro simulated gastrointestinal digestion [214]. Isorhamnetin glucosyl-rhamnosyl-rhamnoside and isorhamnetin glucosyl-pentoside in Opuntia ficus-indica cladodes showed bioaccessibility values of 58% and 38% [215].
It was also reported that the antidiabetic, anti-inflammatory, and antiallergic activities of flavonoid glycosides were similar or even higher than those of aglycones when provided orally [216,217,218,219]. The effect of flavonoid glycosides is beneficial, probably due to the fact that flavonoid glycosides maintain higher plasma concentrations and have a longer mean residence time in the blood than aglycones [220]. Typhaneoside (45) and isorhamnetin-3-O-neohesperidoside (15) were detected immediately after the oral administrations of pollen typhae extract in rats, indicating that they were rapidly absorbed after oral administration [86,221]. IGs in sea buckthorn berries were monoglucuronidated in humans and were readily bioavailable [222]. Following the ingestion of lightly fried onions, flavonols were absorbed into the plasma of humans as glycosides, with a higher accumulation of isorhamnetin-4′-glucoside (9) in the plasma and urine than quercetin conjugates, which indicated that 9 may be preferentially absorbed [223]. Similarly, the results of a randomized crossover supplementation trial in female volunteers showed that 9 underwent significant elevation in the plasma after the ingestion of onion powder [224]. Antunes-Ricardo et al. reported that IGs found naturally in O. ficus-indica have a longer elimination half-life than isorhamnetin, suggesting that they can maintain constant plasma concentrations, and thus, prolong their biological effects [8].
Planar lipophilic polyphenols, such as curcumin, epigallocatechin gallate, quercetin, and genistein, are known as Pan-Assay Interference Compounds (PAINS) or Invalid Metabolic Panaceas (IMPS) because of their ability to interfere with membrane dipole potential [225]. Ana Marta de Matos et al. demonstrated that compounds produced via C-glycosylation are no longer able to alter the membrane dipole potential [226]. However, O-glycosylated compounds are easily hydrolyzed in the gut, so they are not suitable for this strategy. There are no more studies on the interference of isorhamnetin glycosides on membrane dipole potential, so further research in this field is warranted.
7. Marketed Products Related to IGs
In recent years, there has been increased interest in natural phytonutrients. Phytonutrients, such as beta-carotene (representative food, e.g., carrots), lutein (collard), isoflavones (soybeans), resveratrol (red wine), and anthocyanins (grapeseed), are known to provide a variety of significant benefits to humans and improve human well-being [227]. IGs as phytonutrients have been used in food and as a remedy against different health disorders, and processed into various products.
7.1. Food and Functional Food Products Using Opuntia ficus-indica
The cultivation for Opuntia ficus-indica is scattered across various parts of the world, such as Central and South America, Southern Spain, the Mediterranean Sea, Angola, Australia, India, and South Africa [228,229,230]. Opuntia ficus-indica has long been marketed in different forms, such as fresh, frozen, or pre-cooked, and used as fresh greens and in salads in Mexico, Latin America, South Africa, and the Mediterranean area [231]. As a popular dietary supplement in the United States, Opuntia ficus-indica products could be potentially utilized for body weight control and liver function support.
Opuntia ficus-indica can be processed into many food products (Figure 6). Its cladodes have been used as a vegetable, usually eaten freshly peeled, in salads, cooked (boiled, fried, or deep-fried), or made into a juice or sauce [232,233]. Its fruit can be squeezed and used to produce juices, jams, candies, beverages, ice creams, and teas [234,235,236], and has also been added to rice field bean flour to produce an innovative gluten-free pasta [237]. Its peel has been utilized as a substitute for vitamin E, as an antioxidant in margarine preservation [238]. Its seed can be used to make oil [239]. Freeze-dried pulp can be added to rice or corn flour, resulting in a puffed flavanol-rich snack [240]. Its cladodes, pulp, or seeds, or whole plant, can be made into flour, which can partly substitute wheat or corn flour in doughs, bread, cookies, snacks, or desserts [18,241,242]. Opuntia ficus-indica-related products on the market have been listed in Table 3.

Table 3.
Selected examples of marketed Opuntia ficus-indica products.
During the processing of Opuntia ficus-indica products, the processing technology used preserves the fruit’s nutritional and sensory characteristics, and increases the content of IGs. It was reported that the extrusion or the preparation of concentrated juice pretreated with a pulsed electric field of Opuntia ficus-indica allowed for an increase in isorhamnetin glycoside content, especially isorhamnetin-3-O-rutinoside (24) [243,244].
7.2. Food and Functional Food Products of Hippophae rhamnoides
Hippophae rhamnoides possesses abundant bioactive compounds that can be utilized in the preparation of functional food products [19]. The berries, seeds, leaves, and even bark can be processed into supplemental products that gave the body all-natural assistance for many different functions. Hippophae rhamnoides leaves have gradually begun to be used in the food industry for tea processing [245]. A wide variety of products—jams, jellies, juices, powder, and seed oils—can be formulated from Hippophae rhamnoides berries [76]. Over the years, Hippophae rhamnoides products have increased in popularity (Table 4) [246]. Hippophae rhamnoides product consumption as part of the regular diet is common in Asia, the United States, and some European countries [247].

Table 4.
Selected examples of marketed Hippophae rhamnoides products.
It was found that isorhamnetin derivatives were the most important flavonoids in Hippophae rhamnoides fruit juice [248]. The treatment of by-products in juice production via solvent-free microwave hydrogenation diffusion and gravity technology obtained more flavonoids, such as isorhamnetin, isorhamnetin 3-O-glucoside (4), isorhamnetin 3-O-rutinoside (24), than conventional solvent extraction [249].
8. Conclusions and Prospects
IGs are bioactive flavonoids found in various plants, such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba. Routine and innovative assay methods, such as IR, TLC, NMR, UV, MS, HPLC, UPLC, and HSCCC, have been widely used for the characterization and quantification of IGs. Numerous lines of findings have elucidated the pharmacological activities of IGs. These studies have focused on multiple properties of IGs, such as their antioxidant, anti-inflammatory, or anticancer capacities. In recent years, IGs have attracted more attention due to their health-promoting effects on diabetes, obesity, liver injury, and thrombosis. Furthermore, the sugar residues of IGs make them more bioaccessible than aglycones. Meanwhile, IGs maintain higher plasma concentrations and longer average residence time in the blood than aglycons. This indicates that IGs are potent phytonutrients with potential health-promoting effects.
Growing evidence based on observational and clinical studies suggests that a plant-based diet based on fruits, vegetables, and whole grains has a significant effect on preventing various chronic diseases, including cancer, diabetes, and obesity [5]. IG traces have been identified in Hippophae rhamnoides, Opuntia ficus-indica, Vaccinium corymbosum, Vaccinium myrtillus, Brassica juncea, rice, onions, Ginkgo biloba, pollen Typhae, Microctis folium, Sambucus nigra, and Calendula officinalis, among their dietary and medicinal components [8,9,10]. People are more comfortable consuming phytochemicals and nutrients in their daily diets, such as fruit, vegetable juice, and tea [250]. They make vegetables and fruits into salads, blend them in juices, and process them into by-products. Hippophae rhamnoides could be served in pure juices, wine, and health supplements [251]. Meanwhile, Opuntia ficus-indica is used in many forms, including in food, feed, health, and nutrition, and is also used in formulated products, including teas, jams, and juices [252]. Additionally, IGs could be ingested from these plants. The extensive studies herein provide a sufficiently solid basis to discuss the health claims and health-promoting biological activities of IGs in humans. However, the clinical pharmacological effects of Igs still require further study so that their protective effects can be fully exploited in medical or pharmaceutical settings. The pharmacological mechanism of IGs also needs to be further elucidated to provide a material basis for their clinical investigation and application.
Author Contributions
F.G., L.C. (Liang Chen), and Y.L. organized and supervised this study; H.W. and L.C. (Lijia Chen) wrote the manuscript and prepared the tables and figures; B.Y. and J.D. contributed to checking the tables and figures; F.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were modified from Servier Medical Art (http://smart.servier.com/) (accessed on 12 January 2023), licensed under the Creative Commons Attribution 3.0 Generic License (https://creativecommons.org/licenses/by/3.0/)(accessed on 4 September 2022).
Conflicts of Interest
There are no conflicts to declare.
Abbreviations
| 5-HMF | 5-hydroxymethylfurfural |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) |
| ADH | alcohol dehydrogenase |
| AGEs | advanced glycation end products |
| ALDH | aldehyde dehydrogenase |
| ALT | alanine aminotransferase |
| Ara | l-arabinose |
| AST | aspartate aminotransferase |
| ATM | ataxia telangiectasia mutated gene |
| ATR | ATM and Rad3-related gene |
| BB | bee read |
| CCl4 | carbon tetrachloride |
| Chk1 | checkpoint kinase1 |
| Chk2 | checkpoint kinase2 |
| CAA | cellular antioxidant activity assay |
| COX-2 | cyclooxygenase-2 |
| CUPRAC | CUPric reducing antioxidant capacity |
| DAD | diode array detection |
| DPPH | 2,2-diphenyl-1-picrylhydrazil |
| DPP-IV | dipeptidyl peptidase-IV |
| DW | dry weight |
| ERK | extracellular regulated kinases |
| ESI | electrospray ionization |
| FRAP | ferric reducing antioxidant power |
| Gal | d-galactose |
| Glc | d-glucose |
| Glccur | d-glucuronic |
| HFD | high-fat diet |
| HHP | high hydrostatic pressure |
| HMGB1 | high-mobility-group protein 1 |
| HO-1 | heme oxygenase-1 |
| HPLC | high-performance liquid chromatography |
| HSCCC | high-speed counter-current chromatography |
| HSCs | hepatic stellate cells |
| IGs | isorhamnetin glycosides |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| IR | infrared spectroscopy |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MEOS | microsomal ethanol oxidizing system |
| MS | mass spectrometry |
| NF-κB | nuclear factor kappa-B |
| NMR | nuclear magnetic resonance |
| Nrf2 | nuclear factor E2-related factor 2 |
| OFI-E | opuntia ficus-indica extract |
| ONOO(-) | peroxynitrite |
| ORAC | oxygen radical absorbance capacity |
| PAI-1 | plasminogen activator inhibitor type 1 |
| PBMC | human peripheral blood mononuclear cells |
| PSC | peroxyl radical-scavenging capacity |
| PTP1B | protein tyrosine phosphatase 1B |
| Rha | l-rhamnose |
| ROS | reactive oxygen species |
| Xyl | D-xylose |
| TGF-β | transforming growth factor-β |
| TLC | thin-layer chromatography |
| TNF-α | tumor necrosis factor |
| tPA | tissue-type plasminogen activator |
| UPLC | ultra-performance liquid chromatography |
| UV | ultraviolet radiation |
| α-SMA | alpha-smooth muscle actin |
References
- Monjotin, N.; Amiot, M.; Fleurentin, J.; Morel, J.; Raynal, S. Clinical Evidence of the Benefits of Phytonutrients in Human Healthcare. Nutrients 2022, 14, 1712. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.; Cabrita, A.; Malushi, N.; Oliveira, H.; Papa, L.; Rodrigues, J.; Fonseca, A.; Maia, M. Unravelling the phytonutrients and antioxidant properties of European Vicia faba L. seeds. Food Res. Int. 2019, 116, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Saraei, R.; Marofi, F.; Naimi, A.; Talebi, M.; Ghaebi, M.; Javan, N.; Salimi, O.; Hassanzadeh, A. Leukemia therapy by flavonoids: Future and involved mechanisms. J. Cell. Physiol. 2018, 234, 8203–8220. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Bai, Z.S.; Guo, T.T.; Li, J.Y.; Li, Y.W.; Hou, Y.; Chen, G.; Li, N. Dietary flavonoids and human top-ranked diseases: The perspective of in vivo bioactivity and bioavailability. Trends Food Sci. Technol. 2022, 120, 374–386. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Tao, H.; Li, L.; He, Y.; Zhang, X.; Zhao, Y.; Wang, Q.; Hong, G. Flavonoids in vegetables: Improvement of dietary flavonoids by metabolic engineering to promote health. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Marilena, A.R.; César, R.-R.; Janet, G.-U.; Eduardo, C.C.E.; Sergio, S.-S. Bioaccessibility, Intestinal Permeability and Plasma Stability of Isorhamnetin Glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar]
- Wang, L.; Fan, X.; Jian, Y.; Dong, M.; Yang, Q.; Meng, D.; Fu, Y. A sensitive and selective multiple reaction monitoring mass spectrometry method for simultaneous quantification of flavonol glycoside, terpene lactones, and biflavonoids in Ginkgo biloba leaves. J. Pharm. Biomed. Anal. 2019, 170, 335–340. [Google Scholar] [CrossRef]
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trépanier, M.; Kallio, H.; Yang, B. Flavonol glycosides in berries of two major subspecies of sea buckthorn (Hippophaë rhamnoides L.) and influence of growth sites. Food Chem. 2016, 200, 189–198. [Google Scholar] [CrossRef]
- Hyun, S.; Jung, Y.; Chung, H.; Jung, H.; Choi, J. Isorhamnetin glycosides with free radical and ONOO-scavenging activities from the stamens of Nelumbo nucifera. Arch. Pharmacal. Res. 2006, 29, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Abdel Motaal, A.; Salem, H.; Almaghaslah, D.; Alsayari, A.; Bin Muhsinah, A.; Alfaifi, M.; Elbehairi, S.; Shati, A.; El-Askary, H. Flavonol Glycosides: In Vitro Inhibition of DPPIV, Aldose Reductase and Combating Oxidative Stress are Potential Mechanisms for Mediating the Antidiabetic Activity of Cleome droserifolia. Molecules 2020, 25, 5864. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Song, N.; Nam, T.; Shrestha, S.; Park, H.; Lyu, H.; Kim, D.; Lee, G.; Woo, Y.; Jeong, T.; et al. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 10354–10359. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Han, M.S.; Bae, J.S. Down-regulation of endothelial protein C receptor shedding by persicarin and isorhamnetin-3-O-galactoside. Thromb. Res. 2013, 132, e58–e63. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Hernández-Reyes, A.; Uscanga-Palomeque, A.C.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Gutiérrez-Uribe, J.A. Isorhamnetin glycoside isolated from Opuntia ficus-indica (L.) MilI induces apoptosis in human colon cancer cells through mitochondrial damage. Chem. Biol. Interact. 2019, 310, 108734. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, H.I.; Kim, K.M.; Kim, S.J.; Choi, J.S.; Kim, Y.S.; Lee, S.M. Isorhamnetin-3-O-galactoside Protects against CCl4-Induced Hepatic Injury in Mice. Biomol. Ther. 2012, 20, 406–412. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.; Abbas, G.; Adekenov, S.; Hussain, W.; Ali, I. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: Patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.S.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia Ficus Indica Edible Parts: A Food and Nutritional Security Perspective. Food Rev. Int. 2022, 38, 930–952. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Liao, X. Bioactive compounds, health benefits and functional food products of sea buckthorn: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782. [Google Scholar] [CrossRef]
- Ciesarova, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolkova, B.; Koplik, R.; Belajova, E.; Kukurova, K.; Dasko, L.; Panovska, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Yang, M.C.; Choi, S.Z.; Lee, S.O.; Chung, A.K.; Nam, J.H.; Lee, K.H.; Lee, K.R. Flavonoid constituents and their antioxidant activity of Laportea bulbifera Weddell. Saengyak Hakhoechi 2003, 34, 18–24. [Google Scholar]
- Jia, Z.; Zhu, G.; Wang, J. Flavonoid constituents of the seeds of Nitraria tangutorum Bolor. Lanzhou Daxue Xuebao Ziran Kexueban 1991, 27, 102. [Google Scholar]
- Park, Y.-K.; Lee, C.Y. Identification of Isorhamnetin 4’-Glucoside in Onions. J. Agric. Food Chem. 1996, 44, 34. [Google Scholar] [CrossRef]
- Fattorusso, E.; Iorizzi, M.; Lanzotti, V.; Taglialatela-Scafati, O. Chemical composition of shallot (Allium ascalonicum Hort.). J. Agric. Food Chem. 2002, 50, 5686–5690. [Google Scholar] [CrossRef]
- Kassem, M.E.S.; Afifi, M.S.; Marzouk, M.M.; Mostafa, M.A. Two new flavonol glycosides and biological activities of Diplotaxis harra (Forssk.) Boiss. Nat. Prod. Res. 2013, 27, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; Behar, I.; D’Agostino, M.; De Simone, F.; Schettino, O.; Pizza, C. Phytochemical investigation on Mercurialis annua. Biochem. Syst. Ecol. 1987, 15, 667. [Google Scholar] [CrossRef]
- Bechlem, H.; Mencherini, T.; Bouheroum, M.; Benayache, S.; Cotugno, R.; Braca, A.; De Tommasi, N. New Constituents from Gymnocarpos decander. Planta Med. 2017, 83, 1200–1206. [Google Scholar] [CrossRef]
- Batyuk, V.S.; Vasil’chenko, E.A.; Kovaleva, S.N. Flavonoids of Solidago virgaurea L. and S. canadensis L. and their pharmacological properties. Rastit. Resur. 1988, 24, 92. [Google Scholar]
- Litvinenko, V.I.; Bubenchikova, V.N. Phytochemical study of Centaurea cyanus. Chem. Nat. Compd. 1988, 792, 672–674. [Google Scholar] [CrossRef]
- Oksuz, S.; Putun, E. Flavonoids of Centaurea kotschyi var. kotschyi. Doga: Kim. Ser. 1987, 11, 66–71. [Google Scholar]
- Singh, K.N.; Pandey, V.B. Isorhamnetin 7-glucoside from Cnicus wallichi. Phytochemistry 1986, 25, 2683. [Google Scholar] [CrossRef]
- Butayarov, A.V.; Batirov, E.K.; Tadzhibaev, M.M.; Melibaev, S.; Malikov, V.M. Flavonoids from aerial parts of Russowia sogdiana. Chem. Nat. Compd. 1993, 29, 807–808. [Google Scholar] [CrossRef]
- Abdala, L.R. Flavonoids of the aerial parts from Tagetes lucida (Asteraceae). Biochem. Syst. Ecol. 1999, 27, 753–754. [Google Scholar] [CrossRef]
- He, A.; Wang, M. Flavonoids from stringy stonecrop (Sedum sarmentosum). Zhongcaoyao 1997, 28, 517–522. [Google Scholar]
- Grace, M.H.; Mohamed, T.K.; Khattab, A.M. Flavonoids of Carduncellus eriocephalus. Egypt. J. Pharm. Sci. 1999, 39, 409–416. [Google Scholar]
- Zhang, Z.-x.; Zhang, J.; Luo, J.-q.; Zhang, T.-h. Chemical constituents of the seeds of Atriplex centralasiatica. Shenyang Yaoke Daxue Xuebao 2008, 25, 708–710. [Google Scholar]
- Awaad, A.S.; Grace, M.H. Flavonoids and pharmacological activity of Vernonia galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert. Egypt. J. Pharm. Sci. 2001, 40, 117–128. [Google Scholar]
- Krzeminski, K.; Krzeminska, K. Flavonoid heterosides in the herb of Raphanus raphanistrum L. Herba Pol. 1977, 23, 291. [Google Scholar]
- Zhang, S.; Shi, J.; Sun, Z.; Hu, C. Studies on chemical constituents from Caragana intermedia. Zhongyaocai 2006, 29, 19–21. [Google Scholar]
- Paskhov, D.; Marichkova, L. Flavonoids of Astragalus centralpinus and their effect on the smooth muscle of the gastrointestinal tract. Probl. Farm. 1983, 11, 36–42. [Google Scholar]
- Christensen, L.P.; Kaack, K.; Frette, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Vidal-Ollivier, E.; Elias, R.; Faure, F.; Babadjamian, A.; Crespin, F.; Balansard, G.; Boudon, G. Flavonol glycosides from Calendula officinalis flowers. Planta Med. 1989, 55, 73. [Google Scholar] [CrossRef]
- Merfort, I.; Wendisch, D. Flavonoid glucuronides from the flowers of Arnica montana. Planta Med. 1988, 54, 247. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.Y.; Park, P.S.; Bang, S.H.; Lee, K.M.; Lee, Y.R.; Jang, Y.H.; Kim, M.J.; Chun, W.; Heo, M.Y.; et al. Flavonoid glycosides as acetylcholinesterase inhibitors from the whole plants of Persicaria thunbergii. Nat. Prod. Sci. 2014, 20, 191–195. [Google Scholar]
- Mezache, N.; Derbre, S.; Akkal, S.; Laouer, H.; Seraphin, D.; Richomme, P. Fast counter current chromatography of n-butanolic fraction from Senecio giganteus (Asteraceae). Nat. Prod. Commun. 2009, 4, 1357–1362. [Google Scholar] [CrossRef]
- Granica, S.; Czerwinska, M.E.; Zyzynska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Cheng, W.; Sui, C.; Yuan, J.; Zhang, H. Flavonoids of argun groundsel (Senecio argunensis). Zhongcaoyao 1999, 30, 727–729. [Google Scholar]
- Zaki, A.A.; Xu, X.; Wang, Y.; Shie, P.-H.; Qiu, L. A new anti-inflammatory flavonoid glycoside from Tetraena aegyptia. Nat. Prod. Res. 2021, 35, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wu, J.; Azi, g.; Li, R.; Lin, G. Chemical constituents of Aertaihuanqi (Astragalus altaicus). Zhongcaoyao 1994, 25, 563. [Google Scholar]
- Saleh, N.A.M.; Mansour, R.M.A.; Markham, K.R. An acylated isorhamnetin glycoside from Aerva javanica. Phytochemistry 1990, 29, 1344. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Y.; Ma, C.; Yang, S.; Zhou, H.; Zhao, D.; Liu, D.; Li, S. Flavonoid constituents of the pollen of Typha angustfolia L. (Puhuang). Yaoxue Xuebao 1986, 21, 441. [Google Scholar]
- Olszewska, M.A.; Kwapisz, A. Metabolite profiling and antioxidant activity of Prunus padus L. flowers and leaves. Nat. Prod. Res. 2011, 25, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, P.; Qu, L.; Ruan, J.; Yang, S.; Yu, H.; Zhang, Y.; Wang, T. Bioactive constituents obtained from the seeds of Lepidium apetalum willd. Molecules 2017, 22, 540. [Google Scholar] [CrossRef] [PubMed]
- Hoerhammer, L.; Wagner, H.; Kraemer, H.; Farkas, L. Isorhamnetin glycosides. II. Isolation and composition of new glycosides from Brassica napus and Sinapis arvensis. Chem. Ber. 1967, 100, 2301. [Google Scholar] [CrossRef]
- Halim, A.F.; Saad, H.-E.A.; Hashish, N.E. Flavonol glycosides from Nitraria retusa. Phytochemistry 1995, 40, 349. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lou, F.; Wang, J.; Li, Y.; Zhuang, S. Coumaroyl flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry 2001, 58, 1251–1256. [Google Scholar] [CrossRef]
- Wang, D.-M.; Pu, W.-J.; Wang, Y.-H.; Zhang, Y.-J.; Wang, S.-S. A new isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules 2012, 17, 4595–4603. [Google Scholar] [CrossRef]
- Schoensiegel, I.; Egger, K. Flavonol glycosides in the petals of Narcissus pseudonarcissus. Z. Naturforsch. B 1969, 24, 1215. [Google Scholar] [CrossRef]
- Zhang, Z.-l.; Cai, M.-t.; Zuo, Y.-m.; Wang, Y.-y. Studies on chemical constituents in fruits of Trillium tschonoskii Maxim. Shizhen Guoyi Guoyao 2014, 25, 541–543. [Google Scholar] [CrossRef]
- Yoshitama, K.; Shida, Y.; Oyamada, T.; Takasaki, N.; Yahara, S. "Studies of the flavonoids of the genus Trillium". 3. Flavonol glycosides in the leaves of Trillium apetalon Makino and T. kamtschaticum Pallas. J. Plant Res. 1997, 110, 443–448. [Google Scholar] [CrossRef]
- Rodriguez, E.; Shen, M.C.; Mabry, T.J.; Dominguez, X.A. Isorhamnetin 3-0-glucoside 7-0-arabinoside from Eschscholzia mexicana. Phytochemistry 1973, 12, 2069. [Google Scholar] [CrossRef]
- Li, H.; Kim, U.H.; Yoon, J.H.; Ji, H.S.; Park, H.M.; Park, H.Y.; Jeong, T.S. Suppression of Hyperglycemia and Hepatic Steatosis by Black-Soybean-Leaf Extract via Enhanced Adiponectin-Receptor Signaling and AMPK Activation. J. Agric. Food Chem. 2019, 67, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kijima, H.; Ide, T.; Otsuka, H.; Takeda, Y. Alangiflavoside, a new flavonol glycoside from the leaves of Alangium premnifolium. J. Nat. Prod. 1995, 58, 1753. [Google Scholar] [CrossRef]
- Yasukawa, K.; Sekine, H.; Takido, M. Studies of the constituents of genus Lysimachia. Part 4. Two flavonol glycosides from Lysimachia fortunei. Phytochemistry 1989, 28, 2215. [Google Scholar] [CrossRef]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef]
- Kaouadji, M.; Doucoure, A.; Mariotte, A.M.; Chulia, A.J.; Thomasson, F. Flavonol triglycosides from Blackstonia perfoliata. Phytochemistry 1990, 29, 1283. [Google Scholar] [CrossRef]
- El-Sayed, N.H.; Abu Dooh, A.M.; El-Khrisy, E.A.M.; Mabry, T.J. Flavonoids of Cassia italica. Phytochemistry 1992, 31, 2187. [Google Scholar] [CrossRef]
- Trineeva, O.V.; Perova, I.B.; Slivkin, A.I.; Eller, K.I. Study the composition of flavonoids fruits of sea buckthorn. Sorbtsionnye Khromatogr. Protsessy 2017, 17, 87–93. [Google Scholar]
- Skalski, B.; Lis, B.; Pecio, L.; Kontek, B.; Olas, B.; Zuchowski, J.; Stochmal, A. Isorhamnetin and its new derivatives isolated from sea buckthorn berries prevent H(2)O(2)/Fe—Induced oxidative stress and changes in hemostasis. Food Chem. Toxicol. 2019, 125, 614–620. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Torres, N.; Gutiérrez-Uribe, J.; Noriega, L.; Torre-Villalvazo, I.; Leal-Díaz, A.; Antunes-Ricardo, M.; Márquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.; et al. The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.; Guyot, S.; Sotin, H.; Ayadi, M. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef]
- FAO. Cactus (Opuntia spp.) as Forage; Food and Agriculture Organization: Rome, Italy, 2001. [Google Scholar]
- Santos-Zea, L.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Comparative analyses of total phenols, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. J. Agric. Food Chem. 2011, 59, 7054–7061. [Google Scholar] [CrossRef] [PubMed]
- Albergamo, A.; Potortí, A.; Di Bella, G.; Amor, N.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Lo Turco, V. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Garg, P.; Dviwedi, A.; Ali, A.; Kapoor, V.; Kapoor, D.; Kulshrestha, S.; Lal, U.; Negi, P. Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: A review. J. Ethnopharmacol. 2021, 266, 113434. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Skalski, B. Preparations from Various Organs of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) as Important Regulators of Hemostasis and Their Role in the Treatment and Prevention of Cardiovascular Diseases. Nutrients 2022, 14, 991. [Google Scholar] [CrossRef]
- Pop, R.; Socaciu, C.; Pintea, A.; Buzoianu, A.; Sanders, M.; Gruppen, H.; Vincken, J. UHPLC/PDA-ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaë rhamnoides L. varieties. Phytochem. Anal. PCA 2013, 24, 484–492. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.; Ferreres, F.; Moreno, D.; Nowicka, P. UPLC-PDA-Q/TOF-MS profiling of phenolic and carotenoid compounds and their influence on anticholinergic potential for AChE and BuChE inhibition and on-line antioxidant activity of selected Hippophaë rhamnoides L. cultivars. Food Chem. 2020, 309, 125766. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Wang, P.; Wang, M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol. 2020, 162, 1897–1905. [Google Scholar] [CrossRef]
- Gray, D.; Messer, D.; Porter, A.; Hefner, B.; Logan, D.; Harris, R.; Clark, A.; Algaier, J.; Overstreet, J.; Smith, C. Analysis of flavonol aglycones and terpenelactones in Ginkgo biloba extract: A comparison of high-performance thin-layer chromatography and column high-performance liquid chromatography. J. AOAC Int. 2007, 90, 1203–1209. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y. Multifaceted Therapeutic Benefits of Ginkgo biloba L.: Chemistry, Efficacy, Safety, and Uses. J. Food Sci. 2010, 73, R14–R19. [Google Scholar] [CrossRef]
- Eisvand, F.; Razavi, B.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. PTR 2020, 34, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ge, Z.; Deng, R.; Bao, B.; Yao, W.; Cao, Y.; Shan, M.; Cheng, F.; Yan, H.; Chen, P.; et al. Evaluation of VEGF mediated pro-angiogenic and hemostatic effects and chemical marker investigation for Typhae Pollen and its processed product. J. Ethnopharmacol. 2021, 268, 113591. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. Notice of the Ministry of Health on Further Standardizing the Management of Health Food Raw Materials. 2002. Available online: http://www.nhc.gov.cn/wjw/gfxwj/201304/e33435ce0d894051b15490aa3219cdc4.shtml (accessed on 15 September 2022).
- Zeng, G.; Wu, Z.; Cao, W.; Wang, Y.; Deng, X.; Zhou, Y. Identification of anti-nociceptive constituents from the pollen of Typha angustifolia L. using effect-directed fractionation. Nat. Prod. Res. 2020, 34, 1041–1045. [Google Scholar] [CrossRef]
- Cao, S.; Ni, B.; Feng, L.; Yin, X.; Dou, H.; Fu, J.; Lin, L.; Ni, J. Simultaneous Determination of Typhaneoside and Isorhamnetin-3-O-Neohesperidoside in Rats After Oral Administration of Pollen Typhae Extract by UPLC-MS/MS. J. Chromatogr. Sci. 2015, 53, 866–871. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Xu, D.; Huang, G.; Xu, Y.; Wang, Z.; Fang, S.; Chen, Y.; Gu, Y. The antiatherogenic effects of components isolated from pollen typhae. Thromb. Res. 1990, 57, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Yang, X.; Gao, X.; Wang, H.; Chang, Y. A rapid and efficient extraction method based on industrial MCM-41-miniaturized matrix solid-phase dispersion extraction with response surface methodology for simultaneous quantification of six flavonoids in Pollen typhae by ultra-high-performance liquid chromatography. J. Sep. Sci. 2019, 42, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.; Garcia, P.; Castro, M.; Santos-Buelga, C.; Ferreira, I. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef]
- Dinda, M.; Dasgupta, U.; Singh, N.; Bhattacharyya, D.; Karmakar, P. PI3K-mediated proliferation of fibroblasts by Calendula officinalis tincture: Implication in wound healing. Phytother. Res. PTR 2015, 29, 607–616. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Componential Profile and Amylase Inhibiting Activity of Phenolic Compounds from Calendula officinalis L. Leaves. Sci. World J. 2014, 2014, 654193. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New Isorhamnetin Glycosides and other Phenolic Compounds from Calendula officinalis. Chem. Nat. Compd. 2013, 49, 833–840. [Google Scholar] [CrossRef]
- Bezákova, L.; Masterová, I.; Paulíková, I.; Psenák, M. Inhibitory activity of isorhamnetin glycosides from Calendula officinalis L. on the activity of lipoxygenase. Pharmazie 1996, 51, 126–127. [Google Scholar] [PubMed]
- Sriseadka, T.; Wongpornchai, S.; Rayanakorn, M. Quantification of Flavonoids in Black Rice by Liquid Chromatography-Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 11723–11732. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.; Cho, E.; Choi, J.; Chung, H. Antioxidant effects of isorhamnetin 3,7-di-O-beta-d-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Pbpa, B.; Mtba, D.; Ga, C.; Htg, A.; Hg, C. Phenolics profiling by HPLC-DAD-ESI-MS n aided by principal component analysis to classify Rabbiteye and Highbush blueberries. Food Chem. 2020, 340, 127958. [Google Scholar]
- Chen, Y.G.; Li, P.; Li, P.; Yan, R.; Zhang, X.Q.; Wang, Y.; Zhang, X.T.; Ye, W.C.; Zhang, Q.W. α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium. Molecules 2013, 18, 4221–4232. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Yang-Xue, L.I.; Su-Mei, L.I.; Ai-Li, X.U. Simultaneous Determination of Six Flavonoids in Extract of Microctis Folium by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2016, 36, 589–593. [Google Scholar]
- Tedesco, I.; Carbone, V.; Spagnuolo, C.; Minasi, P.; Russo, G.L. Identification and Quantification of Flavonoids from Two Southern Italian Cultivars of Allium cepa L., Tropea (Red Onion) and Montoro (Copper Onion), and Their Capacity to Protect Human Erythrocytes from Oxidative Stress. J. Agric. Food Chem. 2015, 63, 5229–5238. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C. Analysis of the Major Flavonol Glycosides Present in Four Varieties of Onion (Allium cepa) and Changes in Composition Resulting from Autolysis. J. Sci. Food Agric. 1997, 74, 331–339. [Google Scholar] [CrossRef]
- Aalar, H.G. Elderberry (Sambucus nigra L.). In Nonvitamin Nonmineral Nutritional Supplements; Seyed, M.N., Ana, S.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 211–215. [Google Scholar]
- Bhattacharya, S.; Christensen, K.B.; Olsen, L.C.B.; Christensen, L.P.; Grevsen, K.; FæRgeman, N.J.; Kristiansen, K.; Young, J.F.; Oksbjerg, N. Bioactive Components from Flowers of Sambucus nigra L. Increase Glucose Uptake in Primary Porcine Myotube Cultures and Reduce Fat Accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 11033–11040. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Ultraviolet Spectra of Isoflavones, Flavanones and Dihydroflavonols. In The Systematic Identification of Flavonoids; Mabry, T.J., Markham, K.R., Thomas, M.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1970; pp. 165–226. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Karl, C.; Pedersen, P.A.; Schwarz, C. A new flavonoacetylglucoside from Salix viminalis. Phytochemistry 1977, 16, 1117. [Google Scholar] [CrossRef]
- Markham, K.R. 6—Flavones, Flavonols and their Glycosides. In Methods in Plant Biochemistry; Harborne, J.B., Ed.; Academic Press: Cambridge, MA, USA, 1989; Volume 1, pp. 197–235. [Google Scholar]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv. "Brettacher") by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Ferreira, J.; Semedo, C.; Serra, T.; Duarte, C.; Bronze, M. Contribution to the characterization of Opuntia spp. juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Frison-Norrie, S.; Sporns, P. Identification and quantification of flavonol glycosides in almond seedcoats using MALDI-TOF MS. J. Agric. Food Chem. 2002, 50, 2782–2787. [Google Scholar] [CrossRef]
- Bartnik, M.; Glowniak, K.; Gromek, A. TLC and HPLC analysis of the flavonoid glycosides in the aerial parts of Peucedanum tauricum Bieb. JPC-J. Planar Chromatogr. Mod. 2007, 20, 127–130. [Google Scholar] [CrossRef]
- Slimestad, R.; Andersen, Ø.M.; Francis, G.W.; Marston, A.; Hostettmann, K. Syringetin 3-O-(6″-acetyl)-β-glucopyranoside and other flavonols from needles of norway spruce, Picea abies. Phytochemistry 1995, 40, 1537–1542. [Google Scholar] [CrossRef]
- Lee, J.; Mitchell, A.E. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): Varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J. Agric. Food Chem. 2011, 59, 857–863. [Google Scholar] [CrossRef]
- Arimboor, R.; Arumughan, C. HPLC-DAD-MS/MS profiling of antioxidant flavonoid glycosides in sea buckthorn (Hippophae rhamnoides L.) seeds. Int. J. Food Sci. Nutr. 2012, 63, 730–738. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Gurny, R.; Veuthey, J.; Guillarme, D. Coupling ultra high-pressure liquid chromatography with mass spectrometry: Constraints and possible applications. J. Chromatogr. A 2013, 1292, 2–18. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, Y.; Li, L.; Li, Y.; Zhou, P.; Luo, L.; Sun, B. Preparative HSCCC isolation of phloroglucinolysis products from grape seed polymeric proanthocyanidins as new powerful antioxidants. Food Chem. 2015, 188, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Y.; Aisa, H.A.; Xin, X.; Ma, H.; Yili, A.; Zhao, Y. Bioassay-guided isolation of antioxidants from Astragalus altaicus by combination of chromatographic techniques. J. Sep. Sci. 2012, 35, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wei, X.; Ju-Wu, H.; Zhen, G.; Jian-Guo, X.; Chuan-Ling, S.; Young-Soo, B.; Gang, X. Purification of Four Flavonoid Glycosides from Lotus (Nelumbo nucifera Gaertn) plumule by Macroporous Resin Combined with HSCCC. J. Chromatogr. Sci. 2018, 56, 108–114. [Google Scholar]
- Quispe, C.; Viveros-Valdez, E.; Yarleque, J.A.; Arones, M.R.; Paniagua, J.C.; Schmeda-Hirschmann, G. High speed centrifugal countercurrent chromatography (hsccc) isolation and identification by lc-ms n analysis of the polar phenolics from Vasconcellea quercifolia. J. Chil. Chem. Soc. 2017, 58, 1830–1835. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, M.; Park, H.; Chung, H.; Kang, S. Further isolation of peroxynitrite and 1,1-diphenyl-2-picrylhydrazyl radical scavenging isorhamnetin 7-O-glucoside from the leaves of Brassica juncea L. Arch. Pharmacal Res. 2002, 25, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.; Abou El-Kassem, L.; Shaher, F.; Al-Farawati, R. In vitro inhibition of Hepatitis C virus protease and antioxidant by flavonoid glycosides from the Saudi costal plant. Nat. Prod. Res. 2019, 33, 3364–3371. [Google Scholar] [CrossRef]
- Chen, P.; Cao, Y.; Bao, B.; Zhang, L.; Ding, A. Antioxidant capacity of Typha angustifolia extracts and two active flavonoids. Pharm. Biol. 2017, 55, 1283–1288. [Google Scholar] [CrossRef]
- Qi, J.; Gui, X.; Chen, G. Research on active component extraction of nitraria sibirica pall. fruit and their antioxidant activity. Mod. Chin. Med. 2013, 15, 827–831. [Google Scholar] [CrossRef]
- Bouhlel, I.; Limem, I.; Skandrani, I.; Nefatti, A.; Ghedira, K.; Dijoux-Franca, M.; Leila, C. Assessment of isorhamnetin 3-O-neohesperidoside from Acacia salicina: Protective effects toward oxidation damage and genotoxicity induced by aflatoxin B1 and nifuroxazide. J. Appl. Toxicol. JAT 2010, 30, 551–558. [Google Scholar] [CrossRef]
- Demirkiran, O.; Sabudak, T.; Ozturk, M.; Topcu, G. Antioxidant and tyrosinase inhibitory activities of flavonoids from Trifolium nigrescens Subsp. petrisavi. J. Agric. Food Chem. 2013, 61, 12598–12603. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Tawfik, W.; Abou-Setta, L. The flavonoid constitunts of Leucaena leucocephala. Growing in Egypt, and their biological activity. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 67–72. [Google Scholar]
- Yuca, H.; Özbek, H.; Demirezer, L.; Kasil, H.G.; Güvenalp, Z. trans-Tiliroside: A potent α-glucosidase inhibitor from the leaves of Elaeagnus angustifolia L. Phytochemistry 2021, 188, 112795. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Flowers of Astragalus membranaceus var. mongholicus as a Novel High Potential By-Product: Phytochemical Characterization and Antioxidant Activity. Molecules 2019, 24, 434. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, J.; Ben Sghaier, M.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Isorhamnetin 3-O-robinobioside from Nitraria retusa leaves enhance antioxidant and antigenotoxic activity in human chronic myelogenous leukemia cell line K562. BMC Complement. Altern. Med. 2012, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.; Esmat, A. Antioxidant and anti-inflammatory activities of the major phenolics from Zygophyllum simplex L. J. Ethnopharmacol. 2017, 205, 51–56. [Google Scholar] [CrossRef]
- Bouhlel, I.; Skandrani, I.; Nefatti, A.; Valenti, K.; Ghedira, K.; Mariotte, A.M.; Hininger-Favier, I.; Laporte, F.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. Antigenotoxic and antioxidant activities of isorhamnetin 3-O neohesperidoside from Acacia salicina. Drug Chem. Toxicol. 2009, 32, 258–267. [Google Scholar] [CrossRef]
- Yeskaliyeva, B.; Mesaik, M.; Abbaskhan, A.; Kulsoom, A.; Burasheva, G.; Abilov, Z.; Choudhary, M.; Atta-ur-Rahman. Bioactive flavonoids and saponins from Climacoptera obtusifolia. Phytochemistry 2006, 67, 2392–2397. [Google Scholar] [CrossRef]
- Matias, A.; Nunes, S.; Poejo, J.; Mecha, E.; Serra, A.; Madeira, P.; Bronze, M.; Duarte, C. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 2014, 5, 3269–3280. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, H.Y.; Lee, C.; Park, S.Y. Nitric oxide inhibitory constituents from fruits of Opuntia humifusa. Nat. Prod. Sci. 2014, 20, 211–215. [Google Scholar]
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases? Expert Opin. Ther. Targets 2018, 22, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ku, S.; Bae, J. Anti-inflammatory activities of isorhamnetin-3-O-galactoside against HMGB1-induced inflammatory responses in both HUVECs and CLP-induced septic mice. J. Cell. Biochem. 2013, 114, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Koh, M.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lee, H.; Kim, S.; Kwon, Y.; Chun, W. Isorhamnetin-3-O-Glucuronide Suppresses JNK and p38 Activation and Increases Heme-Oxygenase-1 in Lipopolysaccharide-Challenged RAW264.7 Cells. Drug Dev. Res. 2016, 77, 143–151. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Wen, Z.-H.; Bakheit, A.H.; Basudan, O.A.; Ghabbour, H.A.; Al-Ahmari, A.; Feng, C.-W. A Major Diplotaxis harra-Derived Bioflavonoid Glycoside as a Protective Agent against Chemically Induced Neurotoxicity and Parkinson’s Models; In Silico Target Prediction; and Biphasic HPTLC-Based Quantification. Plants 2022, 11, 648. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, Y.; Sun, Y.; Liu, X.; Yi, J.; Cai, S. Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro. Nutrients 2022, 14, 1026. [Google Scholar] [CrossRef]
- Jin, H.; Ko, H.; Chowdhury, M.; Lee, D.; Woo, E. A new indole glycoside from the seeds of Raphanus sativus. Arch. Pharmacal Res. 2016, 39, 755–761. [Google Scholar] [CrossRef]
- Osman, S.; El Kashak, W.; Wink, M.; El Raey, M. New Isorhamnetin Derivatives from Salsola imbricata Forssk. Leaves with Distinct Anti-inflammatory Activity. Pharmacogn. Mag. 2016, 12, S47–S51. [Google Scholar] [CrossRef]
- Ameur, A.S.; Negab, I.; Zouzou, F.; Legseir, B. Anti-inflammatory and antispasmodic activities of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) mill. Flowers. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 432–437. [Google Scholar]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.; Martínez-Vitela, C.; Serna-Saldívar, S. Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.-C.; Li, X.-H.; Li, Q.-Y.; Yang, H.-L.; Wang, H.-L.; Zhang, H.; Zhao, L.; Jiang-Yong, S.-L.; Meng, X.-L.; Zhang, Y.; et al. Total flavonoids from sea buckthorn ameliorates lipopolysaccharide/cig arette smoke-induced airway inflammation. Phytother. Res. PTR 2019, 33, 2102–2117. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; El-Sharkawy, E.R.; Matloub, A.A. Cytotoxic flavonoids from Diplotaxis harra (Forssk.) Boiss. growing in Sinai. J. Med. Plant Res. 2013, 520, 5099–5103. [Google Scholar]
- Tofighi, Z.; Asgharian, P.; Goodarzi, S.; Hadjiakhoondi, A.; Ostad, S.N.; Yassa, N. Potent cytotoxic flavonoids from Iranian Secur. Securidaca. Med. Chem. Res. 2014, 23, 1718–1724. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Bonesi, M.; Menichini, F.; Statti, G.; Menichini, F. In vitro cytotoxic activity of Salsola oppositifolia Desf. (Amaranthaceae) in a panel of tumour cell lines. Z. Fur Naturforschung. C J. Biosci. 2008, 63, 347–354. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.Y.; Liu, P.; Huang, J.; Algradi, A.M.; Pan, J.; Guan, W.; Zhou, Y.Y.; Yang, B.Y.; Kuang, H.X. New flavonoids from the aerial part of Bupleurum chinense DC. Fitoterapia 2020, 147, 104739. [Google Scholar] [CrossRef]
- Pfeffer, C.; Singh, A. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Yao, N.; Li, Y.; Lei, Y.; Hu, N.; Chen, W.; Yao, Z.; Yu, M.; Liu, J.; Ye, W.; Zhang, D. A piperazidine derivative of 23-hydroxy betulinic acid induces a mitochondria-derived ROS burst to trigger apoptotic cell death in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. CR 2016, 35, 192. [Google Scholar] [CrossRef]
- Rademaker, G.; Boumahd, Y.; Peiffer, R.; Anania, S.; Wissocq, T.; Liégeois, M.; Luis, G.; Sounni, N.; Agirman, F.; Maloujahmoum, N.; et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022, 53, 102324. [Google Scholar] [CrossRef]
- Mendes, S.; Sá, R.; Magalhães, M.; Marques, F.; Sousa, M.; Silva, E. The Role of ROS as a Double-Edged Sword in (In)Fertility: The Impact of Cancer Treatment. Cancers 2022, 14, 1585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agric. Food Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Guardado-Félix, D.; Rocha-Pizaña, M.; Garza-Martínez, J.; Acevedo-Pacheco, L.; Gutiérrez-Uribe, J.; Villela-Castrejón, J.; López-Pacheco, F.; Serna-Saldívar, S. Opuntia ficus-indica Extract and Isorhamnetin-3-O-Glucosyl-Rhamnoside Diminish Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice through the Activation of Apoptosis Intrinsic Pathway. Plant Foods Hum. Nutr. 2021, 76, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Moreno-García, B.; Gutiérrez-Uribe, J.; Aráiz-Hernández, D.; Alvarez, M.; Serna-Saldivar, S. Induction of apoptosis in colon cancer cells treated with isorhamnetin glycosides from Opuntia ficus-indica pads. Plant Foods Hum. Nutr. 2014, 69, 331–336. [Google Scholar] [CrossRef]
- Jihed, B.; Bhouri, W.; Ben Sghaier, M.; Bouhlel, I.; Kriffi, M.; Skandrani, I.; Dijoux, F.; Ghedira, K.; Chekir-Ghedira, L. Flavonoids products from Nitraria retusa leaves promote lymphoblastoid cells apoptosis. Nutr. Cancer 2012, 64, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]
- Hoang Anh, N.; Tam, K.; Tuan, N.; Thien, D.; Quan, T.; Tam, N.; Bao, N.; Do, T.; Nga, N.; Thuy, T.; et al. Chemical constituents of Oldenlandia pinifolia and their antiproliferative activities. Nat. Prod. Res. 2019, 33, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, J.; Bhouri, W.; Ben Sghaier, M.; Ghedira, K.; Dijoux Franca, M.G.; Chekir-Ghedira, L. Ethyl acetate extract and its major constituent, isorhamnetin 3-O-rutinoside, from Nitraria retusa leaves, promote apoptosis of human myelogenous erythroleukaemia cells. Cell Prolif. 2011, 44, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Kim, Y.; Kim, M.; Park, J.; Kim, J.; Kim, S.; Lee, B.; Nam, T.; Seo, Y. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2008, 22, 1742–1748. [Google Scholar] [CrossRef]
- Nasri, I.; Chawech, R.; Girardi, C.; Mas, E.; Ferrand, A.; Vergnolle, N.; Fabre, N.; Mezghani-Jarraya, R.; Racaud-Sultan, C. Anti-inflammatory and anticancer effects of flavonol glycosides from Diplotaxis harra through GSK3beta regulation in intestinal cells. Pharm. Biol. 2017, 55, 124–131. [Google Scholar] [CrossRef]
- Highton, A.; Schuster, I.; Degli-Esposti, M.; Altfeld, M. The role of natural killer cells in liver inflammation. Semin. Immunopathol. 2021, 43, 519–533. [Google Scholar] [CrossRef]
- Lücke, J.; Sabihi, M.; Zhang, T.; Bauditz, L.; Shiri, A.; Giannou, A.; Huber, S. The good and the bad about separation anxiety: Roles of IL-22 and IL-22BP in liver pathologies. Semin. Immunopathol. 2021, 43, 591–607. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassanein, E.; Hassan, S.; Hussein, O.E.; Mahmoud, A.M. Flavonoids-mediated SIRT1 signaling activation in hepatic disorders. Life Sci. 2020, 259, 118173. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Mikami, T.; Takahashi, Y.; Sato, H. Comparison of the preventive activity of isorhamnetin glycosides from atsumi-kabu (red turnip, Brassica, campestris L.) leaves on carbon tetrachloride-induced liver injury in mice. Biosci. Biotechnol. Biochem. 2008, 72, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.; Mondello, M.; Lauriano, E.; Taviano, M.; Galluzzo, M.; Miceli, N. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride-induced injury. Phytother. Res. PTR 2005, 19, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, D.; Yogendra Kumar, M.; Verma, S.; Singh, V.; Singh, S. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Tang, Z.; Wang, X.; Yang, B.; Wang, Z.; Wang, Q. Chemical constituents from Sambucus williamsii Hance fruits and hepatoprotective effects in mouse hepatocytes. Nat. Prod. Res. 2018, 32, 2008–2016. [Google Scholar] [CrossRef]
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm. Biol. 2017, 55, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cheng, J.; Zheng, L.; Xu, W.; Xie, Y. Mechanochemical-Assisted Extraction and Hepatoprotective Activity Research of Flavonoids from Sea Buckthorn (Hippophaë rhamnoides L.) Pomaces. Molecules 2021, 26, 7615. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Liu, P. Active Components from Sea Buckthorn (Hippophae rhamnoides L.) Regulate Hepatic Stellate Cell Activation and Liver Fibrogenesis. J. Agric. Food Chem. 2018, 66, 12257–12264. [Google Scholar] [CrossRef]
- Hur, J.M.; Park, S.H.; Choi, J.W.; Park, J.C. Effects of extract and isorhamnetin glycoside from Brassica juncea on hepatic alcohol-metabolizing enzyme system in rats. Nat. Prod. Sci. 2012, 18, 190–194. [Google Scholar]
- Ran, B.; Guo, C.; Li, W.; Li, W.; Wang, Q.; Qian, J.; Li, H. Sea buckthorn (Hippophae rhamnoides L.) fermentation liquid protects against alcoholic liver disease linked to regulation of liver metabolome and the abundance of gut microbiota. J. Sci. Food Agric. 2021, 101, 2846–2854. [Google Scholar] [CrossRef]
- Tanveer, A.; Akram, K.; Farooq, U.; Hayat, Z.; Shafi, A. Management of diabetic complications through fruit flavonoids as a natural remedy. Crit. Rev. Food Sci. Nutr. 2017, 57, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Jia, W.; Zhang, Y.; Wang, H.; Lin, Z.; Chen, S. An on-line detection system for screening small molecule inhibitors of α-Amylase and α-Glucosidase in Prunus mume. J. Chromatogr. A 2022, 1663, 462754. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Inhibitory effects on the digestive enzyme alpha-amylase of three Salsola species (Chenopodiaceae) in vitro. Pharm. Die 2007, 62, 473–475. [Google Scholar]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. alpha-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef]
- Siegień, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osińska, E.; Melzig, M.; Czerwińska, M. Pancreatic lipase and α-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chem. 2021, 355, 129414. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Watanabe, M.; Morikawa-Ichinose, T.; Fujino, K.; Yamamoto, M.; Nishioka, S.; Inoue, C.; Ogawa, F.; Yonekura, M.; Nakasone, A.; et al. Metabolic Profiling for Evaluating the Dipeptidyl Peptidase-IV Inhibitory Potency of Diverse Green Tea Cultivars and Determining Bioactivity-Related Ingredients and Combinations. J. Agric. Food Chem. 2022, 70, 6455–6466. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Fu, Y.; Yi, J.; Gao, A.; Jia, Y.; Cai, S. Effects of Different Dietary Flavonoids on Dipeptidyl Peptidase-IV Activity and Expression: Insights into Structure-Activity Relationship. J. Agric. Food Chem. 2020, 68, 12141–12151. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, L.; Tao, W. Potent protein tyrosine phosphatase 1B (PTP1B) inhibiting constituents from Anoectochilus chapaensis and molecular docking studies. Pharm. Biol. 2015, 53, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Kousaxidis, A.; Petrou, A.; Lavrentaki, V.; Fesatidou, M.; Nicolaou, I.; Geronikaki, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur. J. Med. Chem. 2020, 207, 112742. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Lee, H.; Kim, B.; Ohuchi, K.; Shin, K. Inhibitory effects of isorhamnetin-3-O-beta-d-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jung, Y.; Hyun, S.; Lee, Y.; Choi, J. Rat lens aldose reductase inhibitory constituents of Nelumbo nucifera stamens. Phytother. Res. PTR 2006, 20, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Kwon, E.; Lee, J.; Kim, Y.; Do, A.; Choi, J.; Cho, S.; Jung, U.; Lee, M.; Park, Y.; Choi, M. Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity. Nutrients 2017, 9, 569. [Google Scholar] [CrossRef]
- Yang, Z.; Wen, X.; Li, Y.; Matsuzaki, K.; Kitanaka, S. Inhibitory effects of the constituents of Hippophae rhamnoides on 3T3-L1 cell differentiation and nitric oxide production in RAW264.7 cells. Chem. Pharm. Bull. 2013, 61, 279–285. [Google Scholar] [CrossRef]
- Kong, C.S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-beta-d-glucopyranoside from Salicornia herbacea. Immunopharmacol. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbi, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Tounsi, M.; Ghiringhelli, F.; Rialland, M.; et al. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Pang, Z.; Pan, M.; Zhang, W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 mice. Pharm. Biol. 2017, 55, 1207–1214. [Google Scholar] [CrossRef]
- Mulati, A.; Ma, S.; Zhang, H.; Ren, B.; Zhao, B.; Wang, L.; Liu, X.; Zhao, T.; Kamanova, S.; Sair, A.; et al. Sea-Buckthorn Flavonoids Alleviate High-Fat and High-Fructose Diet-Induced Cognitive Impairment by Inhibiting Insulin Resistance and Neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. In vivo thrombus formation. J. Thromb. Haemost. JTH 2007, 5 (Suppl. S1), 12–17. [Google Scholar] [CrossRef]
- Mega, J.L.; Simon, T. Pharmacology of antithrombotic drugs: An assessment of oral antiplatelet and anticoagulant treatments. Lancet 2015, 386, 281–291. [Google Scholar] [CrossRef]
- Ku, S.K.; Kim, T.H.; Lee, S.; Kim, S.M.; Bae, J.S. Antithrombotic and profibrinolytic activities of isorhamnetin-3-O-galactoside and hyperoside. Food Chem. Toxicol. 2013, 53, 197–204. [Google Scholar] [CrossRef]
- Stochmal, A.; Rolnik, A.; Skalski, B.; Zuchowski, J.; Olas, B. Antiplatelet and Anticoagulant Activity of Isorhamnetin and Its Deriva tives Isolated from Sea Buckthorn Berries, Measured in Whole Blood. Molecules 2022, 27, 4429. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Che Jalil, N.A.; Wan Ghazali, W.S.; Mohamed, M. Bee bread attenuates the progression of atherosclerosis by activating Nrf2/Keap1 and modulating TNF-alpha/NF-kappabeta-associated mast cell migration and a mitochondrial-dependent apoptotic pathway in the obese rat model. Food Funct. 2022, 13, 8119–8130. [Google Scholar] [CrossRef] [PubMed]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Duenas, M.; Tomas, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Budzianowska, A.; Toton, E.; Romaniuk-Drapala, A.; Kikowska, M.; Budzianowski, J. Cytotoxic Effect of Phenylethanoid Glycosides Isolated from Plantago lanceolata L. Life 2023, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Daowtak, K.; Tima, S.; Okonogi, S.; Mueller, M.; Toegel, S.; Khonkarn, R. Hydrolyzed Flavonoids from Cyrtosperma johnstonii with Superior Antioxidant, Antiproliferative, and Anti-Inflammatory Potential for Cancer Prevention. Molecules 2022, 27, 3226. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Borompichaichartkul, C.; Iwamoto, S. Bioaccessibility and antioxidant activity of phenolic acids, flavonoids, and anthocyanins of encapsulated Thai rice bran extracts during in vitro gastrointestinal digestion. Food Chem. 2021, 361, 130161. [Google Scholar] [CrossRef] [PubMed]
- Mrudulakumari Vasudevan, U.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylat ion and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Busselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.L.; Yang, J.L.; Gupta, V.K.; Jiang, Y.M. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.L.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion—ScienceDirect. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef]
- De Santiago, E.; Gill, C.I.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and Colonic Fermentation of Raw and Cooked Opuntia ficus-indica Cladodes Impacts Bioaccessibility and Bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, J.M.; Yokozawa, T.; Sakata, K.; Lee, S. Protective activity of flavonoid and flavonoid glycosides against gluc ose-mediated protein damage. Food Chem. 2010, 126, 892–895. [Google Scholar] [CrossRef]
- Yu, L.; Chen, C.; Wang, L.-F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.-R. Neuroprotective effect of kaempferol glycosides against brain injury a nd neuroinflammation by inhibiting the activation of NF-κB and STAT3 i n transient focal stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef]
- Michael, H.N.; Salib, J.Y.; Eskander, E.F. Bioactivity of Diosmetin Glycosides Isolated from the Epicarp of Date Fruits, Phoenix dactylifera, on the Biochemical Profile of Alloxan Diabetic Male Rats. Phytother. Res. 2012, 27, 699–704. [Google Scholar] [CrossRef]
- Makino, T.; Kanemaru, M.; Okuyama, S.; Shimizu, R.; Tanaka, H.; Mizukami, H. Anti-allergic effects of enzymatically modified isoquercitrin (α-oligo glucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglu cosyl rutin, and quercetin, when administered orally to mice. J. Nat. Med. 2013, 67, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better bi ological significance? Crit. Rev. Food Sci. Nutr. 2015, 57, 1874–1905. [Google Scholar] [CrossRef]
- Zeng, H.; Xue, P.; Su, S.; Huang, X.; Shang, E.; Guo, J.; Qian, D.; Tang, Y.; Duan, J. Comparative Pharmacokinetics of three major bioactive components in rats after oral administration of Typhae Pollen-Trogopterus Feces drug pair before and after compatibility. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2016, 24, 2. [Google Scholar] [CrossRef]
- Lehtonen, H.M.; Lehtinen, O.; Suomela, J.P.; Viitanen, M.; Kallio, H. Flavonol glycosides of sea buckthorn (Hippophae rhamnoides ssp. sinensis) and lingonberry (Vaccinium vitis-idaea) are bioavailable in humans and monoglucuronidated for excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Edwards, C.A.; Lean, M.E.; Crozier, A. Absorption and excretion of conjugated flavonols, including quercetin-4’-O-beta-glucoside and isorhamnetin-4’-O-beta-glucoside by human volunteers after the consumption of onions. Free Radic. Res. 1998, 29, 257–269. [Google Scholar] [CrossRef]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Kyle, J.A.; Collins, A.R. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000, 39, 213–223. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- de Matos, A.M.; Blazquez-Sanchez, M.T.; Sousa, C.; Oliveira, M.C.; de Almeida, R.F.M.; Rauter, A.P. C-Glucosylation as a tool for the prevention of PAINS-induced membrane dipole potential alterations. Sci. Rep. 2021, 11, 4443. [Google Scholar] [CrossRef]
- Ditu, L.-M.; Grigore, M.E.; Camen-Comanescu, P.; Holban, A.M. Introduction in Nutraceutical and Medicinal Foods; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- de Albuquerque, J.; Escalona-Buendía, H.; de Souza Aquino, J.; da Silva Vasconcelos, M. Nopal beverage (Opuntia ficus-indica) as a non-traditional food: Sensory properties, expectations, experiences, and emotions of low-income and food-insecure Brazilian potential consumers. Food Res. Int. 2022, 152, 110910. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.; Cano, M. Characterization, stability, and bioaccessibility of Betalain and phenolic compounds from Opuntia stricta var. Dillenii fruits and products of their industrialization. Foods 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Shin, J.; Koh, E.; Ryu, H.; Kim, H.; Lee, S. Opuntia ficus-indica seed attenuates hepatic steatosis and promotes M2 macrophage polarization in high-fat diet-fed mice. Nutr. Res. 2016, 36, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and dietary intervention with Opuntia ficus-indica (Nopal) in women with obesity improves health condition through gut microbiota adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.; Pérez-Ramírez, I.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef]
- Silva, M.; Albuquerque, T.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.; Costa, H. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef]
- Bellafiore, M.; Pintaudi, A.; Thomas, E.; Tesoriere, L.; Bianco, A.; Cataldo, A.; Cerasola, D.; Traina, M.; Livrea, M.; Palma, A. Redox and autonomic responses to acute exercise-post recovery following Opuntia ficus-indica juice intake in physically active women. J. Int. Soc. Sport. Nutr. 2021, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Ennouri, M.; Khemakhem, B.; Ben Hassen, H.; Ammar, I.; Belghith, K.; Attia, H. Purification and characterization of an amylase from Opuntiaficus-indica seeds. J. Sci. Food Agric. 2013, 93, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Bolaños, J.; Fato-Aldeco, E.; Gutiérrez-Moreno, K.; Botello-Álvarez, J.; Jiménez-Islas, H.; Rico-Martínez, R. A strategy to design efficient fermentation processes for traditional beverages production: Prickly pear wine. J. Food Sci. 2013, 78, M1560–M1568. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, A.E. Opuntia Fruits as Food Enriching Ingredient, the First Step towards New Functional Food Products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Koshak, A.; Algandaby, M.; Mujallid, M.; Abdel-Naim, A.; Alhakamy, N.; Fahmy, U.; Alfarsi, A.; Badr-Eldin, S.; Neamatallah, T.; Nasrullah, M.; et al. Wound Healing Activity of Opuntia ficus-indica Fixed Oil Formulated in a Self-Nanoemulsifying Formulation. Int. J. Nanomed. 2021, 16, 3889–3905. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.; Youssef, K.; El-Samahy, S.; Kroh, L.; Rohn, S. Flavonol profile of cactus fruits (Opuntia ficus-indica) enriched cereal-based extrudates: Authenticity and impact of extrusion. Food Res. Int. 2015, 78, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Anchondo-Trejo, C.; Loya-Carrasco, J.; Galicia-García, T.; Estrada-Moreno, I.; Mendoza-Duarte, M.; Castellanos-Gallo, L.; Márquez-Meléndez, R.; Portillo-Arroyo, B.; Soto-Figueroa, C. Development of a Third Generation Snack of Rice Starch Enriched with Nopal Flour (Opuntia ficus indica). Molecules 2020, 26, 54. [Google Scholar] [CrossRef]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of Prickly Pear Peel Flour as a Bioactive and Functional Ingredient in Bread Preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Namir, M.; Elzahar, K.; Ramadan, M.F.; Allaf, K. Cactus pear peel snacks prepared by instant pressure drop texturing: Effect of process variables on bioactive compounds and functional properties. J. Food Meas. Charact. 2017, 11, 388–400. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.; Jaeger, H.; Youssef, K.; Knorr, D.; El-Samahy, S.; Kroh, L.; Rohn, S. Technological characteristics and selected bioactive compounds of Opuntia dillenii cactus fruit juice following the impact of pulsed electric field pre-treatment. Food Chem. 2016, 210, 249–261. [Google Scholar] [CrossRef]
- Ma, X.; Moilanen, J.; Laaksonen, O.; Yang, W.; Tenhu, E.; Yang, B. Phenolic compounds and antioxidant activities of tea-type infusions processed from sea buckthorn (Hippophaë rhamnoides) leaves. Food Chem. 2019, 272, 1–11. [Google Scholar] [CrossRef]
- Beveridge, T.; Harrison, J.; Drover, J. Processing effects on the composition of sea buckthorn juice from Hippophae rhamnoides L. Cv. Indian Summer. J. Agric. Food Chem. 2002, 50, 113–116. [Google Scholar] [CrossRef]
- Tulsawani, R. Ninety day repeated gavage administration of Hipphophae rhamnoides extract in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 2483–2489. [Google Scholar] [CrossRef]
- Arimboor, R.; Venugopalan, V.; Sarinkumar, K.; Arumughan, C.; Sawhney, R.C. Integrated processing of fresh Indian sea buckthorn (Hippophae rhamnoides) berries and chemical evaluation of products. J. Sci. Food Agric. 2006, 86, 2345–2353. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from Sea Buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.; Daglia, M.; Atanasov, A.; et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Shang, D.; Sang, Z.; Dong, J. Structurally diverse flavonolignans with immunosuppressive and neuroprotective activities from the fruits of Hippophae rhamnoides L. J. Agric. Food Chem. 2020, 68, 6564–6575. [Google Scholar] [CrossRef] [PubMed]
- Khémiri, I.; Bitri, L. Effectiveness of Opuntia ficus indica L. Seed oil in the protection and the healing of experimentally induced gastric mucosa ulcer. Oxidative Med. Cell. Longev. 2019, 2019, 1568720. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).