Natural Pigments Production and Their Application in Food, Health and Other Industries
Abstract
1. Introduction
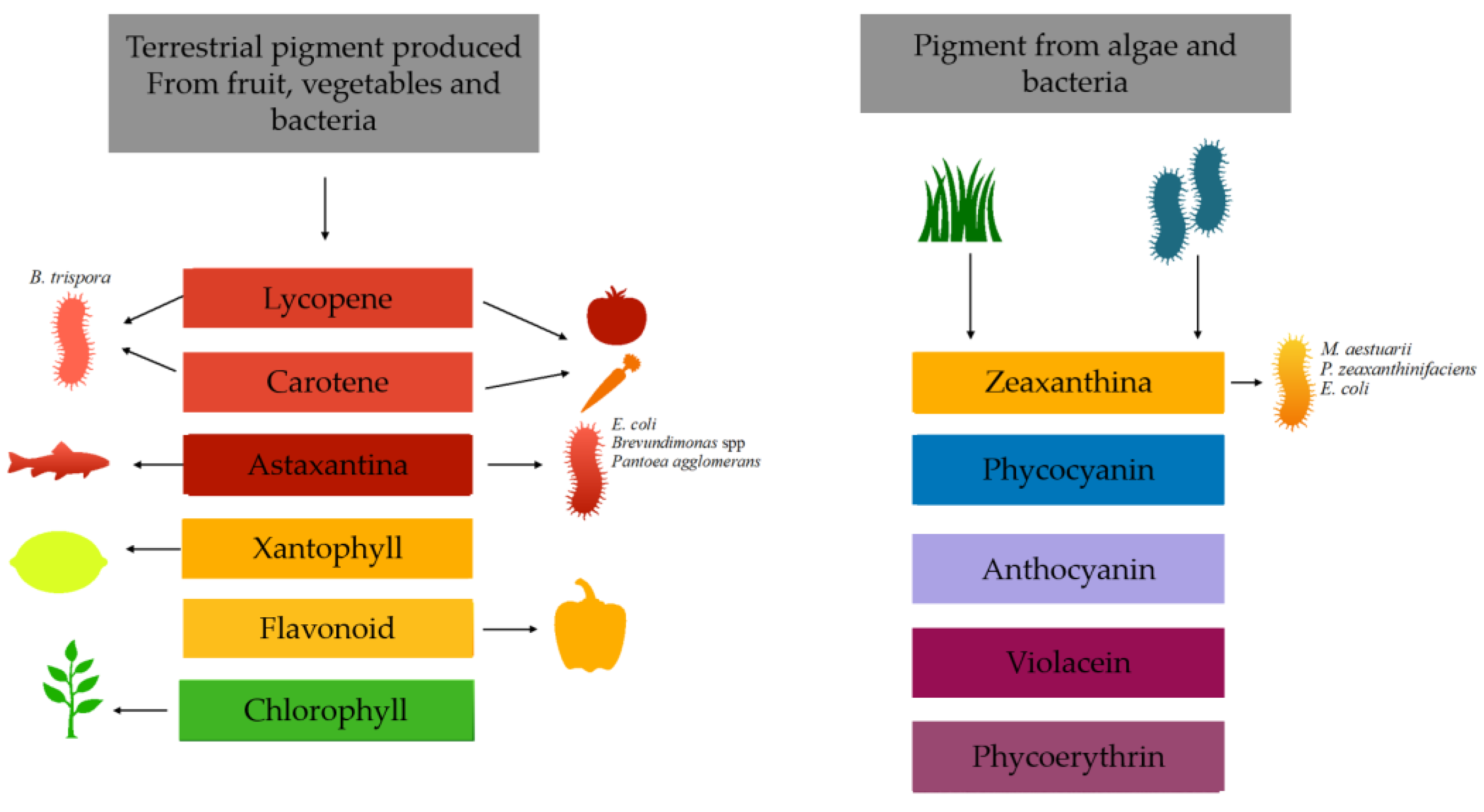
2. Pigments Produced by Microorganisms from Waste
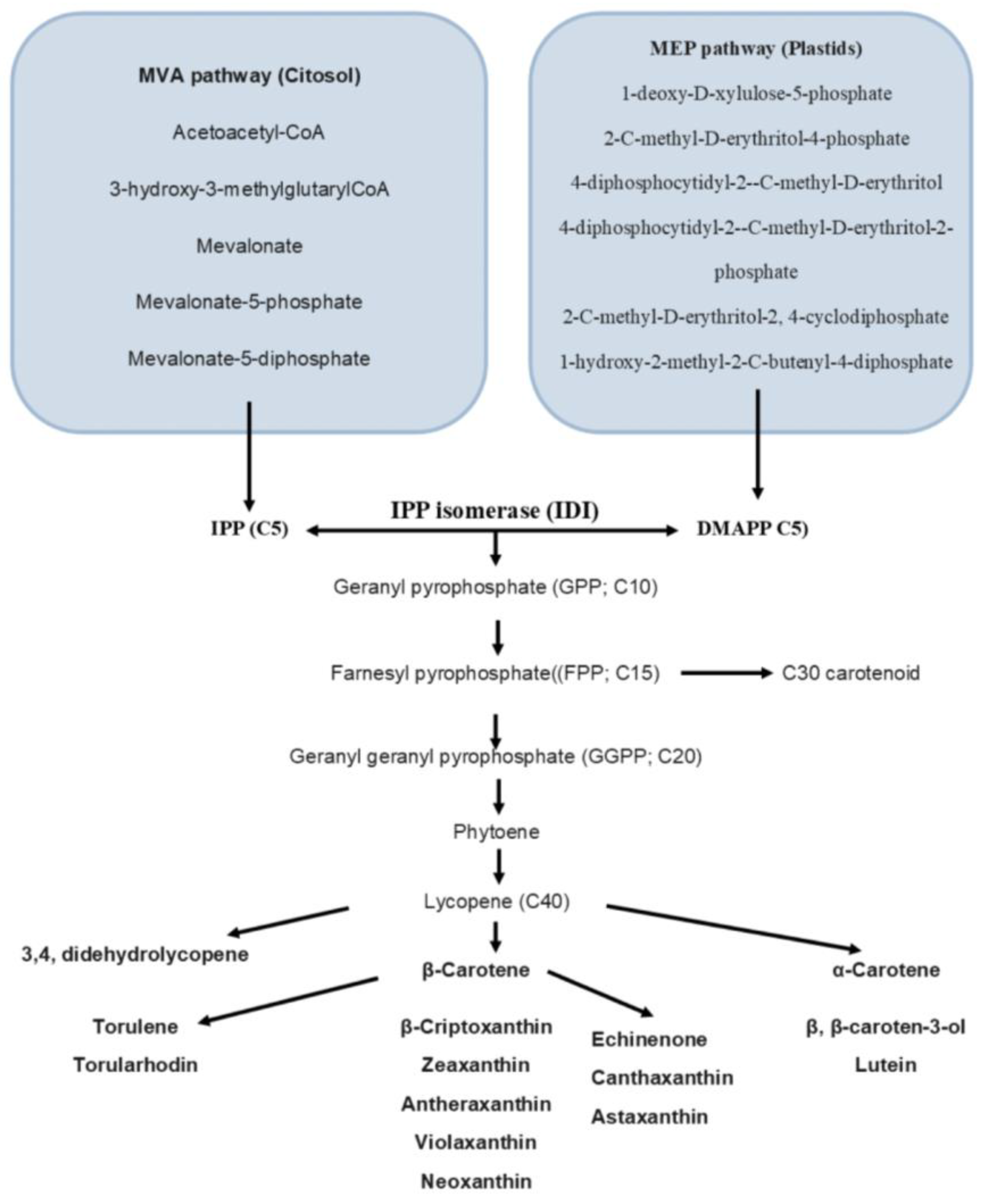
2.1. Carotenoids
- Xanthophylls, consisting of chains containing oxygen atoms; this group includes astaxanthin, lutein and zeaxanthin
- Carotenes are made up of oxygen-free molecules and are made up only of hydrogen and, in addition to carbon. The best-known classes are toluene, lycopene and carotene, which gives its name to the class [30].
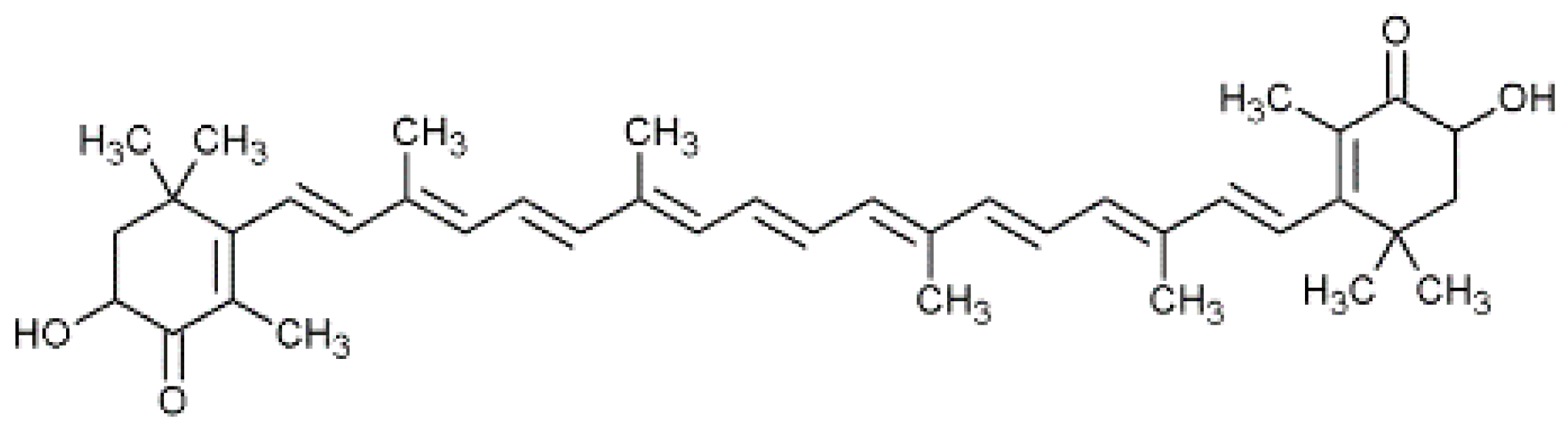
2.2. Astaxantine
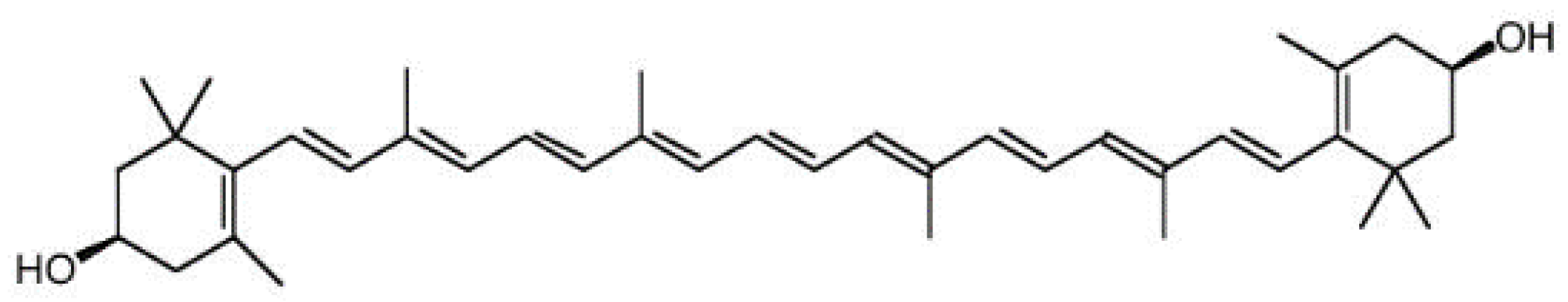
2.3. Zaexanthina
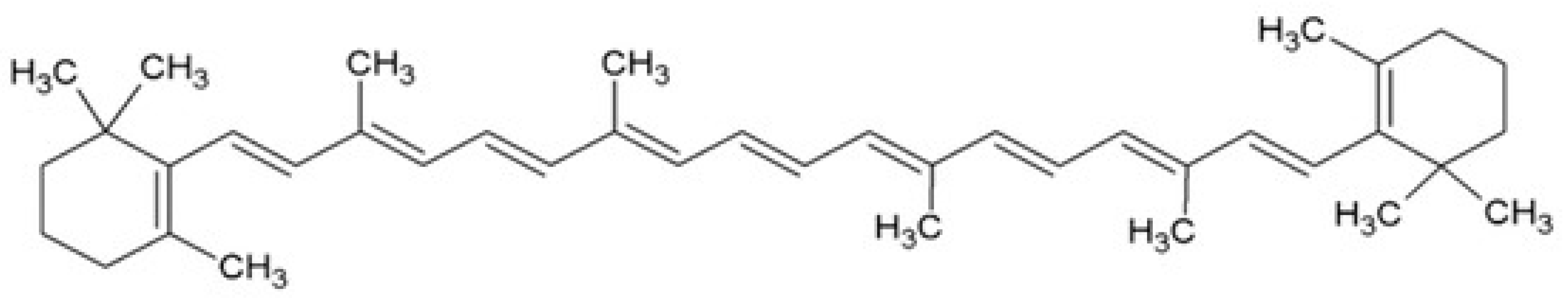
2.4. β-Carotene
2.5. Lycopene
3. Betalains
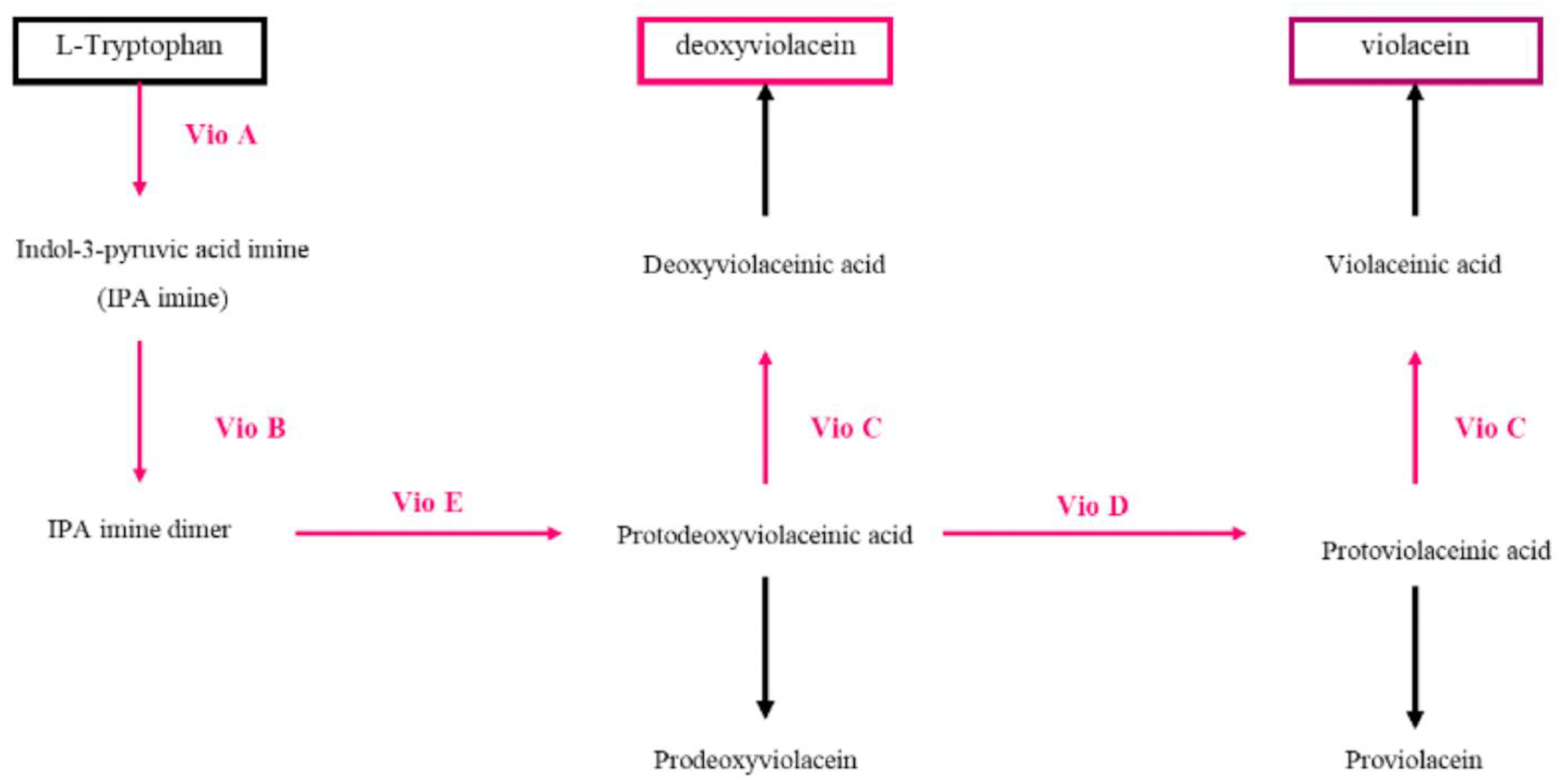
4. Violacein
Oxiviolacein and Deoxiviolacein
5. Prodigiosin
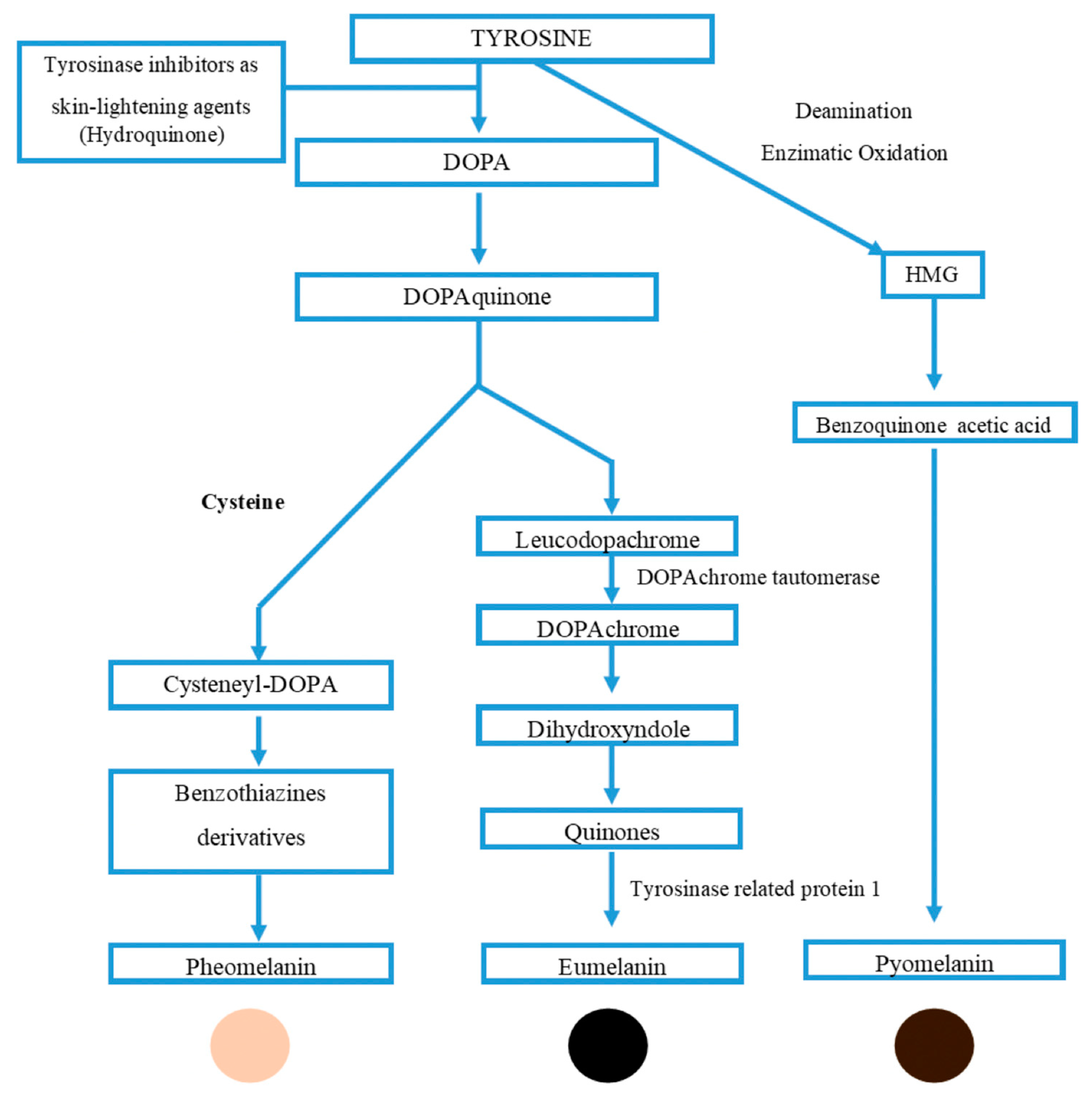
6. Melanin
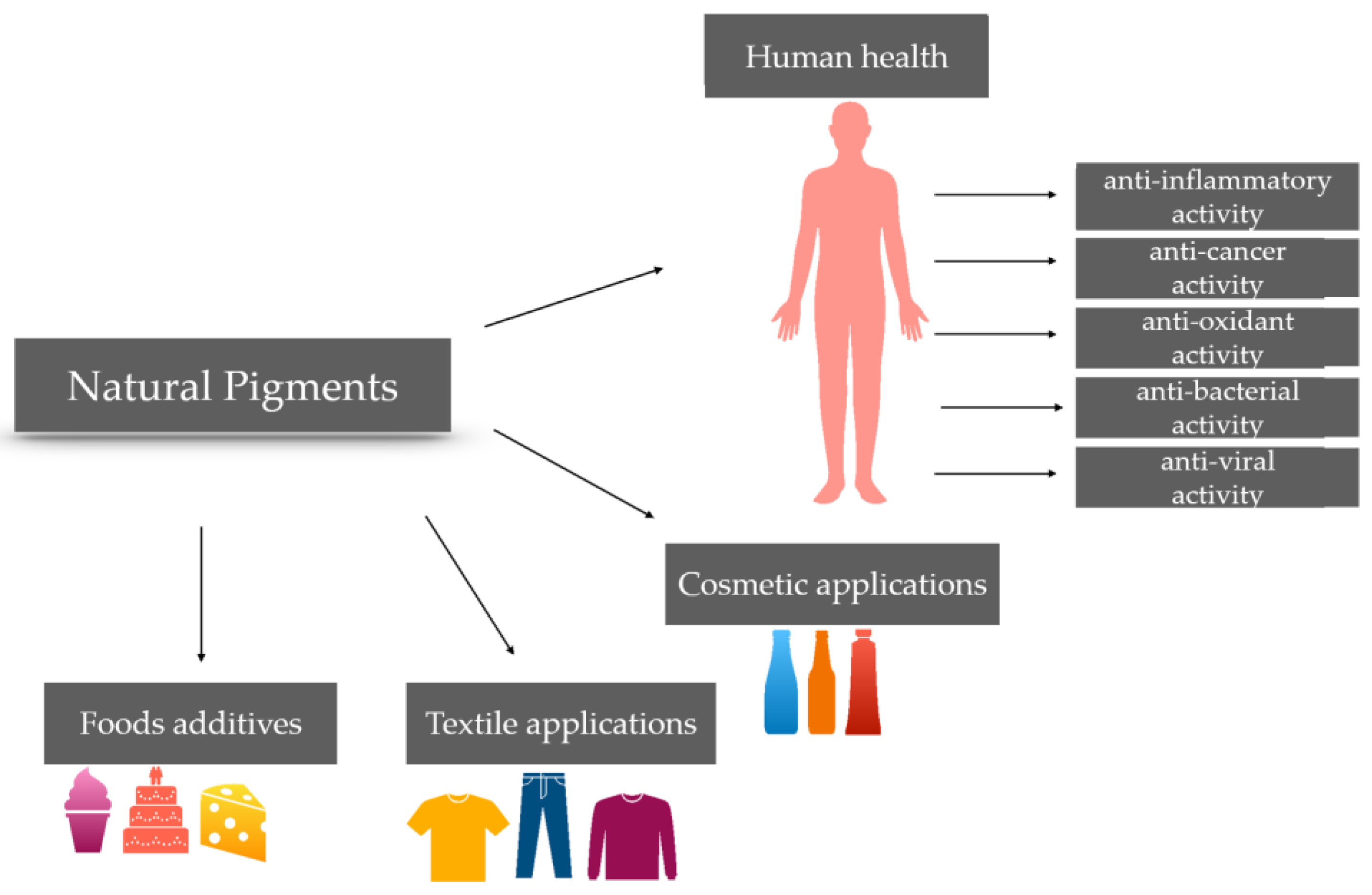
7. Application of Pigments
7.1. Food Industry
7.2. Pharmacological and Nutraceutical Industry
7.3. Cosmetic Industry
7.4. Textile Industry
7.5. Future Perspectives and Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar] [CrossRef]
- Venil, C.K. An Insightful Overview on Microbial Pigment, Prodigiosin. eJBio 2009, 5, 49–61. [Google Scholar]
- Souza Mesquita, L.M.; Martins, M.; Pisani, L.P.; Ventura, S.P.M.; Rosso, V.V. Insights on the Use of Alternative Solvents and Technologies to Recover Bio-based Food Pigments. Compr. Rev. Food Sci. Food Saf. 2020, 20, 787–818. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Mariano-Silva, G.; Leite, M.O.; Mura, F.B.; Verma, M.L.; da Silva, S.S.; Chandel, A.K. Production of Fungal and Bacterial Pigments and Their Applications. Biotechnol. Prod. Bioact. Compd. 2020, 1, 327–361. [Google Scholar] [CrossRef]
- Subhasree, R.S.; Babu, P.D.; Vidyalakshmi, R.; Mohan, V.C. Effect of Carbon and Nitrogen Sources on Stimulation of Pigment Production by Monascus Purpureus on Jackfruit Seeds. Int. J. Microbiol. Res. IJMR 2011, 2, 184–187. [Google Scholar]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; ul Haq, I. An Overview on Industrial and Medical Applications of Bio-Pigments Synthesized by Marine Bacteria. Microorganisms 2020, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Kumar, A.; Vishwakarma, H.S.; Singh, J.; Kumar, M. Microbial Pigments: Production and Their Applications in Various Industries. IJPCBS 2015, 5, 203–212. [Google Scholar]
- Seyfi, R.; Kahaki, F.A.; Ebrahimi, T.; Montazersaheb, S.; Eyvazi, S.; Babaeipour, V.; Tarhriz, V. Antimicrobial Peptides (AMPs): Roles, Functions and Mechanism of Action. Int. J. Pept. Res. Ther. 2019, 26, 1451–1463. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of Lipids and Carotenoids by Rhodotorula Gracilis ATCC 10788 Yeast in a Bioreactor Using Low-Cost Wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of Carotenoids Production by Rhodotorula Mucilaginosa (MTCC-1403) Using Agro-Industrial Waste in Bioreactor: A Statistical Approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-Utilization of Fruits and Vegetables Waste to Produce β-Carotene in Solid-State Fermentation: Characterization and Antioxidant Activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, G.; Arora, A.; Paul, D. Carotenoid Production by Red Yeast Isolates Grown in Agricultural and “Mandi” Waste. Waste Biomass Valorization 2020, 12, 3939–3949. [Google Scholar] [CrossRef]
- Simova, E.D.; Frengova, G.I.; Beshkova, D.M. Synthesis of Carotenoids by Rhodotorula Rubra GED8 Co-Cultured with Yogurt Starter Cultures in Whey Ultrafiltrate. J. Ind. Microbiol. Biotechnol. 2004, 31, 115–121. [Google Scholar] [CrossRef]
- Kanzy, H.; Nasr, N.; El-Shazly, H.; Barakat, O. Optimization of Carotenoids Production by Yeast Strains of Rhodotorula Using Salted Cheese Whey. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 456–469. [Google Scholar]
- Patthawaro, S.; Lomthaisong, K.; Saejung, C. Bioconversion of Agro-Industrial Waste to Value-Added Product Lycopene by Photosynthetic Bacterium Rhodopseudomonas Faecalis and Its Carotenoid Composition. Waste Biomass Valorization 2019, 11, 2375–2386. [Google Scholar] [CrossRef]
- Eroglu, E.; Gunduz, U.; Yucel, M.; Eroglu, I. Photosynthetic Bacterial Growth and Productivity under Continuous Illumination or Diurnal Cycles with Olive Mill Wastewater as Feedstock. Int. J. Hydrog. Energy 2010, 35, 5293–5300. [Google Scholar] [CrossRef]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of Olive Mill Waste as Substrate for Carotenoid Production by Rhodotorula Mucilaginosa. Bioresour. Bioprocess. 2020, 7, 52. [Google Scholar] [CrossRef]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and Carotenoid Production from Wheat Straw Hydrolysates by Different Oleaginous Yeasts. J. Clean. Prod. 2020, 249, 119308. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Sun, L.; Jin, Y.-S.; Dien, B.; Singh, V. Production of Xylose Enriched Hydrolysate from Bioenergy Sorghum and Its Conversion to β-Carotene Using an Engineered Saccharomyces Cerevisiae. Bioresour. Technol. 2020, 308, 123275. [Google Scholar] [CrossRef]
- Petrik, S.; Obruča, S.; Benešová, P.; Márová, I. Bioconversion of Spent Coffee Grounds into Carotenoids and Other Valuable Metabolites by Selected Red Yeast Strains. Biochem. Eng. J. 2014, 90, 307–315. [Google Scholar] [CrossRef]
- Otero, D.M.; Bulsing, B.A.; da M. Huerta, K.; Rosa, C.A.; Zambiazi, R.C.; Burkert, C.A.V.; de M. Burkert, J.F. Carotenoid-Producing Yeasts in the Brazilian Biodiversity: Isolation, Identification And Cultivation In Agroindustrial Waste. Braz. J. Chem. Eng. 2019, 36, 117–129. [Google Scholar] [CrossRef]
- YAZGIN, O.; KESKIN-GUNDOGDU, T. Production of Biogas and Astaxanthin from Fruit and Vegetable Wastes Using an Integrated System. Int. J. Second. Metab. 2020, 35–46. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Benameur, Q.; Gervasi, C.; Santini, A.; Cicero, N.; Dugo, G. Valorization of Raw Materials from Agricultural Industry for Astaxanthin and β-Carotene Production by Xanthophyllomyces Dendrorhous. Nat. Prod. Res. 2018, 32, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Poddar, K.; Padhan, B.; Sarkar, D.; Sarkar, A. Purification and Optimization of Pink Pigment Produced by Newly Isolated Bacterial Strain Enterobacter Sp. PWN1. SN Appl. Sci. 2021, 3, 105. [Google Scholar] [CrossRef]
- Bezirhan Arikan, E.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus Carbonarius. J. Fungi 2020, 6, 240. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Masalamany, S.R.L.; Venil, C.K.; Ahmad, W.A. Antibacterial Mode of Action of Violacein from Chromobacterium Violaceum UTM5 against Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Aureus (MRSA). Environ. Sci. Pollut. Res. 2018, 25, 5164–5180. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological Production of Carotenoids by Yeasts: An Overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cámara, S.; Ibañez, A.; Rubio, S.; Barreiro, C.; Barredo, J.-L. Main Carotenoids Produced by Microorganisms. Encyclopedia 2021, 1, 1223–1245. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Manimala, M.R.A.; Murugesan, R. In Vitro Antioxidant and Antimicrobial Activity of Carotenoid Pigment Extracted from Sporobolomyces sp. Isolated from Natural Source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Korumilli, T.; Mishra, S. Carotenoid Production by Bacillus Clausii Using Rice Powder as the Sole Substrate: Pigment Analyses and Optimization of Key Production Parameters. J. Biochem. Technol. 2014, 5, 788–794. [Google Scholar]
- Sun, L.; Atkinson, C.A.; Lee, Y.-G.; Jin, Y.-S. High-level Β-carotene Production from Xylose by Engineered Saccharomyces Cerevisiae without Overexpression of a Truncated HMG1 (THMG1). Biotechnol. Bioeng. 2020, 117, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a Microalgal Metabolite for Aquaculture: A Review on the Synthetic Mechanisms, Production Techniques, and Practical Application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces Dendrorhous for the Industrial Production of Astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Dufossé, L. A Review of Natural Astaxanthin Production in a Circular Bioeconomy Context Using Paracoccus Carotinifaciens. Bioresour. Technol. 2023, 369, 128499. [Google Scholar] [CrossRef]
- Niizawa, I.; Espinaco, B.Y.; Zorrilla, S.E.; Sihufe, G.A. Astaxanthin Production by Autotrophic Cultivation of Haematococcus Pluvialis: A Success Story. In Global Perspectives on Astaxanthin; Academic Press: Cambridge, MA, USA, 2021; pp. 71–89. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, W.; Mou, H.; Sun, H. Improving Astaxanthin Production of Haematococcus Pluvialis by an Efficient Fed-Batch Strategy in a Photobioreactor. Algal Res. 2021, 60, 102539. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Silva, P.G.P.; Amantino, C.F.; Burkert, J.F.M.; Primo, F.L.; Pessoa, A.; Santos-Ebinuma, V.C. Production of Natural Astaxanthin by Phaffia Rhodozyma and Its Potential Application in Textile Dyeing. Biochem. Eng. J. 2022, 187, 108658. [Google Scholar] [CrossRef]
- Lien, N.T.K.; Lan, N.N.; Thoa, N.K.; Phuong, N.T.D.; Huy, N.Q.; Hoang, N.H. Bacterial astaxanthin: Production and application in aquaculture—A review. Vietnam. J. Biotechnol. 2018, 16, 393–405. [Google Scholar] [CrossRef]
- Pan, X.; Wang, B.; Duan, R.; Jia, J.; Li, J.; Xiong, W.; Ling, X.; Chen, C.; Huang, X.; Zhang, G.; et al. Enhancing Astaxanthin Accumulation in Xanthophyllomyces Dendrorhous by a Phytohormone: Metabolomic and Gene Expression Profiles. Microb. Biotechnol. 2020, 13, 1446–1460. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, H.; Tang, J.; Bi, C.; Li, Q.; Zhang, X. Coordinated Expression of Astaxanthin Biosynthesis Genes for Improved Astaxanthin Production in Escherichia Coli. J. Agric. Food Chem. 2020, 68, 14917–14927. [Google Scholar] [CrossRef]
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; De Pasquale, P.; Dugo, G.; Cicero, N. Astaxanthin Production by Xanthophyllomyces Dendrorhous Growing on a Low Cost Substrate. Agrofor. Syst. 2020, 94, 1229–1234. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, Y.M.; Baik, K.S.; Choi, K.S.; Ka, J.-O.; Seong, C.N. Mesoflavibacter Aestuarii Sp. Nov., a Zeaxanthin-Producing Marine Bacterium Isolated from Seawater. Int. J. Syst. Evol. Microbiol. 2014, 64, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Singhal, R.S. Modelling and Optimization of Zeaxanthin Production by Paracoccus Zeaxanthinifaciens ATCC 21588 Using Hybrid Genetic Algorithm Techniques. Biocatal. Agric. Biotechnol. 2016, 8, 228–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Sun, J.; Xue, C.; Mao, X. Biotechnological Production of Zeaxanthin by Microorganisms. Trends Food Sci. Technol. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production. Biology 2018, 7, 14. [Google Scholar] [CrossRef]
- Nanou, K.; Roukas, T.; Papadakis, E. Improved Production of Carotenes from Synthetic Medium by Blakeslea Trispora in a Bubble Column Reactor. Biochem. Eng. J. 2012, 67, 203–207. [Google Scholar] [CrossRef]
- Muhammad, A.; Feng, X.; Rasool, A.; Sun, W.; Li, C. Production of Plant Natural Products through Engineered Yarrowia Lipolytica. Biotechnol. Adv. 2020, 43, 107555. [Google Scholar] [CrossRef] [PubMed]
- Hladnik, L.; Vicente, F.A.; Grilc, M.; Likozar, B. β-Carotene Production and Extraction: A Case Study of Olive Mill Wastewater Bioremediation by Rhodotorula Glutinis with Simultaneous Carotenoid Production. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčák, S.; Čertík, M. Dual Production of Polyunsaturated Fatty Acids and Beta-Carotene with Mucor Wosnessenskii by the Process of Solid-State Fermentation Using Agro-Industrial Waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef]
- Nurkanto, A.; Handayani, R.; Purnaningsih, I.; Kusmiati, M.; Setianingrum, N.; Mulyadi, M.; Kusdiyanti, E.; Dinoto, A. Biosynthesis and Profiling of Single Cell Carotenoids of Phaf Ia Rhodozyma in Waste-Based Cultivation Media. JMSB 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xia, Q.; Zhang, H.; Zhang, R.; Yang, J. Metabolic Engineering of Different Microbial Hosts for Lycopene Production. J. Agric. Food Chem. 2020, 68, 14104–14122. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Kondo, K.; Saito, T.; Shimada, H.; Fraser, P.D.; Misawa, N. Production of the Carotenoids Lycopene, β-Carotene, and Astaxanthin in the Food Yeast Candida Utilis. Appl. Environ. Microbiol. 1998, 64, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Lampila, L.E.; Wallen, S.E.; Bullerman, L.B. A Review of Factors Affecting Biosynthesis of Carotenoids by the Order Mucorales. Mycopathologia 1985, 90, 65–80. [Google Scholar] [CrossRef]
- Sevgili, A.; Erkmen, O. Improved Lycopene Production from Different Substrates by Mated Fermentation of Blakeslea Trispora. Foods 2019, 8, 120. [Google Scholar] [CrossRef]
- Murillo, F.J.; Calderón, I.L.; López-Díaz, I.; Cerdá-Olmedo, E. Carotene-Superproducing Strains of Phycomyces. Appl. Environ. Microbiol. 1978, 36, 639–642. [Google Scholar] [CrossRef]
- Hohmann, H.-P.; Pasamontes, L.; Tessier, M.; Van Loon, A. Fermentative Carotenoid Production. Google Patents EP0747483B1, 2000. [Google Scholar]
- Choudhari, S.M.; Ananthanarayan, L.; Singhal, R.S. Use of Metabolic Stimulators and Inhibitors for Enhanced Production of β-Carotene and Lycopene by Blakeslea Trispora NRRL 2895 and 2896. Bioresour. Technol. 2008, 99, 3166–3173. [Google Scholar] [CrossRef]
- Wang, H.-B.; Xu, R.-G.; Yu, L.-J.; Luo, J.; Zhang, L.-W.; Huang, X.-Y.; Zou, W.-A.; Zhao, Q.; Lu, M.-B. Improved SS-Carotene and Lycopene Production by Blakeslea Trispora with Ultrasonic Treatment in Submerged Fermentation. Z. Für Nat. C 2014, 69, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Jeong, S.-W.; Yang, J.E.; Choi, Y.J. High-Yield Production of Lycopene from Corn Steep Liquor and Glycerol Using the Metabolically Engineered Deinococcus Radiodurans R1 Strain. J. Agric. Food Chem. 2020, 68, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Yu, C.; Lu, S.; Xiong, S.; Yuan, Y. Continuous Self-Cycling Fermentation Leads to Economical Lycopene Production by Saccharomyces Cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shi, B.; Ye, Z.; Li, X.; Liu, M.; Chen, Y.; Xia, J.; Nielsen, J.; Deng, Z.; Liu, T. Lipid Engineering Combined with Systematic Metabolic Engineering of Saccharomyces Cerevisiae for High-Yield Production of Lycopene. Metab. Eng. 2019, 52, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Giridhar, P. Plant Betalains: Chemistry and Biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.S. Betalains: Application in Functional Foods. Bioact. Mol. Food 2019, 1471–1498. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Akbar Hussain, E.; Sadiq, Z.; Zia-Ul-Haq, M. Processing of Betalains. Betalains Biomol. Asp. 2018, 185–187. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Betalains. In Encyclopedia of Food Chemistry; Academic Press: Cambridge, MA, USA, 2019; pp. 35–39. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent Advances in Betalain Research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Grewal, P.S.; Modavi, C.; Russ, Z.N.; Harris, N.C.; Dueber, J.E. Bioproduction of a Betalain Color Palette in Saccharomyces Cerevisiae. Metab. Eng. 2018, 45, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, X.; Li, S.; Zhang, X.; Yu, S.; Zhao, G.-R. Metabolic Engineering of Escherichia Coli for de Novo Production of Betaxanthins. J. Agric. Food Chem. 2020, 68, 8370–8380. [Google Scholar] [CrossRef] [PubMed]
- Akita, T.; Hina, Y.; Nishi, T. Production of Betacyanins by a Cell Suspension Culture of Table Beet (Beta vulgaris L.). Biosci. Biotechnol. Biochem. 2000, 64, 1807–1812. [Google Scholar] [CrossRef]
- Gasztonyi, M.N.; Daood, H.; Hájos, M.T.; Biacs, P. Comparison of Red Beet (Beta Vulgarisvarconditiva) Varieties on the Basis of Their Pigment Components. J. Sci. Food Agric. 2001, 81, 932–933. [Google Scholar] [CrossRef]
- Balibar, C.J.; Walsh, C.T. In Vitro Biosynthesis of Violacein from L-Tryptophan by the Enzymes VioA−E from Chromobacterium Violaceum. Biochemistry 2006, 45, 15444–15457. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T. Violacein and Related Tryptophan Metabolites Produced by Chromobacterium Violaceum: Biosynthetic Mechanism and Pathway for Construction of Violacein Core. Appl. Microbiol. Biotechnol. 2011, 91, 1463–1475. [Google Scholar] [CrossRef]
- Cauz, A.C.G.; Carretero, G.P.B.; Saraiva, G.K.V.; Park, P.; Mortara, L.; Cuccovia, I.M.; Brocchi, M.; Gueiros-Filho, F.J. Violacein Targets the Cytoplasmic Membrane of Bacteria. ACS Infect. Dis. 2019, 5, 539–549. [Google Scholar] [CrossRef]
- Durán, M.; Ponezi, A.N.; Faljoni-Alario, A.; Teixeira, M.F.S.; Justo, G.Z.; Durán, N. Potential Applications of Violacein: A Microbial Pigment. Med. Chem. Res. 2011, 21, 1524–1532. [Google Scholar] [CrossRef]
- Kanade, Y.; Mohan, W.; Patwardhan, R. Violacein: A Promising Bacterial Secondary Metabolite. Res. J. Chem. Environ. 2022, 26, 165–177. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lim, S.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Biotechnological Activities and Applications of Bacterial Pigments Violacein and Prodigiosin. J. Biol. Eng. 2021, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Dike-Ndudim, J.N.; Ugenyi, L.C.; Ndubueze, C.W. Assessment of Antifungal Potentials of Violacein Extract from Chromobacterium Violaceum Isolated from Domestic and Recreational Water Sources in Owerri, Imo State, Nigeria. World J. Adv. Res. Rev. 2021, 10, 168–172. [Google Scholar] [CrossRef]
- Sedláček, I.; Holochová, P.; Sobotka, R.; Busse, H.-J.; Švec, P.; Králová, S.; Šedo, O.; Pilný, J.; Staňková, E.; Koublová, V.; et al. Classification of a Violacein-Producing Psychrophilic Group of Isolates Associated with Freshwater in Antarctica and Description of Rugamonas Violacea Sp. Nov. Microbiol. Spectr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Aranda, S.; Montes-Borrego, M.; Landa, B.B. Purple-Pigmented Violacein-Producing Duganella spp. Inhabit the Rhizosphere of Wild and Cultivated Olives in Southern Spain. Microb. Ecol. 2011, 62, 446–459. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, H.; Zhang, C.; Lou, K.; Xing, X.-H. Reconstruction of the Violacein Biosynthetic Pathway from Duganella sp. B2 in Different Heterologous Hosts. Appl. Microbiol. Biotechnol. 2009, 86, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Thøgersen, M.S.; Delpin, M.W.; Melchiorsen, J.; Kilstrup, M.; Månsson, M.; Bunk, B.; Spröer, C.; Overmann, J.; Nielsen, K.F.; Gram, L. Production of the Bioactive Compounds Violacein and Indolmycin Is Conditional in a MaeA Mutant of Pseudoalteromonas Luteoviolacea S4054 Lacking the Malic Enzyme. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Timmermans, M.L.; Picott, K.J.; Ucciferri, L.; Ross, A.C. Culturing Marine Bacteria from the GenusPseudoalteromonason a Cotton Scaffold Alters Secondary Metabolite Production. MicrobiologyOpen 2018, 8, e00724. [Google Scholar] [CrossRef]
- Agematu, H.; Suzuki, K.; Tsuya, H. Massilia sp. BS-1, a Novel Violacein-Producing Bacterium Isolated from Soil. Biosci. Biotechnol. Biochem. 2011, 75, 2008–2010. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Gayke, M.; Narode, H.; Alzahrani, K.J.; Singh, V. Enhanced Production of Violacein by Chromobacterium Violaceum Using Agro-industrial Waste Soybean Meal. J. Appl. Microbiol. 2021, 132, 1121–1133. [Google Scholar] [CrossRef]
- Park, H.; Park, S.; Yang, Y.-H.; Choi, K.-Y. Microbial Synthesis of Violacein Pigment and Its Potential Applications. Crit. Rev. Biotechnol. 2021, 41, 879–901. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Göcke, Y.; Bolten, C.; Brock, N.L.; Dickschat, J.S.; Wittmann, C. Microbial Production of the Drugs Violacein and Deoxyviolacein: Analytical Development and Strain Comparison. Biotechnol. Lett. 2011, 34, 717–720. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, H.; Xiao, S.; Fang, M.; Zhang, R.; He, S.; Lou, K.; Xing, X.-H. Pathway Redesign for Deoxyviolacein Biosynthesis in Citrobacter Freundii and Characterization of This Pigment. Appl. Microbiol. Biotechnol. 2012, 94, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Darshan, N.; Manonmani, H.K. Prodigiosin Inhibits Motility and Activates Bacterial Cell Death Revealing Molecular Biomarkers of Programmed Cell Death. AMB Express 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Siva, R.; Subha, K.; Bhakta, D.; Ghosh, A.R.; Babu, S. Characterization and Enhanced Production of Prodigiosin from the Spoiled Coconut. Appl. Biochem. Biotechnol. 2011, 166, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Aruldass, C.A.; Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Brown Sugar as a Low-Cost Medium for the Production of Prodigiosin by Locally Isolated Serratia Marcescens UTM1. Int. Biodeterior. Biodegrad. 2014, 95, 19–24. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Chen, S.-P.; Nguyen, T.H.; Nguyen, M.T.; Tran, T.T.T.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Novel Efficient Bioprocessing of Marine Chitins into Active Anticancer Prodigiosin. Mar. Drugs 2019, 18, 15. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.; Ribera, J. Microbial Production of Melanin and Its Various Applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Gao, Q.; Garcia-Pichel, F. Microbial Ultraviolet Sunscreens. Nat. Rev. Microbiol. 2011, 9, 791–802. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production of Melanin by Soil Microbial Isolate on Fruit Waste Extract: Two Step Optimization of Key Parameters. Biotechnol. Rep. 2014, 4, 139–146. [Google Scholar] [CrossRef]
- Müjdeci, G.N. Experimental Modeling and Optimization of Melanin Production by Aureobasidium Pullulans NBRC 100716 in Carrot Peel Extract. Environ. Prog. Sustain. Energy 2022, 41, e13919. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, K.; Zhao, S.; Wang, W.; Ru, X.; Song, J.; Cong, H.; Yang, Q. Screening and Identification of a Strain of Aureobasidium Pullulans and Its Application in Potato Starch Industrial Waste. Environ. Res. 2022, 214, 113947. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Scognamiglio, M.; Mirpoor, S.F.; Cammarota, M.; Ventriglia, R.; Giosafatto, C.V.L.; Fiorentino, A.; Porta, R.; Schiraldi, C. Enhanced Streptomyces Roseochromogenes Melanin Production by Using the Marine Renewable Source Posidonia Oceanica Egagropili. Appl. Microbiol. Biotechnol. 2022, 106, 7265–7283. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tao, J.; Wei, D.; Shen, Y.; Tong, W. Development of an Adsorption Procedure for the Direct Separation and Purification of Prodigiosin from Culture Broth. Biotechnol. Appl. Biochem. 2004, 40, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial Pigments: A Colorful Palette Reservoir for Biotechnological Applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.d.O. Nanoencapsulation of Carotenoids: A Focus on Different Delivery Systems and Evaluation Parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef]
- Pasamontes, L.; Hug, D.; Tessier, M.; Hohmann, H.-P.; Schierle, J.; van Loon, A.P. Isolation and Characterization of the Carotenoid Biosynthesis Genes of Flavobacterium Sp. Strain R1534. Gene 1997, 185, 35–41. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S. Safety and Efficacy of Lutein and Lutein/Zeaxanthin Extracts from Tagetes Erecta for Poultry for Fattening and Laying (except Turkeys). EFSA J. 2019, 17, e05698. [Google Scholar]
- Surai, P.F. The Antioxidant Properties of Canthaxanthin and Its Potential Effects in the Poultry Eggs and on Embryonic Development of the Chick. Part 2. World’s Poult. Sci. J. 2012, 68, 717–726. [Google Scholar] [CrossRef]
- Simon, J.E.; Decker, E.A.; Ferruzzi, M.G.; Giusti, M.M.; Mejia, C.D.; Goldschmidt, M.; Talcott, S.T. Establishing Standards on Colors from Natural Sources. J. Food Sci. 2017, 82, 2539–2553. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Tsou, T.-Y.; Liu, H.-L. Response Surface Optimization of Microbial Prodigiosin Production from Serratia Marcescens. J. Taiwan Inst. Chem. Eng. 2011, 42, 217–222. [Google Scholar] [CrossRef]
- Namazkar, S.; Ahmad, W.A. Spray-Dried Prodigiosin from Serratia Marcescens as A Colorant. Biosci. Biotechnol. Res. Asia 2013, 10, 69–76. [Google Scholar] [CrossRef]
- Durán, N.; Menck, C.F.M. Chromobacterium Violaceum: A Review of Pharmacological and Industiral Perspectives. Crit. Rev. Microbiol. 2001, 27, 201–222. [Google Scholar] [CrossRef]
- Pantanella, F.; Berlutti, F.; Passariello, C.; Sarli, S.; Morea, C.; Schippa, S. Violacein and Biofilm Production in Janthinobacterium Lividum. J. Appl. Microbiol. 2007, 102, 992–999. [Google Scholar] [CrossRef]
- Pauer, H.; Hardoim, C.C.P.; Teixeira, F.L.; Miranda, K.R.; Barbirato, D.d.S.; de Carvalho, D.P.; Antunes, L.C.M.; Leitão, Á.A.d.C.; Lobo, L.A.; Domingues, R.M.C.P. Impact of Violacein from Chromobacterium Violaceum on the Mammalian Gut Microbiome. PLoS ONE 2018, 13, e0203748. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a Photosensitizer. Effects on Human Health and Food Quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-Overproducing Strains of Lactobacillus Fermentum for Riboflavin-Enriched Bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Y.; Chen, F. Monascus Pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef]
- Vidyalakshmi, R.; Paranthaman, R.; Murugesh, S.; Singaravadivel, K. Microbial Bioconversion of Rice Broken to Food Grade Pigments. 2009. Available online: https://www.researchgate.net/publication/237812123_Microbial_Bioconversion_of_Rice_Broken_to_Food_Grade_Pigments (accessed on 1 February 2023).
- Hamano, P.S.; Kilikian, B.V. Production of Red Pigments by Monascus Ruber in Culture Media Containing Corn Steep Liquor. Braz. J. Chem. Eng. 2006, 23, 443–449. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From Molecule to Function. Mol. Nutr. Food Res. 2017, 61, 1600469. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kim, S.; Moon, B. Optimization by Response Surface Methodology of Lutein Recovery from Paprika Leaves Using Accelerated Solvent Extraction. Food Chem. 2016, 205, 140–145. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of Antioxidant High Dietary Fiber Powder from Carrot Peels. LWT Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Alves Rezende, C.; Alexandre Rodrigues, R.; Fernández Barbero, G.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple Peels as a Value-Added Food Ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Kumar, R.R.; Prasad, S. Metabolic Engineering of Bacteria. Indian J. Microbiol. 2011, 51, 403–409. [Google Scholar] [CrossRef]
- Nagpal, N.; Munjal, N.; Chatterjee, S. Microbial Pigments with Health Benefits—A Mini Review. 2011. Available online: https://www.researchgate.net/publication/280067897_Microbial_Pigments_with_Health_Benefits_-_A_Mini_Review_Neeraj_Nagpal_Neera_Munjal_and_Sayan_Chatterjee_Trends_in_Biosciences_Volume_4_No_2_2011_pg_157 (accessed on 1 February 2023).
- Jayaseelan, S.; Ramaswamy, D.; Dharmaraj, S. Pyocyanin: Production, Applications, Challenges and New Insights. World J. Microbiol. Biotechnol. 2014, 30, 1159–1168. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, G.; Zhang, Y.; Yu, J.; Wang, Y.; Ma, X. Active Edible Films with Plant Extracts: A Updated Review of Their Types, Preparations, Reinforcing Properties, and Applications in Muscle Foods Packaging and Preservation. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- You, Z.; Zhang, S.; Liu, X.; Zhang, J.; Wang, Y.; Peng, Y.; Wu, W. Insights into the Anti-Infective Properties of Prodiginines. Appl. Microbiol. Biotechnol. 2019, 103, 2873–2887. [Google Scholar] [CrossRef]
- Dodou, H.; Batista, A.D.M.; Sales, G.; de Medeiros, S.; Rodrigues, M.; Nogueira, P.; Silveira, E.; Nogueira, N. Violacein Antimicrobial Activity on Staphylococcus Epidermidis and Synergistic Effect on Commercially Available Antibiotics. J. Appl. Microbiol. 2017, 123, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid. Based Complement. Altern. Med. 2011, 2011, e670349. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, J.; Wang, X.; Kong, D.; Du, W.; Li, H.; Hse, C.-Y.; Shupe, T.; Zhou, D.; Zhao, K. Biological Potential and Mechanism of Prodigiosin from Serratia Marcescens Subsp. Lawsoniana in Human Choriocarcinoma and Prostate Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 3465. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, C.; Liu, R.; Chen, J.; Li, R.; Wang, X.; Bai, W.; Liu, X.; Xiang, T.; Zhang, L.; et al. Antiviral Activity and Specific Modes of Action of Bacterial Prodigiosin against Bombyx Mori Nucleopolyhedrovirus in Vitro. Appl. Microbiol. Biotechnol. 2016, 100, 3979–3988. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Koujah, L.; Patil, C.D.; Ames, J.M.; Agelidis, A.; Yadavalli, T.; Patil, S.V.; Shukla, D. Bacterial Pigment Prodigiosin Demonstrates a Unique Antiherpesvirus Activity That Is Mediated through Inhibition of Prosurvival Signal Transducers. J. Virol. 2020, 94, e00251-20. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, T.M.; Alharbi, N.S.; Theruvinthalakal, A.M.; Thekkangil, A.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Sankar, K.; Innasimuthu, G.M.; Alanzi, K.F.; et al. Characterization and Antifungal Activity of the Yellow Pigment Produced by a Bacillus Sp. DBS4 Isolated from the Lichen Dirinaria Agealita. Saudi J. Biol. Sci. 2020, 27, 1403–1411. [Google Scholar] [CrossRef]
- Sasidharan, A.; Sasidharan, N.K.; Amma, D.B.N.S.; Vasu, R.K.; Nataraja, A.V.; Bhaskaran, K. Antifungal Activity of Violacein Purified from a Novel Strain of Chromobacterium sp. NIIST (MTCC 5522). J. Microbiol. 2015, 53, 694–701. [Google Scholar] [CrossRef]
- Leon, L.L.; Miranda, C.C.; De Souza, A.O.; Durán, N. Antileishmanial Activity of the Violacein Extracted from Chromobacterium Violaceum. J. Antimicrob. Chemother. 2001, 48, 449–450. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, A.; Hosokawa, K.; Soliev, A.B.; Osaka, I.; Arakawa, R.; Enomoto, K. Cytotoxic Prodigiosin Family Pigments from Pseudoalteromonas sp. 1020R Isolated from the Pacific Coast of Japan. Biosci. Biotechnol. Biochem. 2012, 76, 1229–1232. [Google Scholar] [CrossRef]
- Srilekha Investigation of In Vitro Cytotoxic Activity of Pigment Extracted from Salinicoccus sp. Isolated from Nellore Sea Coast. Available online: https://www.marinemedicalsociety.in/article.asp?issn=0975-3605;year=2018;volume=20;issue=1;spage=31;epage=33;aulast=Srilekha (accessed on 30 January 2023).
- Campàs, C.; Dalmau, M.; Montaner, B.; Barragán, M.; Bellosillo, B.; Colomer, D.; Pons, G.; Pérez-Tomás, R.; Gil, J. Prodigiosin Induces Apoptosis of B and T Cells from B-Cell Chronic Lymphocytic Leukemia. Leukemia 2003, 17, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Núñez Selles, A.J.; Daglia, M.; Rastrelli, L. The Potential Role of Mangiferin in Cancer Treatment through Its Immunomodulatory, Anti-Angiogenic, Apoptopic, and Gene Regulatory Effects. BioFactors 2016, 42, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković,, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and Cell Growth Activities of Beet Root Pomace Extracts. J. Funct. Foods 2012, 4, 670–678. [CrossRef]
- Rubino, S.; Busà, R.; Attanzio, A.; Alduina, R.; Di Stefano, V.; Girasolo, M.A.; Orecchio, S.; Tesoriere, L. Synthesis, Properties, Antitumor and Antibacterial Activity of New Pt(II) and Pd(II) Complexes with 2,2′-Dithiobis(Benzothiazole) Ligand. Bioorg. Med. Chem. 2017, 25, 2378–2386. [Google Scholar] [CrossRef]
- Melo, P.S.; Maria, S.S.; Vidal, B.C.; Haun, M.; Durán, N. Violacein Cytotoxicity and Induction of Apoptosis in V79 Cells. Vitr. Cell Dev. Biol. Anim. 2000, 36, 539–543. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Subash-Babu, P.; Antonisamy, P. Violacein Induces Apoptosis in Human Breast Cancer Cells through up Regulation of BAX, P53 and down Regulation of MDM2. Exp. Toxicol. Pathol. 2016, 68, 89–97. [Google Scholar] [CrossRef]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas Aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; El-Ewasy, S.M. Bioproduction, Characterization, Anticancer and Antioxidant Activities of Extracellular Melanin Pigment Produced by Newly Isolated Microbial Cell Factories Streptomyces Glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]
- Lee, M.E.; Aswani, A.; Han, A.S.; Tomlin, C.J.; Dueber, J.E. Expression-Level Optimization of a Multi-Enzyme Pathway in the Absence of a High-Throughput Assay. Nucleic Acids Res. 2013, 41, 10668–10678. [Google Scholar] [CrossRef]
- Antonisamy, P.; Ignacimuthu, S. Immunomodulatory, Analgesic and Antipyretic Effects of Violacein Isolated from Chromobacterium Violaceum. Phytomedicine 2010, 17, 300–304. [Google Scholar] [CrossRef]
- Keith, K.E.; Killip, L.; He, P.; Moran, G.R.; Valvano, M.A. Burkholderia Cenocepacia C5424 Produces a Pigment with Antioxidant Properties Using a Homogentisate Intermediate. J. Bacteriol. 2007, 189, 9057–9065. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Wen, J.-Y.; Hsu, Y.-W.; Pan, T.-M. Monascus-Fermented Yellow Pigments Monascin and Ankaflavin Showed Antiobesity Effect via the Suppression of Differentiation and Lipogenesis in Obese Rats Fed a High-Fat Diet. J. Agric. Food Chem. 2013, 61, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Egeland, E.S. Carotenoids. In The Physiology of Microalgae; Developments in Applied Phycology; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 507–563. ISBN 978-3-319-24945-2. [Google Scholar]
- Liu, S.; Zhang, S.; Lv, X.; Lu, J.; Ren, C.; Zeng, Z.; Zheng, L.; Zhou, X.; Fu, H.; Zhou, D.; et al. Limonin Ameliorates Ulcerative Colitis by Regulating STAT3/MiR-214 Signaling Pathway. Int. Immunopharmacol. 2019, 75, 105768. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A Dietary Anticancer Molecule with Multiple Mechanisms of Action: Recent Trends and Advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Extraction and Bioconversion of Kaempferol Metabolites from Cauliflower Outer Leaves through Fungal Fermentation. Biochem. Eng. J. 2016, 116, 27–33. [Google Scholar] [CrossRef]
- Lee, M.; Nam, T.G.; Lee, I.; Shin, E.J.; Han, A.; Lee, P.; Lee, S.-Y.; Lim, T.-G. Skin Anti-Inflammatory Activity of Rose Petal Extract (Rosa Gallica) through Reduction of MAPK Signaling Pathway. Food Sci. Nutr. 2018, 6, 2560–2567. [Google Scholar] [CrossRef]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of Microbial Products in Cosmetic Industry. Nat. Prod. Bioprospect. 2019, 9, 267–278. [Google Scholar] [CrossRef]
- Srinivasan, M.; Keziah, S.M.; Hemalatha, M.; Devi, C.S. Pigment from Streptomyces Bellus MSA1 Isolated from Marine Sediments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022049. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.d.; Shariff, M.; Kamarudin, M.S. Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic Benefits of Astaxanthin on Humans Subjects. Acta Biochim. Pol. 2012, 59. [Google Scholar] [CrossRef]
- Choksi, J.; Vora, J.; Shrivastava, N. Bioactive Pigments from Isolated Bacteria and Its Antibacterial, Antioxidant and Sun Protective Application Useful for Cosmetic Products. Indian J. Microbiol. 2020, 60, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Chandi, G.K.; Gill, B.S. Production and Characterization of Microbial Carotenoids as an Alternative to Synthetic Colors: A Review. Int. J. Food Prop. 2011, 14, 503–513. [Google Scholar] [CrossRef]
- Alihosseini, F.; Ju, K.-S.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial Colorants: Characterization of Prodiginines and Their Applications on Textile Materials. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Durán, M.; Brocchi, M.; Cordi, L.; Tasic, L.; Castro, G.R.; Nakazato, G. Advances in Chromobacterium Violaceum and Properties of Violacein-Its Main Secondary Metabolite: A Review. Biotechnol. Adv. 2016, 34, 1030–1045. [Google Scholar] [CrossRef]
- Fabara, A.N.; Fraaije, M.W. An Overview of Microbial Indigo-Forming Enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 925–933. [Google Scholar] [CrossRef]
- Perumal, K.; Stalin, V.; Chandrasekarenthiran, S.; Sumathi, E.; Saravanakumar, A. Extraction and Characterization of Pigment from Sclerotinia sp. and Its Use in Dyeing Cotton. Text. Res. J. 2009, 79, 1178–1187. [Google Scholar] [CrossRef]



| Pigments | Microorganism | Waste | References |
|---|---|---|---|
| ß-Carotene | R. gracilis ATCC 10788 | Potato wastewater | Kot et al., 2020 [11] |
| Torularhodin, β-carotene, torulene | R. mucilaginosa MTCC-1403 | Agro-industrial waste | Sharma et al., 2020 [12] |
| ß-Carotene | B. trispora MTCC 884 | Fruit and vegetable waste | Kaur et al., 2019 [13] |
| Total carotenoids | R. toruloides ATCC204091 | Vegetable waste | Sinha et al., 2021 [14] |
| Total carotenoids | R. glutinis Y1 | Vegetable waste | Sinha et al., 2021 [14] |
| Total carotenoids | R. rubra GED8 | Whey ultrafltrate | Simova et al., 2004 [15] |
| Total carotenoids | R. glutinis | Salted cheese whey | Kanzy et al., 2015 [16] |
| Lycopene | R. faecalis PA2 | Agro-industrial waste | Patthawaro et al., 2020 [17] |
| Total carotenoids | R. sphaeroides O.U.001 | Olive mill wastewater | Eroglu et al., 2010 [18] |
| Torularhodin, β-carotene, torulene | R. mucilaginosa | Olive mill wastewater | Ghilardi et al., 2020 [19] |
| Total carotenoids | R. toruloides | Wheat straw hydrolysates | Liu et al., 2019 [20] |
| Total carotenoids | L. starkeyi | Wheat straw hydrolysates | Liu et al., 2019 [20] |
| ß-Carotene | S. cerevisiae | Lignocellulosic biomass | Cheng et al., 2020 [21] |
| Total carotenoids | S. roseus | Spent coffee | Petrik et al., 2014 [22] |
| Total carotenoids | P. fermentans | Rice water | Otero et al., 2019 [23] |
| Astaxanthin | H. pluvialis | Fruit and vegetable waste | Yazgin et al., 2020 [24] |
| Astaxanthin | X. dendrorhous | Food waste | Gervasi et al., 2018 [25] |
| Prodigiosin | Enterobacter sp. PWN1 | Casein acid hydrolysate | Poddar et al., 2021 [26] |
| Melanin | A. carbonarius | Fruit and vegetable waste | Arikan et al., 2020 [27] |
| Violacein | C. violaceum UTM5 | Pineapple waste | Aruldass et al., 2018 [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. https://doi.org/10.3390/nu15081923
Di Salvo E, Lo Vecchio G, De Pasquale R, De Maria L, Tardugno R, Vadalà R, Cicero N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients. 2023; 15(8):1923. https://doi.org/10.3390/nu15081923
Chicago/Turabian StyleDi Salvo, Eleonora, Giovanna Lo Vecchio, Rita De Pasquale, Laura De Maria, Roberta Tardugno, Rossella Vadalà, and Nicola Cicero. 2023. "Natural Pigments Production and Their Application in Food, Health and Other Industries" Nutrients 15, no. 8: 1923. https://doi.org/10.3390/nu15081923
APA StyleDi Salvo, E., Lo Vecchio, G., De Pasquale, R., De Maria, L., Tardugno, R., Vadalà, R., & Cicero, N. (2023). Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients, 15(8), 1923. https://doi.org/10.3390/nu15081923











