Abstract
A strong controversy persists regarding the effect of red wine (RW) consumption and health. Guidelines for the prevention of cardiovascular diseases (CVD) and cancers discourage alcohol consumption in any form, but several studies have demonstrated that low RW intake may have positive effects on CVD risk. This review evaluated randomised controlled trials (RCTs), examining the recent literature on the correlations between acute and chronic RW consumption and health. All RCTs published in English on PubMed from 1 January 2000 to 28 February 2023 were evaluated. Ninety-one RCTs were included in this review, seven of which had a duration of more than six months. We assessed the effect of RW on: (1) antioxidant status, (2) cardiovascular function, (3) coagulation pathway and platelet function, (4) endothelial function and arterial stiffness, (5) hypertension, (6) immune function and inflammation status, (7) lipid profile and homocysteine levels, (8) body composition, type 2 diabetes and glucose metabolism, and (9) gut microbiota and the gastrointestinal tract. RW consumption mostly results in improvements in antioxidant status, thrombosis and inflammation markers, lipid profile, and gut microbiota, with conflicting results on hypertension and cardiac function. Notably, beneficial effects were observed on oxidative stress, inflammation, and nephropathy markers, with a modest decrease in CVD risk in five out of seven studies that evaluated the effect of RW consumption. These studies were conducted mainly in patients with type 2 diabetes mellitus, and had a duration between six months and two years. Additional long-term RCTs are needed to confirm these benefits, and assess the potential risks associated with RW consumption.
1. Introduction
Wine is an alcoholic beverage produced by the fermentation of crushed grapes. The different types of grapes and wine-making processes affect the colour and strength of the final beverage. The alcohol content varies from approximately 9 to 15% ethanol (ET) per volume [1]. Red wine (RW) contains other nutrients such as monosaccharides (e.g., glucose and fructose), variable levels of micronutrients (e.g., potassium, calcium, iron, magnesium, copper) and some B vitamins. More than 100 polyphenol compounds, including flavonoids and non-flavonoids, have been identified in RW, and to a lesser extent in white wine (WW) [2].
There is a strong ambiguity surrounding RW consumption and health [3]. Guidelines for the prevention of cardiovascular and neoplastic diseases advise against alcohol consumption, but drinking low-to-moderate amounts of wine may have some beneficial effects on cardiovascular disease risk (CVD) in certain populations [4]. Prospective cohort studies have demonstrated that any form of alcohol increases the risk of cancer [5]. In fact, the European Code Against Cancer advises limiting or eliminating alcohol consumption [6], and the International Agency for Research on Cancer has classified the consumption of alcoholic beverages as carcinogenic to humans (Group 1) in a dose-response manner. High alcohol consumption has been correlated with an increased risk of cancers of mouth, pharynx and larynx, oesophagus (squamous cell carcinoma), liver, colorectum, breast (before and after menopause), and stomach, in addition to many other diseases, such as cirrhosis, infectious diseases, CVD, diabetes, neuropsychiatric conditions, and early dementia [3,4,5].
In contrast, possible health benefits from RW intake have also been revealed, as RW contains less alcohol than spirits, and has greater antioxidant effects due to its higher polyphenol content. In fact, according to O’Keefe and colleagues, lower rates of death, T2DM, CVD, congestive heart failure, and stroke are associated with light-to-moderate habitual RW intake [1]. Even stronger results have been obtained in the framework of a healthy Mediterranean diet (MD) [2]. According to Renaud and de Lorgeril, the “French paradox” is the observation of a low prevalence of ischaemic heart disease despite a high intake of saturated fat: this phenomenon is accredited to the consumption of RW and its cardioprotective effect [7,8]. Haseeb and colleagues [9] described the composition of RW and the effects of its polyphenols on chronic CVD, and according to the authors, the polyphenols in RW could synergistically confer benefits against chronic CVD, if consumption remains within the maximum doses suggested by guidelines (one 125 mL glass for women and two glasses for men).
In consideration of the persistent contradictions, the aim of this review was to systematically evaluate the existing literature regarding the correlations between acute and chronic RW consumption and health.
2. Materials and Methods
We performed a PubMed search for randomised controlled trials (RCT) using the following keywords: “red” AND “wine” AND “humans”. It was decided to limit the search to only one database because PubMed’s search engine is particularly efficient, and the filters and results on other databases were duplicated. We researched papers published from 1 January 2000 to 28 February 2023. After this initial research, 6429 studies were discovered. Of these, 636 papers were excluded as they were duplicate studies. We screened 5793 records and removed studies on the following topics: cytotoxicity, functional foods, supplements (as resveratrol), probiotics, sensory aspect, dysphagia, differences in glasses or labelling, pharmacokinetics of alcohol, nasal or asthma symptoms, osmotic-stress-sensitive wine, biomarkers of wine consumption, wine extract, interaction with drugs, allergens, combination with other foods, hypothalamic–pituitary–adrenal axis, adiponectin or leptin, monocyte migration, catechin, leukocytes functions, natural wine, intelligence test, gastric emptying, diuretic effects, tailor-made beverage intervention, ET only, and hormones (e.g., aromatase). Studies in which the dose of RW was not clear, or the diet was not clearly distinguished from RW were also excluded. We thus did not consider duplicate papers, reviews, conference proceedings, abstracts, short surveys, letters, books, or book chapters. We also did not include in vitro or animal model studies. The 209 studies obtained were combined into one database. The full texts of the selected manuscripts were carefully examined. Two reviewers (M.L. and A.F.) independently judged the appropriateness of inclusion. In cases of disagreement, a third reviewer (A.A.) was invited to participate in the review procedure. Finally, 118 papers were excluded because they focused on WW, derivatives of RW, or nutraceutical or supplement use. After applying all criteria, 91 studies [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] were included in this review. Figure 1 shows the steps for applying the inclusion and exclusion criteria to determine the final group of studies considered in this review. The mean characteristics (Table S1) and topics (Table S2) of all papers are shown in the Supplementary Materials.
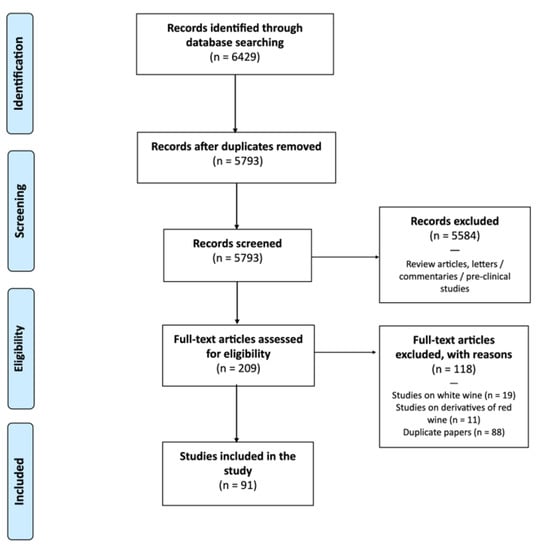
Figure 1.
Flow chart of studies included.
3. Results
3.1. Antioxidant Status
The antioxidant effects of RW consumption were evaluated in 19 studies (Table 1). Moderate amounts of RW, in the context of an MD, showed beneficial effects on the oxidative status of healthy subjects due to the increased expression of antioxidant enzymes involved in the reduction in circulating ROS, such as catalase (CAT), superoxide dismutase 2 (SOD2), and glutathione peroxidase 1 (GPX1) [93]. Antioxidant effects of RW consumption have been described for various conditions, including type 2 diabetes mellitus (T2DM) and acute CVD, as well as in elderly people [14,17,29,34,50]. This protective effect seems to be related to its high polyphenolic content. The plasma concentration of polyphenols was particularly higher in individuals consuming RW, compared with those consuming WW, resulting in the higher inhibition rate of oxidative stress. Of note, the described antioxidant effect may be dependent on a synergism between the different polyphenols contained in RW [32]. Accordingly, RW may have more powerful antioxidant effects than gin [59] and WW [56], for example, which have a lower content of polyphenols. The antioxidant effect of RW is also mediated by other bioactive compounds. It has been shown that moderate doses of RW increase blood concentrations of homovanillic acid, a typical biomarker of dopamine turnover, in healthy volunteers. Homovanillic acid is also a phenolic metabolite, so the increased blood concentrations of this biomarker could result from the metabolism of RW phenolic compounds [85]. RW’s antioxidants also suppress the post-prandial activation of NF-κB, an oxidative-stress-related transcription factor involved in the regulation of inflammatory responses [17,70]. Similarly, oxidized guanine species and protein carbonyl levels were significantly decreased in the RW group in CAD patients [100]. Importantly, it is well established that the beneficial effects observed after RW consumption, in terms of the increased activity of antioxidant enzymes, are not due to the alcohol content in wine, but to the polyphenolic composition. De-alcoholised red wine (DRW) could therefore be an excellent source of antioxidants with protective properties in conditions linked to oxidative stress [68]. On the other hand, plasma antioxidant capacity, measured through the ferric reducing antioxidant power (FRAP) in healthy subjects, was similarly increased by DRW and RW deprived of polyphenols. The authors hypothesised that increased FRAP after polyphenol-stripped RW consumption could be primarily explained by the increase in plasma urate, which is also a strong antioxidant with free radical scavenging and metal chelating capabilities [51]. Conversely, Blackhurst et al. demonstrated that RW consumption increased plasma antioxidant catechin levels, but did not affect the postprandial peroxidation status of chylomicrons after a high-fat meal. Moreover, RW did not increase the oxygen radical absorbance capacity (ORAC) in fasting subjects after a meal [39]. Interestingly, one glass of RW displayed lower amounts of bioavailable flavonols than one glass of tea or 15 g of red onion (RO), although urinary excretion of quercetin after wine consumption did not differ from that measured after onion consumption and was higher than that after tea, suggesting that RW has similar antioxidant activity and could therefore prevent LDL oxidation [15]. In contrast, Chiu et al. demonstrated that RO has greater antioxidant protection on plasma LDL [88]. It is notable that the alcohol content of 375 mL RW/day increased oxidative stress in a 4-week RCT [90]. Similarly, Addolorato and colleagues demonstrated that ET could increase lipid peroxidation parameters and reduce antioxidant capacity, although these effects were attenuated when ET was consumed in beer or RW [45].

Table 1.
Overview of studies that evaluated the antioxidant effects of red wine consumption.
3.2. Cardiovascular Function
The effects of RW consumption on cardiovascular function were evaluated in seven studies (Table 2). Acute RW consumption, in comparison with gin, had a greater down-regulatory effect on genes related to atherosclerosis progression in men with high CVD risk, probably due to the higher phenolic content [96]. In patients with stable angina pectoris, the acute intake of low doses of RW did not significantly affect ischemic pre-conditioning (IPC) during exercise stress testing [57]. Acute RW and DRW consumption did not improve coronary epicardial diameters or flow rate, even though both beverages reduced vasoconstrictive peptide endothelin-1 levels [64]. Moderate consumption of RW over 1–2 weeks did not reduce CVD risks by altering either the coronary microcirculation or haemorrheology [62]. In a two-year randomised controlled trial, moderate wine intake did not affect total carotid plaque volume, although the subjects with the greatest baseline plaque severity displayed a small regression in plaque burden [94]. In young healthy individuals, low blood concentrations of ET following RW intake had an acute depressant effect on left ventricular (LV) performance, but an increase in some indices of right ventricular function [65], suggesting that low ET doses may impair LV function. A lifestyle modification intervention (including RW consumption) did not affect the blood flow velocity of the internal carotid or middle cerebral artery in patients with carotid atherosclerosis [76]. Most of the enrolled patients were receiving statin therapy, however, which could have hidden the beneficial effects on blood flow velocity and thus affected the study results. Overall, low consumption of RW did not significantly improve CVD parameters even in long-term studies, suggesting that other factors modulated by RW account for the CVD risk. None of the RCTs included in this review assessed the effects of RW on atrial fibrillation and other cardiac arrhythmias.

Table 2.
Overview of studies that evaluated the cardiovascular effects of red wine consumption.
3.3. Coagulation Pathway and Platelet Function
The effect of RW consumption on coagulation and platelet function was evaluated in eleven studies (Table 3). These studies were conducted for up to three weeks, although one study ran for three months [20]. The intake of RW during a meal significantly decreased thrombotic activation, in terms of the plasma levels of prothrombin fragments 1 + 2 and activated factor VII [14], and fibrinogen concentrations [41], indicating inhibitory effects on the coagulation system. A sustained viscosity-reducing effect of plasma was observed after three weeks of RW consumption. A reduced viscosity was retained after three weeks of abstention [41]. RW intake led to a significant reduction in the aggregation ability of platelets against platelet activating factor (PAF), regardless of the ET percentage content [89]. RW decreased [17] or did not affect [87] concentrations of plasminogen activator inhibitor-1 (PAI-1), a risk factor for thrombosis and atherosclerosis, more negatively than ET itself [87]. RW and beer could reduce von Willebrand factor (vWF) levels. The vWF factor promotes the adhesion of platelets to damaged blood vessel walls and then acts as a bridge between one platelet and another, promoting clot formation. RW consumption did not increase the PAI-1/tPA ratio, which is associated with increased CV risk, an effect observed after the intake of other alcoholic beverages [58]. Thus, RW, but not ET alone, reduced the specific activity of platelet-activating factor and RW had a weak but significant inhibition effect on platelet activation. This RW effect is greater than that of WW, probably due to the higher polyphenol content [21]. Three studies did not demonstrate that RW had a favourable effect on coagulation and platelet function. The consumption of RW or WW with dinner did not affect the platelet count, platelet function, or viscoelastic properties of the blood taken the next morning [23]. In a study comparing a MD with a high-fat diet, the moderate consumption of RW resulted in a significant increase in platelet aggregation and secretion, but did not significantly modify the bleeding time or plasma vWF concentrations [20]. Similarly, there were no differences in fibrinogen and D-dimer levels after two weeks of drinking RW [62]. In contrast, Banach et al. observed a significant increase in PAI-1 concentration in the RW-drinking group [72]. Overall, RW intake shows the ability to reduce platelet aggregation and the process of coagulation.

Table 3.
Effects of red wine consumption on the coagulation pathway.
3.4. Endothelial Function and Arterial Stiffness
The effect of RW consumption on endothelial function was evaluated in 15 studies (Table 4). The consumption of RW may have positive effects on endothelial function according to most of the studies considered. These effects, in term of increased flow-mediated dilatation (FMD), have been clearly demonstrated in patients with hypercholesterolemic [27] and CAD [25]. Polyphenols were shown to play an important role in inducing RW-mediated acute vasodilation and a reduction in von Willebrand factor levels in healthy subjects [40,58]. These data suggest that polyphenols play a role in mediating RW effects on endothelial function [40]. Notably, in healthy subjects, a daily consumption of 100 mL of RW for three weeks led to increased levels of circulating endothelial progenitor cells (EPC), probably mediated by the increased bioavailability of plasma nitric oxide, with potential cardiovascular protective effects. In vitro studies showed that resveratrol could recapitulate the effects of RW on EPC function [61]. When flow-mediated vasodilation was examined, a single dose of DRW increased endothelium-dependent vasodilation in response to hyperaemia, whereas ingestion of RW induced vasodilation without affecting the percentage increase in artery diameter [10]. ET consumption dilates the brachial artery and increases muscle sympathetic nerve activity, heart rate, and cardiac output with dose-dependent effects. These acute effects are not modified by RW polyphenols [54], suggesting that the content of polyphenols in RW, rather than ethanol, exerts cardiovascular protective actions [27]. Accordingly, RW’s antioxidants can counteract the acute effects of smoking on the endothelium [48].

Table 4.
Overview of studies that evaluated the effects of red wine consumption on endothelial function and arterial stiffness.
Other studies have shown that RW has no effect or an unfavourable effect on endothelial function. Intake of a moderate amount of RW did not increase endothelial function [29]. In postmenopausal dyslipidaemic women, the intake of DRW and RW for six weeks was not associated with significant changes in vascular function or arterial stiffness, in comparison with women consuming water [43]. Similarly, the regular daily consumption of RW for a four-week period did not alter endothelial function [36]. Of note, Banach and colleagues demonstrated that a high acute consumption of RW in a previously abstinent population can lead to a significant increase in plasma concentrations of a potent vasoconstrictor (endothelin-1) [72]. Similarly, an intake of 375 mL RW/day for four weeks increased vasoconstrictor eicosanoids [71,90]. Although rapid alcohol consumption causes vasodilation at the level of the distributing artery as well as at the arteriolar level, a reduction in FMD has been observed in subjects consuming RW or an alcoholic beverage with a low content of polyphenols, suggesting that the polyphenols contained in RW may not be able to preserve the endothelial function [49].
3.5. Hypertension
The effect of RW consumption on blood pressure (BP) was evaluated in 13 studies (Table 5). Six studies lasted up to four weeks [36,54,66,69,78,86,90]. Only one study evaluated the effect over a longer time span of six months [79]. The ingestion of 250 mL RW at lunch resulted in a reduction in postprandial BP in subjects with hypertension and central obesity [18]. Consumption of either RW or DRW resulted in decreased systolic blood pressure (SBP) in patients with coronary artery disease [31]. These data were confirmed in another study that evaluated the effects of RW in combination with smoking on haemodynamic parameters. Both RW and DRW were able to prevent the increase in peripheral SBP induced by smoking. Notably, both RW and DRW were able to decrease postprandial wave reflexes, with RW having a more pronounced effect, suggesting that the alcohol present in RW contributes to reducing the augmentation index [44].

Table 5.
Effects of red wine consumption on hypertension.
Interestingly, Chiva-Blanch et al. [66] demonstrated that DRW consumption for four weeks, compared to RW or gin, led to a reduction in SBP and diastolic blood pressure (DBP), with a parallel increase in plasma NO concentration, which may mediate the observed effects on BP.
Other studies have found no effect from moderate RW consumption on BP. In patients with T2DM who were alcohol abstainers, consuming RW for six months had no effect on their mean 24-h BP. A more pronounced BP-lowering effect was noted among fast ET metabolisers, however [79]. In subjects consuming different beverages (i.e., RW, ethanol, water) no intervention was able to affect blood pressure [54]. RW containing 24–31 g of alcohol per day, administered for four weeks, increased 24-h BP and heart rate (HR) but reduced waking BP in well-controlled T2DM subjects [86].
RW was described as having adverse effects on BP by Barden and colleagues, who showed that a dose of 375 mL RW/day for four weeks increased BP, plasma levels of CYP450 vasoconstrictor eicosanoids and oxidative stress [90]. It has been suggested that increased plasma levels in the vasoconstrictor 20-HETE promote BP elevation and potentially contribute to the BP elevation associated with a binge drinking pattern [71]. Similarly, regular consumption of 200–300 mL RW/day in healthy premenopausal women raised HR and 24-h SBP and DBP. In the DRW group, BP values were similarly elevated [82], and accordingly, Zilkens et al. also demonstrated that polyphenols do not play a significant role in mitigating the effects of alcohol in increasing BP in men [36]. Other authors have shown that postprandial RW ingestion has a moderate effect on increasing DBP, but not SBP [78].
3.6. Immune Function and Inflammatory Status
The effects of RW consumption on the immune system and inflammatory status were evaluated by 14 studies (Table 6). Most were conducted for a period of up to four weeks, and only one study was conducted for 12 weeks [92]. The serum levels of pro-inflammatory cytokines (IL-6 and TNF-a) and serum levels of acute-phase proteins (hs-CRP and fibrinogen) did not show a significant change after the acute consumption of various alcoholic beverages, including RW [58]. Watzl et al. also reported that postprandial consumption [19] and moderate daily consumption for two weeks [22] of RW or DRW in healthy men had no negative effects on human immune cell function. Similarly, RW did not affect the plasma levels of lipid mediators of inflammation resolution (SPMs) in patients with T2DM [92]. No significant changes were demonstrated in the plasma levels of hs-CRP, IL-6, IL-18, VCAM-1, CASP-1, MMP-9, TIMP-1, APO B, cystatin C, or ICAM-1 [95].

Table 6.
Overview of studies that evaluated the effects of red wine consumption on immune function and inflammatory status.
Avellone et al. [28] showed that LDL/HDL, fibrinogen, factor VII, plasma C-reactive protein, and antibodies to oxidised LDL were markedly reduced, while HDL-C, Apo A1, TGFbeta1, t-PA, PAI, and total plasma antioxidant capacity were significantly elevated, indicating the beneficial effects of RW on levels of CV risk biomarkers. Conversely, the study by Banach et al. [72] demonstrated a negative effect on the fibrinolytic system and endothelial function (increased tPA:Ag, PAI:Ag and E-1) of consuming different types of alcoholic beverages, including RW, on haemostatic factors. Several studies have shown that the intake of RW led to a decrease in inflammatory or immune biomarkers in the serum. Moderate alcohol consumption, such as RW, marginally reduced fibrinogen levels in healthy subjects [33]. RW was more effective than WW in promoting a reduction in serum inflammatory biomarkers, CAM expression on monocyte surface membranes, and monocyte adhesion to endothelial cells [51]. Another study detected favourable effects of RW only in participants with high cytokine levels, in particular for cytokines that promote initial inflammation such as TNF-α, IL-6, and IFN-γ [77]. Furthermore, lower secretion of ΤNFα was observed after 8 weeks of intake in the RW group versus the ET group [97]. A Cava rosé wine, with a medium-level polyphenol content, has been shown to reduce the inflammatory markers of atherosclerosis (adhesion molecules, cytokines, and the CD40/CD40L system) to a greater extent than other alcoholic beverages without polyphenols [55]. Consumption of 375 mL of RW/day for four weeks also increased SPMs, in particular, resulting in higher levels of 18 -HEPE, RvD1, and 17R-RvD1, capable of attenuating excessive inflammation [90]. In contrast to the other studies, Williams and colleagues demonstrated that, in men with CVD, the consumption of moderate amounts of RW acutely increased plasma IL-6 levels, probably in response to alcohol-induced oxidative stress in the liver [26].
3.7. Lipid Profile and Homocysteine Levels
The effects of RW consumption on the lipid profile were evaluated by 23 studies (Table 7). The moderate consumption of RW could have a modest beneficial effect on lipoproteins and cellular cholesterol efflux compared with an alcohol solution [12]. Accordingly, alcohol consumption could promote a reverse cholesterol transport pathway (RCTP), the process by which cholesterol is directed from the peripheral tissues to the liver via HDL for subsequent excretion in the bile, regardless of alcoholic beverage type (RW, beer, or spirits) [16]. Similarly, Chiva-Blanch et al. reported that RW may reduce plasma concentrations of lipoprotein (a), which is responsible for transporting cholesterol in the blood and is considered a CVD risk factor that does not respond to standard therapy for low density lipoprotein (LDL) reduction [67]. Two Sicilian RWs were shown to have positive effects on several cardiovascular risk factors, including HDL and Apo A1, in a total of 48 subjects of both sexes [28]. A study by Tsang and colleagues also revealed that oxidised LDL levels were reduced, while HDL cholesterol concentrations increased modestly after RW consumption in healthy volunteers [34]. Another study reported a 34% reduction in LDL in the RW group, compared with controls [47]. A randomised cross-over trial comparing the effects of a moderate intake of RW with alcoholic beverages without polyphenols on plasma antioxidant vitamins, lipid profile, and the oxidability of LDL particles, demonstrated that RW would provide additional lipid benefits due to its antioxidant effects by decreasing plasma levels of MDA, SOD, and oxidised LDL [59]. Notably, RW daily consumption associated with lifestyle changes, including moderate physical exercise for 20 weeks, improved the LDL/HDL ratio in patients with carotid atherosclerosis [74]. A study investigating the potential contribution of ET components in inducing beneficial effects on the lipid profile level demonstrated that RW consumption for four weeks induced an improvement in HDL and fibrinogen plasma levels compared with control groups drinking water, with or without red grape extract [30]. In a recent study by Briansó-Llort and colleagues, RW rich in resveratrol had positive effects on total cholesterol, in healthy volunteers [98].

Table 7.
Effects of red wine consumption on lipid profile and homocysteine levels.
Other studies did not demonstrate beneficial effects of RW consumption on lipid metabolism [27,100]. The ingestion of DRW, but not RW, decreased circulating F2-isoprostanes, a prostaglandin-like compound formed from the free-radical-mediated oxidation of arachidonic acid [13]. It was supposed that phenolic compounds would have a beneficial effect on lipid peroxidation if consumed separately from alcohol. Similarly, consumption of DRW containing 880 mg total polyphenols in dyslipidaemic postmenopausal women did not change triglyceride levels or postprandial chylomicrons. The same study demonstrated a 35% increase in postprandial TG levels following RW consumption, compared to the control group [24]. An intake of 300 mL RW did not reduce LDL oxidation in healthy subjects [35] and did not affect lipid peroxidation in postprandial chylomicrons [11,39]. RW consumption did not lead to any increase in HDL and the lipid profile was unchanged in RW vs. WW [91]. No change in serum HDL cholesterol was observed in diabetic patients with consumption of RW (24–31 g alcohol/day) compared to DRW or water [86]. Similarly, in subjects with T2DM, daily intake of 150 mL of muscadine grape wine (MW) or de-alcoholised MW had no significant effect on the lipid profile compared to the other groups [37]. LDL-cholesterol levels were reduced in subjects without steatosis at baseline, but there were no changes in HDL or triglycerides levels with moderate RW consumption for three months. In contrast, hepatic triglyceride content increased [63]. An intake of RO extract, which is very rich in polyphenols, showed greater cholesterol-lowering efficacy than RW [88].
RW intake was not observed to elevate plasma homocysteine levels in T2DM subjects [86]. Conversely, 24 g/day alcohol given as RW to healthy men for two weeks significantly increased homocysteine levels [56].
3.8. Body Composition, Type 2 Diabetes, and Glucose Metabolism
The effect of RW consumption on body weight, body composition, and adipocytokines levels was evaluated by six studies (Table 8). When part of an energy-restricted regimen, moderate RW consumption, with or without alcohol, improved glycaemic control in diabetic patients, with a parallel improvement in several metabolic parameters related to antioxidant status [37]. Accordingly, moderate wine consumption did not promote weight gain or abdominal adiposity in controlled diabetic patients following the Mediterranean diet [84], nor did fat accumulate in subcutaneous and abdominal fat depots in healthy subjects with a waist circumference above 94 cm [38]. A study that focused on two types of RW with different resveratrol content showed that the consumption of neither RW changed BMI [98]. Interestingly, healthy subjects consuming RW for 14 days displayed increased plasma levels of leptin, the main adipokine with the primary function of regulating energy balance, while no significant increase in plasma levels of adiponectin and other adipocytokines was observed [95]. Djurovic et al. revealed that RW could induce an increase in circulating leptin levels in females, but not in males [46].

Table 8.
Effects of red wine consumption on body weight, obesity, and adipocytokines.
Thirteen studies evaluated the effects of RW consumption on glucose metabolism (Table 9). A significant decrease in insulin and an increase in the fasting glucose-to-insulin ratio were demonstrated among T2DM subjects given DRW [37]. In another study, the RW group exhibited lower insulin levels and HOMA scores compared with the control group, while other metabolic parameters were similar [42]. Increased oxidative stress may play a role in the progression of diabetic nephropathy, a microvascular complication of diabetes. It has been demonstrated that RW consumption reduces urinary protein levels, as well as urinary 8-OHdG and urinary L-FABP, in patients with diabetic nephropathy, thus providing protective effects against the clinical progression of chronic kidney disease [60]. These results suggest that RW, but not WW, has protective effects in diabetic patients, probably due to its ability to reduce oxidative stress. In another study, fasting blood glucose remained unchanged, while basal insulin and HOMA-IR values decreased significantly in the groups in which RW and DRW (30% and 22%, respectively) were consumed compared to gin [67]. A moderate intake of RW in adults with obesity and metabolic syndrome (MetS) resulted in a reduction in MetS risk markers and improved gut microbiota composition [81]. Moderate RW consumption significantly reduced fasting glucose levels, especially in subjects with higher basal A1C levels, but not postprandial levels: the reduction in A1C levels was not statistically significant [53]. On the other hand, RW consumption during a meal induced an increase in plasma glucose and insulin similar to that observed after a meal without wine in diabetic patients. Nevertheless, wine ingestion with the meal counterbalanced the decrease in plasma levels of total radical antioxidant parameters (TRAP) induced by food in the postprandial phase, with beneficial effects on LDL oxidation and thrombotic activation in these patients [14].

Table 9.
Effects of red wine on glycaemic control in individuals with type 2 diabetes.
The insulin sensitivity index (ISI), assessed with a hyperinsulinaemic euglycemic clamp, was not affected by moderate RW consumption, in healthy subjects with visceral fat accumulation [38]. Interestingly, although both RW and WW tended to improve the glucose metabolism after two years in diabetic patients, only WW significantly reduced plasma glucose levels and HOMA-IR scores, compared to the control group [79]. Notably, moderate RW consumption did not alter the plasma mediators of inflammation in patients with T2DM [92]. No significant differences in fasting plasma glucose levels were revealed in a 14-day pilot study that compared two types of RW with different resveratrol contents [98]. Thus, no fasting glycaemic changes were detected in CAD [100] and hypercholesterolaemic [27] patients after ingesting RW. Taken together, these results suggest that RW consumption in association with a meal may improve glycaemic control in diabetic patients, without affecting the body weight of patients with T2DM.
3.9. Gut Microbiota and Gastrointestinal Tract
Seven studies evaluated the effects of RW consumption on the gut microbiota and gastrointestinal tract (Table 10). Moderate consumption of RW (16 g ethanol/day) over three months promoted an increase in hepatic triglyceride content, without developing steatosis, in healthy subjects. Interestingly, the authors also observed a significant reduction in LDL cholesterol levels [63]. A clinical study focused on the effects of different alcoholic beverages on the postprandial functionality of the digestive system in terms of gastric emptying kinetics and orocaecal transit, and demonstrated that RW had an inhibitory effect on the gastric emptying of solid food, as well as on meal-induced gallbladder emptying, compared with beer and whisky, in healthy volunteers. No effect was observed on the orocaecal transit time of food [75]. These results suggest that RW, obtained by fermentation only, affects the motor functions of the digestive tract and of the gallbladder differently from other alcoholic beverages, depending on the ET concentration.

Table 10.
Effects of red wine on the gut and its microbiota.
Three studies evaluated the effects of RW consumption for four weeks on the gut microbiota. First, RW polyphenols inhibit non-beneficial bacteria from the human microbiota and promote the growth of probiotic bacteria such as bifidobacteria in healthy male volunteers. RW particularly modulated the growth of select beneficial gut bacteria species, with a parallel improvement in blood pressure, lipid profile, and inflammatory status, linked to changes in the bifidobacteria number [69]. Another study demonstrated that RW consumption induced a significant increase in amounts of Bifidobacterium and Prevotella with a negative correlation with lipopolysaccharide (LPS) plasma concentration [73]. Similar results in terms of increased faecal microbial diversity, have been obtained both in healthy individuals [81,99] and in patients with metabolic alterations associated with obesity [83]. Plasma trimethylamine N-oxide (TMAO) did not differ between people receiving the RW intervention and ET abstention [99].
Taken together these studies suggest that the inclusion of RW polyphenols in the diet could provide prebiotic benefits for the gut microbiota through stimulating the growth of beneficial species, including Enterococcus, Prevotella, Bacteroides, and Bifidobacterium, with a positive effect on health.
4. Discussion
The Global Burden of Disease consortium estimated that 1.34 billion people consumed harmful amounts of alcohol in 2020 (1.03 billion males and 0.312 billion females) [101]. It is also relevant to note that in the first two years of the COVID-19 pandemic, eating and lifestyle habits changed globally [102]. A recent study by Cicero and colleagues showed a significant increase in daily RW consumption in Northern Italy during the lockdown period (February–April 2020). As RW consumption had been increasing even before the COVID-19 pandemic, it is worth emphasising that long-term ET consumption has been associated with acute and chronic negative effects on health [103]. The deleterious effects of heavy alcohol consumption have been established by numerous studies, and led to an increased risk of driving accidents [104], infections [105], violence [106], foetal alcohol disorders [107], and a high risk of acute CVD events [108]. Most common diseases with a high morbidity and mortality, such as CVD [109], T2DM [110,111], cancer [112], and digestive diseases [113], are related to the total amount of ET ingested, the pattern of consumption, and the type of alcoholic beverage consumed. For most diseases, dose–response curves grow from zero consumption upwards, with many exponential curves [114]. It is therefore evident that even the occasional consumption of beverages with a high ET content (e.g., spirits) and/or chronic abuse of any alcoholic substance is deleterious, and that there is no beneficial reason to start consuming any kind of alcoholic beverage [115,116].
It is crucial to establish the effects of chronic consumption of RW, which has a lower ET content than spirits and a higher flavonoid content than WW, beer, and spirits. Two main questions remain unanswered: (a) Does RW have the same deleterious health effects that have been widely demonstrated for other alcoholic beverages? (b) Do the benefits of resveratrol and flavonoids in RW outweigh the toxic effects of ET? A prospective cohort study by Jani and colleagues showed that consumers of spirits and beer, compared with those who consume RW, had a higher absolute and relative risk of mortality, of experiencing a major adverse CVD event, of liver cirrhosis, and of accidents/self-harm. The relative risk was even higher in subjects who drank alcohol without eating [117].
The analysis of the 91 papers included in this review (Tables S1–S3) show the positive effects of RW consumption on antioxidant status, thrombosis, immune function and inflammation, lipid profile, and gut microbiota, whereas neutral effects were observed on body weight and glucose metabolism (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9 and Table 10). Beneficial effects from RW, in term of oxidative stress, inflammation, and nephropathy markers, with a modest reduction in CVD risk, were observed in five of seven studies analysed, which were performed mainly with T2DM subjects who consumed RW for at least six months [42,60,79,80,94] (Table S3). These seven studies did not detect changes in blood pressure, weight gain, or blood glucose levels, however. There are no RCT that have evaluated the effect of RW on the risk of arrhythmia. Some cohort studies have suggested that RW, when consumed in moderation, may have a protective effect against atrial fibrillation [118], while others have found no significant effects or even potential harm [119,120,121]. A study published in the Journal of the American College of Cardiology [119] demonstrated a J-shaped relationship between total alcohol consumption and risk of AF in a population of middle-aged and older adults. Drinking RW or WW appears to be potentially safer than beer or spirits [119,120]. The dose of RW also seems to be relevant in influencing the risk of arrhythmia [121]. Consumption of two or more RW drinks per day was associated with a small but statistically significant increased AF risk [122].
A review published in 2017 revealed that light to moderate RW consumption is beneficial for the heart [7]. In contrast, a study published two years ago concluded that even low amounts of ET increase the risk of cancer and early death [123]. It is also possible that observational studies overemphasise the positive effects of alcohol on CVD outcomes. Although moderate RW intake is presumably linked to a low number of CVD events, there are many confounding elements, especially genetic and socioeconomic associations with RW consumption, that probably explain most of the association between wine and a reduction in CVD events [124]. It is also important to consider other lifestyle variables that might confound the data. For example, it was found that RW drinkers tended to buy healthier food than beer or spirit drinkers; this tendency of RW drinkers to eat according to nutritional guidelines or have healthier attitudes could explain some of the positive health effects in these subjects [115,125,126]. On the other hand, the specific contribution of RW was emphasised in a longitudinal study from the UK Biobank that showed a lower visceral fat mass (β = −0.023, p < 0.001) in subjects who drank more RW, associated with reduced inflammation and increased HDL. In fact, RW may help to protect against adipogenesis due to its anti-inflammatory/eulipidaemic effects [127].
Most of the studies included in our review suggest that the health benefit of RW is due to polyphenol intake. RW naturally has a high concentration and wide variety of polyphenolic compounds. The considerable presence of polyphenols has led some foods to be defined ‘superfoods’ in order to emphasise their remarkable health benefits. Dietary polyphenols could have benefits for the prevention of MetS, different forms of cancers, and neurodegenerative diseases [128]. Foods rich in flavonoids, such as berries and RW, have thus been associated with a lower risk of mortality [129]. Flavonoids, due to their antioxidant, anti-atherogenic (inhibition of LDL oxidation), and antithrombotic (reduction in platelet aggregation) effects, may have a significant role in preventing atherosclerosis and thrombosis [130]. Patients imbibing drinks with a higher amount of polyphenols, adjusted for potentially confounding factors, had a significantly lower risk of hypertension [131]. Interestingly, the anti-inflammatory effects of RW might be increased by polyphenols combined with ET. An analysis of the cardiovascular contribution of polyphenols and alcohol in RW showed that phenolic compounds could reduce the levels of serum factors, such as intercellular adhesion molecule-1 and E-selectin, which mediate the process of leukocyte adhesion. In contrast, both ET and RW polyphenols could contribute to reducing soluble inflammatory mediators such as CD 40 ligand, IL-16, and MCP-1 [132]. A possible role has been suggested for ET itself as a potential cardioprotective agent. Many epidemiological studies have confirmed a J-shaped curve for ET in a similar way as for the consumption of foods or drinks rich in polyphenols [133,134]. However, a systematic review by Yoon and colleagues, suggested that the presence of comorbidities or age may affect the protective actions of alcohol consumption on CVD [135]. Several papers in our review [30,45,54,65,71,82,90] evaluated the effects of ET in RW. ET increased oxidative stress [90] and reduced antioxidant capacity [45], with detrimental effects on cardiac function through impaired LV function [65], dilation of the brachial artery [54], and increased muscle sympathetic nerve activity [54]. ET increases heart rate [54,82] and cardiac output with dose-dependent effects [54], and may contribute to BP elevation [71,82,90] through a stimulation of CYP450 vasoconstrictor eicosanoids [90]. Conversely, one study showed that ET in RW could have a positive effect on CVD risk reduction through an increase in HDL levels [30]. The potential toxicity and risk of addiction induced by ET might mean that the consumption of polyphenols through foods is favoured over RW. High concentrations of polyphenols are found in spices and herbs, cocoa, berries, seeds such as flaxseed, nuts such as hazelnut, olives, and some vegetables such as artichoke [136]. Due to its quercetin 3-O-glucoside, epicatechin, and epigallocatechin gallate content, and other compounds, green tea has higher antioxidant activity than RW [137]. Cocoa shows higher levels of phenolic compounds (611 mg of gallic acid equivalents, GAE) than black tea, green tea, and RW [138].
On the other hand, the advantages of RW consumption include the greater complexity of the polyphenol composition, due to enzymatic and chemical reactions during fermentation, which distinguish RW from other sources of polyphenols, including grape juice [139]. The RW content of melatonin, with antioxidant, anticarcinogenic and cardioprotective properties, should be also considered [140,141]. Finally, RW has been shown to stimulate the growth of beneficial bacteria present in the gut microbiota, such as Bifidobacterium and Prevotella, with a subsequent reduction in plasma levels of LPS, whose increase is associated with a high risk of insulin resistance and CVD [69,73]. RW demonstrates the ability to affect the gut microbiota composition by increasing the amount of bacteria producing butyrate and reducing the levels of bacterial organisms producing LPS, with protective effects against risk factors for metabolic syndrome [81]. TMAO has been identified as a potential CVD risk factor linked to diet, the gut microbiota, and cardiovascular health. Studies have shown that plant-based diets, such as the MD, vegetarian, and vegan diets, can effectively improve TMAO levels, while animal-based diets appear to have the opposite effect [142,143]. The only recent RCT included in the review [99] that evaluated TMAO concluded that TMAO concentrations were unrelated to RW intake. The addition of RW to a plant-based diet would offer no further benefit in reducing blood and urine values of TMAO, as suggested in a previous paper [144].
Finally, we acknowledge that this review has some limitations. The research was restricted to one database, and only English-language RCTs were included; nevertheless, a large number of studies were identified.
5. Conclusions
Acute and short-term RW consumption seems to exert positive effects on antioxidant status, the lipid profile, thrombosis and inflammation markers, and the gut microbiota. Importantly, a longer duration of treatment with RW has been shown to protect renal and cardiac function parameters in T2DM patients, suggesting that a moderate intake of RW may serve as a dietary supplement in diabetic patients. On the other hand, blood pressure values, homocysteine levels, and gastrointestinal function seem to be impaired by short-term RW intake. The studies selected in this review confirm that the RW intake levels may influence the protection against cardiovascular and metabolic diseases. The deleterious effects observed for ET consumption limit the dose of RW that can be consumed, and additional studies might clarify whether different dosages of RW preserve metabolic and cardiovascular functions of at-risk subjects, such as diabetic patients. This review also suggests that the intake of DRW may be a promising strategy to prevent cardiovascular and metabolic dysfunction. The removal of ET may exclude the detrimental effects of RW consumption, due to the potential risks of addiction and chronic illness, and maintain the content in flavonoids, preserving their protective and healthy actions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15081921/s1, Table S1: Mean features of all papers; Table S2: Mean Topics of all papers; Table S3: Long-term studies.
Author Contributions
Conceptualization, M.L., A.F., M.C. and A.A.; writing—original draft preparation, M.L., A.F., E.C. and A.A.; writing—review and editing, M.L., A.F., E.C., M.C. and A.A.; visualization, A.F. and E.C.; supervision, M.L., M.C. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement:
Data are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AF, atrial fibrillation; BP, blood pressure; CAT, catalase; CVD, cardiovascular diseases; DBP, diastolic blood pressure; DRW, de-alcoholised red wine; EPC, endothelial progenitor cells; ET, ethanol; FMD, flow-mediated dilatation; FRAP, ferric reducing antioxidant power; GPX1, glutathione peroxidase 1; HDL, high-density lipoprotein; HR, heart rate; hs-CRP, high-sensitivity C-reactive protein; IPC, ischemic pre-conditioning; ISI, insulin sensitivity index; LDL low-density lipoprotein; LPS, lipopolysaccharides; LV, left ventricular; MD, Mediterranean diet; MetS, metabolic syndrome; MSNA, muscle sympathetic nerve activity; MW, muscadine grape wine; ORAC, oxygen radical absorbance capacity; PAF, platelet activating factor; PAI-1, plasminogen activator inhibitor-1; RCT, randomized controlled trial; RCTP, reverse cholesterol transport pathway; RO, red onion; RW, red wine; SBP, systolic blood pressure; SOD2, superoxide dismutase 2; SPMs, lipid mediators of inflammation resolution; TMAO, trimethylamine N-oxide; TRAP, total radical antioxidant parameters; T2DM, type 2 diabetes mellitus; vWF, von Willebrand factor; WW, white wine.
References
- O’keefe, J.H.; Bhatti, S.K.; Bajwa, A.; DiNicolantonio, J.J.; Lavie, C.J. Alcohol and Cardiovascular Health: The Dose Makes the Poison or the Remedy. Mayo Clin. Proc. 2014, 89, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narvaez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Favaloro, E.; Targher, G. Moderate Red Wine Consumption and Cardiovascular Disease Risk: Beyond the “French Paradox”. Semin. Thromb. Hemost. 2010, 36, 059–070. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of Red Wine Consumption on Cardiovascular Health. Curr. Med. Chem. 2019, 26, 3542–3566. [Google Scholar] [CrossRef] [PubMed]
- Minzer, S.; Estruch, R.; Casas, R. Wine Intake in the Framework of a Mediterranean Diet and Chronic Non-Communicable Diseases: A Short Literature Review of the Last 5 Years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, C.; Cecchini, M.; Anderson, A.S.; Berrino, F.; Boutron-Ruault, M.-C.; Espina, C.; Key, T.J.; Leitzmann, M.; Norat, T.; Powers, H.; et al. European Code against Cancer 4th Edition: Alcohol drinking and cancer. Cancer Epidemiol. 2016, 45, 181–188. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health: A Comprehensive Review. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef]
- Agewall, S.; Wright, S.; Doughty, R.N.; Whalley, G.A.; Duxbury, M.; Sharpe, N. Does a glass of red wine improve endothelial func-tion? Eur. Heart J. 2000, 21, 74–78. [Google Scholar] [CrossRef]
- Caccetta, R.A.; Croft, K.D.; Beilin, L.J.; Puddey, I.B. Ingestion of red wine significantly increases plasma phenolic acid concentrations but does not acutely affect ex vivo lipoprotein oxidizability. Am. J. Clin. Nutr. 2000, 71, 67–74. [Google Scholar] [CrossRef]
- Senault, C.; Betoulle, D.; Luc, G.; Hauw, P.; Rigaud, D.; Fumeron, F. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 63–69. [Google Scholar]
- Caccetta, R.; Burke, V.; Mori, T.A.; Beilin, L.J.; Puddey, I.B.; Croft, K.D. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free. Radic. Biol. Med. 2001, 30, 636–642. [Google Scholar] [CrossRef]
- Ceriello, A.; Bortolotti, N.; Motz, E.; Lizzio, S.; Catone, B.; Assaloni, R.; Tonutti, L.; Taboga, C. Red wine protects diabetic patients from meal-induced oxidative stress and thrombosis activation: A pleasant approach to the prevention of cardiovascular disease in diabetes. Eur. J. Clin. Investig. 2001, 31, 322–328. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.H.; Hollman, P.C.; van Amersfoort, I.; Olthof, M.R.; Katan, M.B. Red wine is a poor source of bioavailable flavonols in men. J. Nutr. 2001, 131, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Van der Gaag, M.S.; Sierksma, A.; Schaafsma, G.; van Tol, A.; Geelhoed-Mieras, T.; Bakker, M.; Hendriks, H.F. Moderate alcohol con-sumption and changes in postprandial lipoproteins of premenopausal and postmenopausal women: A diet-controlled, ran-domized intervention study. J. Women’s Health Gend.-Based Med. 2000, 9, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Mansvelt, E.P.; van Velden, D.P.; Fourie, E.; Rossouw, M.; van Rensburg, S.J.; Smuts, C.M. The in Vivo Antithrombotic Effect of Wine Consumption on Human Blood Platelets and Hemostatic Factors. Ann. N. Y. Acad. Sci. 2002, 957, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Foppa, M.; Fuchs, F.D.; Preissler, L.; Andrighetto, A.; Rosito, G.A.; Duncan, B.B. Red wine with the noon meal lowers post-meal blood pressure: A randomized trial in centrally obese, hypertensive patients. J. Stud. Alcohol 2002, 63, 247–251. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Briviba, K.; Rechkemmer, G. Acute intake of moderate amounts of red wine or alcohol has no effect on the immune system of healthy men. Eur. J. Nutr. 2002, 41, 264–270. [Google Scholar] [CrossRef]
- Mezzano, D.; Leighton, F.; Strobel, P.; Martínez, C.; Marshall, G.; Cuevas, A.; Castillo, O.; Panes, O.; Muñoz, B.; Rozowski, J.; et al. Mediterranean diet, but not red wine, is associated with beneficial changes in primary haemostasis. Eur. J. Clin. Nutr. 2003, 57, 439–446. [Google Scholar] [CrossRef]
- Pignatelli, P.; Lenti, L.; Pulcinelli, F.M.; Catasca, R.; Saccani, G.; Germanò, G.; Marcoccia, A.; Silvestri, M.A.; Ghiselli, A.; Violi, F. Red and white wine differently affect collagen-induced platelet aggregation. Pathophysiol. Haemost. Thromb. 2002, 32, 356–358. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Pretzer, G.; Roser, S.; Barth, S.W.; Rechkemmer, G. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur. J. Clin. Nutr. 2004, 58, 40–45. [Google Scholar] [CrossRef]
- Kikura, M.; Levy, J.H.; Safon, R.A.; Lee, M.K.; Szlam, F. The influence of red wine or white wine intake on platelet function and viscoelastic property of blood in volunteers. Platelets 2004, 15, 37–41. [Google Scholar] [CrossRef]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of acute red wine polyphenol consumption on postprandial lipaemia in postmenopausal women. Atherosclerosis 2004, 177, 401–408. [Google Scholar] [CrossRef]
- Whelan, A.P.; Sutherland, W.H.; McCormick, M.P.; Yeoman, D.J.; de Jong, S.A.; Williams, M.J. Effects of white and red wine on endo-thelial function in subjects with coronary artery disease. Intern. Med. J. 2004, 34, 224–228. [Google Scholar] [CrossRef]
- Williams, M.J.; Sutherland, W.H.; Whelan, A.P.; McCormick, M.P.; de Jong, S.A. Acute effect of drinking red and white wines on cir-culating levels of inflammation-sensitive molecules in men with coronary artery disease. Metabolism 2004, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.R.; Lage, S.H.; Brandizzi, L.; Yoshida, V.; da Luz, P.L. The action of red wine and purple grape juice on vascular reactivity is independent of plasma lipids in hypercholesterolemic patients. Braz. J. Med. Biol. Res. 2005, 38, 1339–1347. [Google Scholar] [CrossRef]
- Avellone, G.; Di Garbo, V.; Campisi, D.; De Simone, R.; Raneli, G.; Scaglione, R.; Licata, G. Effects of moderate Sicilian red wine con-sumption on inflammatory biomarkers of atherosclerosis. Eur. J. Clin. Nutr. 2006, 60, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Guarda, E.; Godoy, I.; Foncea, R.; Pérez, D.D.; Romero, C.; Venegas, R.; Leighton, F. Red wine reduces oxidative stress in patients with acute coronary syndrome. Int. J. Cardiol. 2005, 104, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Marckmann, P.; Dragsted, L.O.; Finné Nielsen, I.L.; Nielsen, S.E.; Grønbaek, M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005, 59, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.N.; Papamichael, C.M.; Karatzis, E.N.; Papaioannou, T.G.; Aznaouridis, K.A.; Katsichti, P.P.; Stamatelopoulos, K.S.; Zampelas, A.; Lekakis, J.P.; Mavrikakis, M.E. Red wine acutely induces favorable effects on wave reflections and central pressures in coro-nary artery disease patients. Am. J. Hypertens. 2005, 18 Pt 1, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Ghiselli, A.; Buchetti, B.; Carnevale, R.; Natella, F.; Germanò, G.; Fimognari, F.; Di Santo, S.; Lenti, L.; Violi, F. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atherosclerosis 2006, 188, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Retterstol, L.; Berge, K.E.; Braaten, Ø.; Eikvar, L.; Pedersen, T.R.; Sandvik, L. A daily glass of red wine: Does it affect markers of inflammation? Alcohol Alcohol. 2005, 40, 102–105. [Google Scholar] [CrossRef]
- Tsang, C.; Higgins, S.; Duthie, G.G.; Duthie, S.J.; Howie, M.; Mullen, W.; Lean, M.E.; Crozier, A. The influence of moderate red wine consumption on antioxidant status and indices of oxidative stress associated with CHD in healthy volunteers. Br. J. Nutr. 2005, 93, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.; Kostner, K.; Thallinger, C.; Bur, A.; Brunner, M.; Wolzt, M.; Joukhadar, C. Wine ingestion has no effect on lipid peroxida-tion products. Pharmacology 2005, 75, 152–156. [Google Scholar] [CrossRef]
- Zilkens, R.R.; Burke, V.; Hodgson, J.M.; Barden, A.; Beilin, L.J.; Puddey, I.B. Red Wine and Beer Elevate Blood Pressure in Normotensive Men. Hypertension 2005, 45, 874–879. [Google Scholar] [CrossRef]
- Banini, A.E.; Boyd, L.C.; Allen, J.C.; Allen, H.G.; Sauls, D.L. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006, 22, 1137–1145. [Google Scholar] [CrossRef]
- Beulens, J.W.; van Beers, R.M.; Stolk, R.P.; Schaafsma, G.; Hendriks, H.F. The effect of moderate alcohol consumption on fat distribu-tion and adipocytokines. Obesity 2006, 14, 60–66. [Google Scholar] [CrossRef]
- Blackhurst, D.M.; Marais, A.D. Concomitant consumption of red wine and polyunsaturated fatty acids in edible oil does not influence the peroxidation status of chylomicron lipids despite increasing plasma catechin concentration. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 550–558. [Google Scholar] [CrossRef]
- Boban, M.; Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Salamunic, I.; Obad, A.; Palada, I.; Dujic, Z. Red Wine Induced Modulation of Vascular Function: Separating the Role of Polyphenols, Ethanol, and Urates. J. Cardiovasc. Pharmacol. 2006, 47, 695–701. [Google Scholar] [CrossRef]
- Jensen, T.; Retterstøl, L.J.; Sandset, P.M.; Godal, H.C.; Skjønsberg, O.H. A daily glass of red wine induces a prolonged reduction in plasma viscosity: A randomized controlled trial. Blood Coagul. Fibrinolysis 2006, 17, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Cacciapuoti, F.; Siniscalchi, M.; Sasso, F.C.; Marchese, F.; Cinone, F.; Musacchio, E.; Marfella, M.A.; Ruggiero, L.; Chiorazzo, G.; et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet. Med. 2006, 23, 974–981, Erratum in Diabet. Med. 2017, 34, 1488. [Google Scholar] [CrossRef] [PubMed]
- Naissides, M.; Pal, S.; Mamo, J.C.; James, A.P.; Dhaliwal, S. The effect of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. Eur. J. Clin. Nutr. 2006, 60, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.; Karatzi, K.; Karatzis, E.; Papaioannou, T.G.; Katsichti, P.; Zampelas, A.; Lekakis, J. Combined acute effects of red wine consumption and cigarette smoking on haemodynamics of young smokers. J. Hypertens. 2006, 24, 1287–1292. [Google Scholar] [CrossRef]
- Addolorato, G.; Leggio, L.; Ojetti, V.; Capristo, E.; Gasbarrini, G.; Gasbarrini, A. Effects of short-term moderate alcohol administra-tion on oxidative stress and nutritional status in healthy males. Appetite 2008, 50, 50–56. [Google Scholar] [CrossRef]
- Djurovic, S.; Berge, K.E.; Birkenes, B.; Braaten, Ø.; Retterstøl, L. The effect of red wine on plasma leptin levels and vasoactive fac-tors from adipose tissue: A randomized crossover trial. Alcohol Alcohol. 2007, 42, 525–528. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2007, 22, 41–46. [Google Scholar] [CrossRef]
- Karatzi, K.; Papamichael, C.; Karatzis, E.; Papaioannou, T.G.; Voidonikola, P.T.; Lekakis, J.; Zampelas, A. Acute smoking induces en-dothelial dysfunction in healthy smokers. Is this reversible by red wine’s antioxidant constituents? J. Am. Coll. Nutr. 2007, 26, 10–15. [Google Scholar] [CrossRef]
- Hijmering, M.L.; De Lange, D.W.; Lorsheyd, A.; Kraaijenhagen, R.J.; Van De Wiel, A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: A small trial in healthy volunteers. Neth. J. Med. 2007, 65, 29–35. [Google Scholar]
- Micallef, M.; Lexis, L.; Lewandowski, P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr. J. 2007, 6, 27. [Google Scholar] [CrossRef]
- Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Katalinic, V.; Obad, A.; Palada, I.; Dujic, Z.; Boban, M. The increase in human plasma anti-oxidant capacity after red wine consumption is due to both plasma urate and wine polyphenols. Atherosclerosis 2008, 197, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sacanella, E.; Vázquez-Agell, M.; Mena, M.P.; Antúnez, E.; Fernández-Solá, J.; Nicolás, J.M.; Lamuela-Raventós, R.M.; Ros, E.; Estruch, R. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: A randomized trial. Am. J. Clin. Nutr. 2007, 86, 1463–1469. [Google Scholar] [CrossRef]
- Shai, I.; Wainstein, J.; Harman-Boehm, I.; Raz, I.; Fraser, D.; Rudich, A.; Stampfer, M.J. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: A multicenter, randomized, clinical intervention trial. Diabetes Care 2007, 30, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Spaak, J.; Merlocco, A.C.; Soleas, G.J.; Tomlinson, G.; Morris, B.L.; Picton, P.; Notarius, C.F.; Chan, C.T.; Floras, J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H605–H612. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Agell, M.; Sacanella, E.; Tobias, E.; Monagas, M.; Antúnez, E.; Zamora-Ros, R.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Fernández-Solá, J.; Nicolás, J.M.; et al. Inflammatory Markers of Atherosclerosis Are Decreased after Moderate Consumption of Cava (Sparkling Wine) in Men with Low Cardiovascular Risk. J. Nutr. 2007, 137, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.; Woodside, J.V.; Young, I.S.; Sharpe, P.C.; Mercer, C.; Patterson, C.C.; McKinley, M.C.; Kluijtmans, L.A.; Whitehead, A.S.; Evans, A. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers—A randomized, crossover intervention study. QJM 2008, 101, 881–887. [Google Scholar] [CrossRef]
- Marinaccio, L.; Lanza, G.A.; Niccoli, G.; Fabretti, A.; Lamendola, P.; Barone, L.; Di Monaco, A.; Di Clemente, F.; Crea, F. Effect of Low Doses of Alcohol on the Warm-Up Phenomenon in Patients With Stable Angina Pectoris. Am. J. Cardiol. 2008, 102, 146–149. [Google Scholar] [CrossRef]
- Tousoulis, D.; Ntarladimas, I.; Antoniades, C.; Vasiliadou, C.; Tentolouris, C.; Papageorgiou, N.; Latsios, G.; Stefanadis, C. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin. Nutr. 2008, 27, 594–600. [Google Scholar] [CrossRef]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antúnez, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef]
- Nakamura, T.; Fujiwara, N.; Sugaya, T.; Ueda, Y.; Koide, H. Effect of red wine on urinary protein, 8-hydroxydeoxyguanosine, and liver-type fatty acid–binding protein excretion in patients with diabetic nephropathy. Metabolism 2009, 58, 1185–1190. [Google Scholar] [CrossRef]
- Huang, P.-H.; Chen, Y.-H.; Tsai, H.-Y.; Chen, J.-S.; Wu, T.-C.; Lin, F.-Y.; Sata, M.; Chen, J.-W.; Lin, S.-J. Intake of Red Wine Increases the Number and Functional Capacity of Circulating Endothelial Progenitor Cells by Enhancing Nitric Oxide Bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Belcik, T.; Kalvaitis, S.; Jayaweera, A.R.; Choi, S.-W.; Wei, K. Effect of modest alcohol consumption over 1-2 weeks on the coronary microcirculation of normal subjects. Eur. J. Echocardiogr. 2010, 11, 683–689. [Google Scholar] [CrossRef]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, O.; Johansson, L.; Kullberg, J.; Ahlström, H.; Lindström, T.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: A prospective randomized study. Ann. Med. 2011, 43, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, T.O.; Saraste, A.; Lehtimäki, T.; Toikka, J.O.; Saraste, M.; Raitakari, O.T.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. Decreased endothelin-1 levels after acute consumption of red wine and de-alcoholized red wine. Atherosclerosis 2010, 211, 283–286. [Google Scholar] [CrossRef]
- Cameli, M.; Ballo, P.; Garzia, A.; Lisi, M.; Bocelli, A.; Mondillo, S. Acute Effects of Low Doses of Ethanol on Left and Right Ventricular Function in Young Healthy Subjects. Alcohol. Clin. Exp. Res. 2011, 35, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martínez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochem-ical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Schrieks, I.C.; van den Berg, R.; Sierksma, A.; Beulens, J.W.; Vaes, W.H.; Hendriks, H.F. Effect of Red Wine Consumption on Biomarkers of Oxidative Stress. Alcohol Alcohol. 2013, 48, 153–159. [Google Scholar] [CrossRef]
- Barden, A.E.; Croft, K.D.; Beilin, L.J.; Phillips, M.; Ledowski, T.; Puddey, I.B. Acute effects of red wine on cytochrome P450 eicosanoids and blood pressure in men. J. Hypertens. 2013, 31, 2195–2202. [Google Scholar] [CrossRef]
- Banach, J.; Żekanowska, E.; Bujak, R.; Gilewski, W.; Błażejewski, J.; Karasek, D.; Balak, W.; Pietrzak, J.; Sinkiewicz, W. Short-term alcohol consumption may have detrimental effect on fibrinolysis and endothelial function: Preliminary report of prospective randomised study. Kardiol. Polska 2013, 71, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Queipo-Ortuño, M.I.; Boto-Ordoñez, M.; Coin-Aragüez, L.; Roca-Rodriguez, M.M.; Delgado-Lista, J.; Cardona, F.; Andres-Lacueva, C.; Tinahones, F.J. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am. J. Clin. Nutr. 2013, 97, 1053–1061, Erratum in Am. J. Clin. Nutr. 2013, 98, 512. [Google Scholar] [CrossRef] [PubMed]
- Droste, D.W.; Iliescu, C.; Vaillant, M.; Gantenbein, M.; De Bremaeker, N.; Lieunard, C.; Velez, T.; Meyer, M.; Guth, T.; Kuemmerle, A.; et al. A daily glass of red wine associated with lifestyle changes independentlyimproves blood lipids in patients with carotid arteriosclerosis: Results from arandomized controlled trial. Nutr. J. 2013, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Kasicka-Jonderko, A.; Jonderko, K.; Gajek, E.; Piekielniak, A.; Zawislan, R. Sluggish gallbladder emptying and gastrointestinal transit after intake of commonalcoholic beverages. J. Physiol. Pharmacol. 2014, 65, 55–66. [Google Scholar]
- Droste, D.W.; Iliescu, C.; Vaillant, M.; Gantenbein, M.; De Bremaeker, N.; Lieunard, C.; Velez, T.; Meyer, M.; Guth, T.; Kuemmerle, A.; et al. Advice on Lifestyle Changes (Diet, Red Wine and Physical Activity) Does Not Affect Internal Carotid and Middle Cerebral Artery Blood Flow Velocity in Patients with Carotid Arteriosclerosis in a Randomized Controlled Trial. Cerebrovasc. Dis. 2014, 37, 368–375. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Espinosa-Martos, I.; Rodríguez, J.M.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Moderate Consumption of Red Wine Can Modulate Human Intestinal Inflammatory Response. J. Agric. Food Chem. 2014, 62, 10567–10575. [Google Scholar] [CrossRef]
- Fantin, F.; Bulpitt, C.J.; Zamboni, M.; Cheek, E.; Rajkumar, C. Arterial compliance may be reduced by ingestion of red wine. J. Hum. Hypertens. 2015, 30, 68–72. [Google Scholar] [CrossRef]
- Gepner, Y.; Henkin, Y.; Schwarzfuchs, D.; Golan, R.; Durst, R.; Shelef, I.; Harman-Boehm, I.; Spitzen, S.; Witkow, S.; Novack, L.; et al. Differential Effect of Initiating Moderate Red Wine Consumption on 24-h Blood Pressure by Alcohol Dehydrogenase Genotypes: Randomized Trial in Type 2 Diabetes. Am. J. Hypertens. 2015, 29, 476–483. [Google Scholar] [CrossRef]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults With Type 2 Diabetes: A 2-year randomized, controlled trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Beilin, L.J.; Puddey, I.B. Randomized Controlled Intervention of the Effects of Alcohol on Blood Pressure in Premenopausal Women. Hypertension 2015, 66, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.; Muñoz-González, I.; Jiménez, E.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Del Carmen Martínez-Cuesta, M.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2017, 61, 1600620. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Shelef, I.; Shemesh, E.; Henkin, Y.; Schwarzfuchs, D.; Gepner, Y.; Harman-Boehm, I.; Witkow, S.; Friger, M.; Chassidim, Y.; et al. Effects of initiating moderate wine intake on abdominal adipose tissue in adults with type 2 diabetes: A 2-year randomized controlled trial. Public Health Nutr. 2017, 20, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Medina, S.; Martínez-Hernández, P.; Arina, S.; Zafrilla, P.; Mulero, J.; Genieser, H.G.; Ferreres, F.; Gil-Izquierdo, Á. Mel-atonin and hydroxytyrosol-rich wines influence the generation of DNA oxidation catabolites linked to mutagenesis after the ingestion of three types of wine by healthy volunteers. Food Funct. 2016, 7, 4781–4796. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Zilkens, R.R.; Hodgson, J.M.; Beilin, L.J.; Puddey, I.B. The effects of alcohol on ambulatory blood pressure and other cardiovascular risk factors in type 2 diabetes: A randomized intervention. J. Hypertens. 2016, 34, 421–428. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine consumption reduced postprandial platelet sensitivity against platelet activating factor in healthy men. Eur. J. Nutr. 2016, 56, 1485–1492. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Shen, Y.-C.; Huang, T.-Y.; Venkatakrishnan, K.; Wang, C.-K. Cardioprotective Efficacy of Red Wine Extract of Onion in Healthy Hypercholesterolemic Subjects. Phytother. Res. 2016, 30, 380–385. [Google Scholar] [CrossRef]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef]
- Barden, A.E.; Chavez, V.; Phillips, M.; Mas, E.; Beilin, L.J.; Croft, K.D.; Mori, T.A.; Puddey, I.B. A Randomized Trial of Effects of Alcohol on Cytochrome P450 Eicosanoids, Mediators of Inflammation Resolution, and Blood Pressure in Men. Alcohol. Clin. Exp. Res. 2017, 41, 1666–1674. [Google Scholar] [CrossRef]
- Taborsky, M.; Ostadal, P.; Adam, T.; Moravec, O.; Gloger, V.; Schee, A.; Skala, T. Red or white wine consumption effect on atherosclerosis in healthy individuals (In Vino Veritas study). Bratisl. Med. J. 2017, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Shinde, S.; Phillips, M.; Beilin, L.; Mas, E.; Hodgson, J.M.; Puddey, I.; Mori, T.A. The effects of alcohol on plasma lipid mediators of inflammation resolution in patients with Type 2 diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 29–34. [Google Scholar] [CrossRef]
- Di Renzo, L.; Cioccoloni, G.; Salimei, P.S.; Ceravolo, I.; De Lorenzo, A.; Gratteri, S. Alcoholic Beverage and Meal Choices for the Prevention of Noncommunicable Diseases: A Randomized Nutrigenomic Trial. Oxid. Med. Cell. Longev. 2018, 2018, 5461436. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Shai, I.; Gepner, Y.; Harman-Boehm, I.; Schwarzfuchs, D.; Spence, J.D.; Parraga, G.; Buchanan, D.; Witkow, S.; Friger, M.; et al. Effect of wine on carotid atherosclerosis in type 2 diabetes: A 2-year randomized controlled trial. Eur. J. Clin. Nutr. 2018, 72, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.; Elshahat, S.; McAlinden, N.; Dean, K.; Young, I.S.; Sharpe, P.C.; Blankenburg, S.; Patterson, C.C.; McKinley, M.C.; Evans, A.; et al. Effect of Moderate Red Wine versus Vodka Consumption on Inflammatory Markers Related to Cardiovascular Disease Risk: A Randomized Crossover Study. J. Am. Coll. Nutr. 2020, 39, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Roth, I.; Casas, R.; Ribó-Coll, M.; Doménech, M.; Lamuela-Raventós, R.M.; Estruch, R. Acute consumption of Andalusian aged wine and gin decreases the expression of genes related to atherosclerosis in men with high cardiovascular risk: Randomized intervention trial. Clin. Nutr. 2019, 38, 1599–1606. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Argyrou, C.; Detopoulou, M.; Tsitsou, S.; Seremeti, S.; Yannakoulia, M.; Antonopoulou, S.; Kolovou, G.; Kalogeropoulos, P. The effect of moderate wine consumption on cytokine secretion by peripheral blood mononuclear cells: A randomized clinical study in coronary heart disease patients. Cytokine 2021, 146, 155629. [Google Scholar] [CrossRef]
- Briansó-Llort, L.; Simó-Servat, O.; Ramos-Perez, L.; Torres-Torronteras, J.; Hernandez, C.; Simó, R.; Selva, D.M. Effect of Resveratrol Content in Red Wine on Circulating Sex Hormone-Binding Globulin: Lessons from a Pilot Clinical Trial. Mol. Nutr. Food Res. 2022, 66, e2200125. [Google Scholar] [CrossRef]
- Haas, E.A.; Saad, M.J.A.; Santos, A.; Vitulo, N.; Lemos, W.J.F.; Martins, A.M.A.; Picossi, C.R.C.; Favarato, D.; Gaspar, R.S.; Magro, D.O.; et al. A red wine intervention does not modify plasma trimethylamine N-oxide but is associated with broad shifts in the plasma metabolome and gut microbiota composition. Am. J. Clin. Nutr. 2022, 116, 1515–1529. [Google Scholar] [CrossRef]
- Choleva, M.; Argyrou, C.; Detopoulou, M.; Donta, M.-E.; Gerogianni, A.; Moustou, E.; Papaemmanouil, A.; Skitsa, C.; Kolovou, G.; Kalogeropoulos, P.; et al. Effect of Moderate Wine Consumption on Oxidative Stress Markers in Coronary Heart Disease Patients. Nutrients 2022, 14, 1377. [Google Scholar] [CrossRef]
- GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systematic analysis for the Global Burden of Disease Study 2020. Lancet 2022, 400, 185–235. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Guseva, E.; Perrone, M.A.; Müller, A.; Rizzo, G.; Storz, M.A. Changes in Eating Habits and Physical Activity after COVID-19 Pandemic Lockdowns in Italy. Nutrients 2021, 13, 4522. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Mezzadri, M.; Grandi, E.; Borghi, C. The Brisighella Heart Study Group COVID-19-Related Quarantine Effect on Dietary Habits in a Northern Italian Rural Population: Data from the Brisighella Heart Study. Nutrients 2021, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.; Irving, H.; Kanteres, F.; Room, R.; Borges, G.; Cherpitel, C.; Greenfield, T.; Rehm, J. The more you drink, the harder you fall: A systematic review and meta-analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend. 2010, 110, 108–116. [Google Scholar] [CrossRef]
- Trevejo-Nunez, G.; Kolls, J.K.; de Wit, M. Alcohol Use As a Risk Factor in Infections and Healing: A Clinician’s Perspective. Alcohol Res. 2015, 37, 177–184. [Google Scholar]
- Shorey, R.C.; Stuart, G.L.; McNulty, J.K.; Moore, T.M. Acute alcohol use temporally increases the odds of male perpetrated dating violence: A 90-day diary analysis. Addict. Behav. 2014, 39, 365–368. [Google Scholar] [CrossRef]
- Crawford-Williams, F.; Steen, M.; Esterman, A.; Fielder, A.; Mikocka-Walus, A. “My midwife said that having a glass of red wine was actually better for the baby”: A focus group study of women and their partner’s knowledge and experiences relating to alcohol consumption in pregnancy. BMC Pregnancy Childbirth 2015, 15, 79. [Google Scholar] [CrossRef]
- Mostofsky, E.; Chahal, H.S.; Mukamal, K.J.; Rimm, E.B.; Mittleman, M.A. Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation 2016, 133, 979–987. [Google Scholar] [CrossRef]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef]
- Koppes, L.L.; Dekker, J.M.; Hendriks, H.F.; Bouter, L.M.; Heine, R.J. Moderate alcohol consumption lowers the risk of type 2 diabetes: A meta-analysis of prospective observational studies. Diabetes Care 2005, 28, 719–725. [Google Scholar] [CrossRef]
- Solomon, C.G.; Hu, F.B.; Stampfer, M.J.; Colditz, G.A.; Speizer, F.E.; Rimm, E.B.; Willett, W.C.; Manson, J.E. Moderate alcohol consumption and risk of coronary heart disease among women with type 2 diabetes mellitus. Circulation 2000, 102, 494–499. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington, DC, USA, 2018; Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 9 October 2022).
- Shield, K.D.; Parry, C.; Rehm, J. Chronic Diseases and Conditions Related to Alcohol Use. Alcohol Res. Curr. Rev. 2013, 35, 155–171. [Google Scholar]
- Anderson, P. The Impact of Alcoholic Beverages on Human Health. Nutrients 2021, 13, 4417. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol use disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef]
- Friedmann, P.D. Clinical practice. Alcohol use in adults. N. Engl. J. Med. 2013, 368, 365–373, Erratum in N. Engl. J. Med. 2013, 368, 1661; Erratum in N. Engl. J. Med. 2013, 368, 781. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.D.; McQueenie, R.; Nicholl, B.I.; Field, R.; Hanlon, P.; Gallacher, K.I.; Mair, F.S.; Lewsey, J. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: A prospective cohort study. BMC Med. 2021, 19, 8. [Google Scholar] [CrossRef]
- Csengeri, D.; Sprünker, N.-A.; Di Castelnuovo, A.; Niiranen, T.; Vishram-Nielsen, J.K.; Costanzo, S.; Söderberg, S.; Jensen, S.M.; Vartiainen, E.; Donati, M.B.; et al. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur. Heart J. 2021, 42, 1170–1177, Erratum in Eur. Heart J. 2021, 42, 2711. [Google Scholar] [CrossRef]
- Tu, S.J.; Gallagher, C.; Elliott, A.D.; Linz, D.; Pitman, B.M.; Hendriks, J.M.L.; Lau, D.H.; Sanders, P.; Wong, C.X. Risk Thresholds for Total and Beverage-Specific Alcohol Consumption and Incident Atrial Fibrillation. JACC: Clin. Electrophysiol. 2021, 7, 1561–1569. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Tolstrup, J.S.; Friberg, J.; Jensen, G.; Grønbaek, M. Alcohol Consumption and Risk of Atrial Fibrillation in Men and Women: The Copenhagen City Heart Study. Circulation 2005, 112, 1736–1742. [Google Scholar] [CrossRef]
- Surma, S.; Lip, G.Y. Alcohol and Atrial Fibrillation. Rev. Cardiovasc. Med. 2023, 24, 73. [Google Scholar] [CrossRef]
- Conen, D.; Tedrow, U.B.; Cook, N.R.; Moorthy, M.V.; Buring, J.E.; Albert, C.M. Alcohol Consumption and Risk of Incident Atrial Fibrillation in Women. JAMA 2008, 300, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Calvert, C.M.; Toomey, T.; Jones-Webb, R. Are people aware of the link between alcohol and different types of Cancer? BMC Public Health 2021, 21, 734. [Google Scholar] [CrossRef]
- Krittanawong, C.; Isath, A.; Rosenson, R.S.; Khawaja, M.; Wang, Z.; Fogg, S.E.; Virani, S.S.; Qi, L.; Cao, Y.; Long, M.T.; et al. Alcohol Consumption and Cardiovascular Health. Am. J. Med. 2022, 135, 1213–1230.e3. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.; Friis, K.; Skovenborg, E.; Grønbaek, M. Food buying habits of people who buy wine or beer: Cross sectional study. BMJ 2006, 332, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-J.; Tan, L.; Ren, L.; Shao, Y.; Tao, W.; Wang, Y. COVID-19 Risk Appears to Vary Across Different Alcoholic Beverages. Front. Nutr. 2022, 8, 1146. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Powell, L.; Hamer, M.; O’Donovan, G.; Duncan, M.J.; Stamatakis, E. Is There a Link between Different Types of Alcoholic Drinks and Obesity? An Analysis of 280,183 UK Biobank Participants. Int. J. Environ. Res. Public Health 2020, 17, 5178. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Ohishi, T.; Fukutomi, R.; Shoji, Y.; Goto, S.; Isemura, M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules 2021, 26, 453. [Google Scholar] [CrossRef]
- Zhang, X.; Molsberry, S.A.; Yeh, T.S.; Cassidy, A.; Schwarzschild, M.A.; Ascherio, A.; Gao, X. Intake of Flavonoids and Flavonoid-Rich Foods and Mortality Risk Among Individuals With Parkinson Disease: A Prospective Cohort Study. Neurology. Neurology 2022, 98, e1064–e1076. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillén, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D.; et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 326–334, Erratum in Am. J. Clin. Nutr. 2012, 95, 1506. [Google Scholar] [CrossRef]
- Chudzińska, M.; Wołowiec, Ł.; Banach, J.; Rogowicz, D.; Grześk, G. Alcohol and Cardiovascular Diseases—Do the Consumption Pattern and Dose Make the Difference? J. Cardiovasc. Dev. Dis. 2022, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.L. J-curve revisited: Cardiovascular benefits of moderate alcohol use cannot be dismissed. Med. J. Aust. 2013, 198, 419–422. [Google Scholar] [CrossRef]
- Yoon, S.J.; Jung, J.G.; Lee, S.; Kim, J.S.; Ahn, S.K.; Shin, E.S.; Jang, J.E.; Lim, S.H. The protective effect of alcohol consumption on the incidence of cardiovascular diseases: Is it real? A systematic review and meta-analysis of studies conducted in community settings. BMC Public Health 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Pintać, D.; Bekvalac, K.; Mimica-Dukić, N.; Rašeta, M.; Anđelić, N.; Lesjak, M.; Orčić, D. Comparison study between popular brands of coffee, tea and red wine regarding polyphenols content and antioxidant activity. Food Chem. Adv. 2022, 1, 100030. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, A.H.J.; Lee, C.Y. Cocoa Has More Phenolic Phytochemicals and a Higher Antioxidant Capacity than Teas and Red Wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno Ottoboni, A.M.M.; Fiorini, A.M.R.; Guiguer, É.L.; Nicolau, C.C.T.; Goulart, R.A.; Flato, U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Lamont, K.T.; Somers, S.; Lacerda, L.; Opie, L.H.; Lecour, S. Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J. Pineal Res. 2011, 50, 374–380. [Google Scholar] [CrossRef]
- Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G. The Influence of Animal- or Plant-Based Diets on Blood and Urine Trimethylamine-N-Oxide (TMAO) Levels in Humans. Curr. Nutr. Rep. 2022, 11, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G.; Tarsisano, M.G.; Di Renzo, L.; Federici, M.; Caprio, M.; De Lorenzo, A. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients 2021, 13, 1426. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, H.; Xiang, Q.; Shen, L.; Guo, X.; Zhai, C.; Hu, H. Targeting Trimethylamine N-Oxide: A New Therapeutic Strategy for Alleviating Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 864600. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).