Prevention of the Pro-Aggressive Effects of Ethanol-Intoxicated Mice by Schisandrin B
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Animal Treatment
2.4. Hematoxylin & Eosin (H&E) and Sirius Red Staining
2.5. Histopathological Examination and Scoring
2.6. Western Blotting
2.7. Collection of Serum, Cerebrospinal Fluid (CSF), and Liver Lysate
2.8. Measurement of Biochemical Parameters
2.9. Neurofunctional Tests
2.10. Statistical Analysis
3. Results
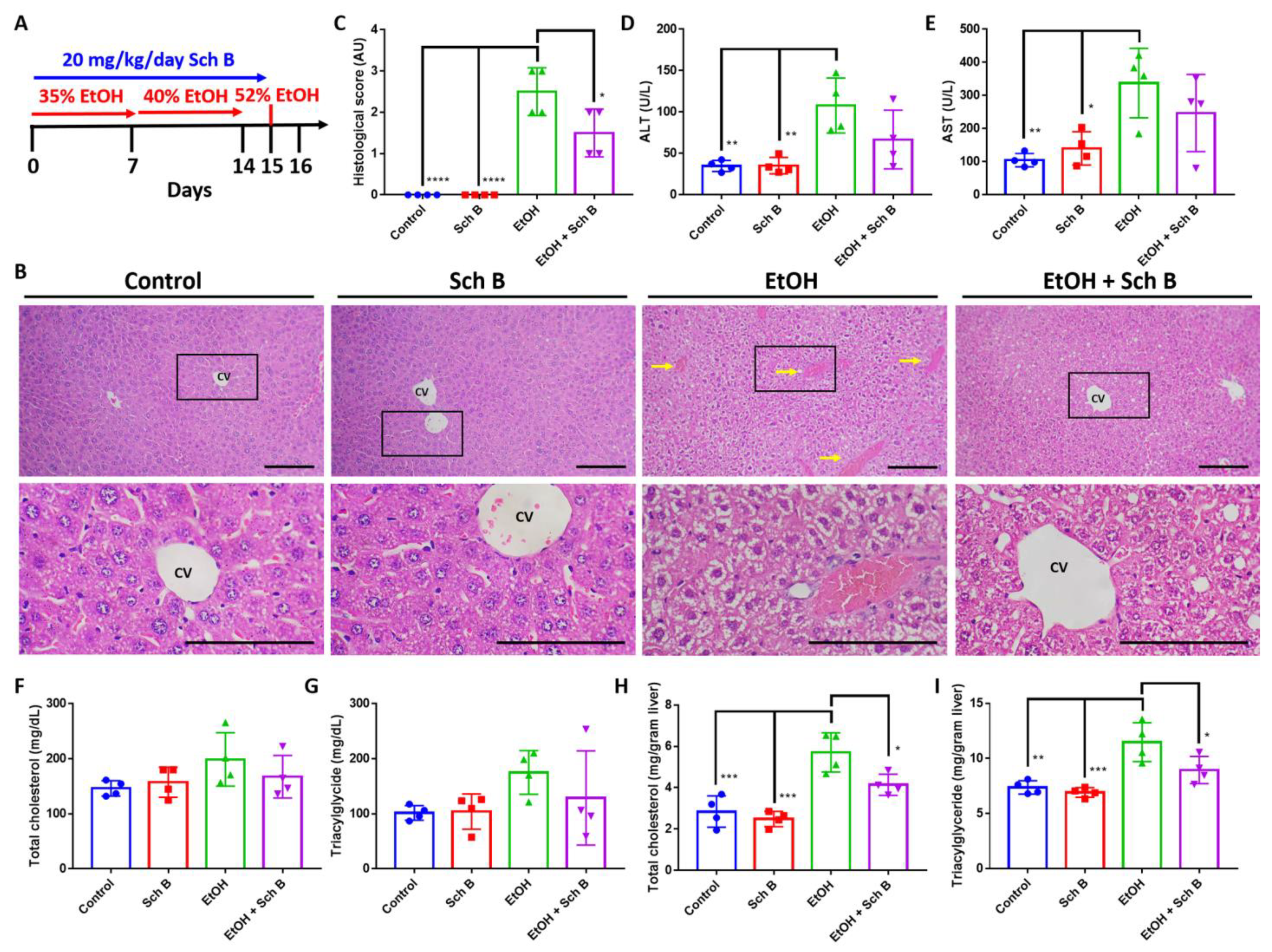
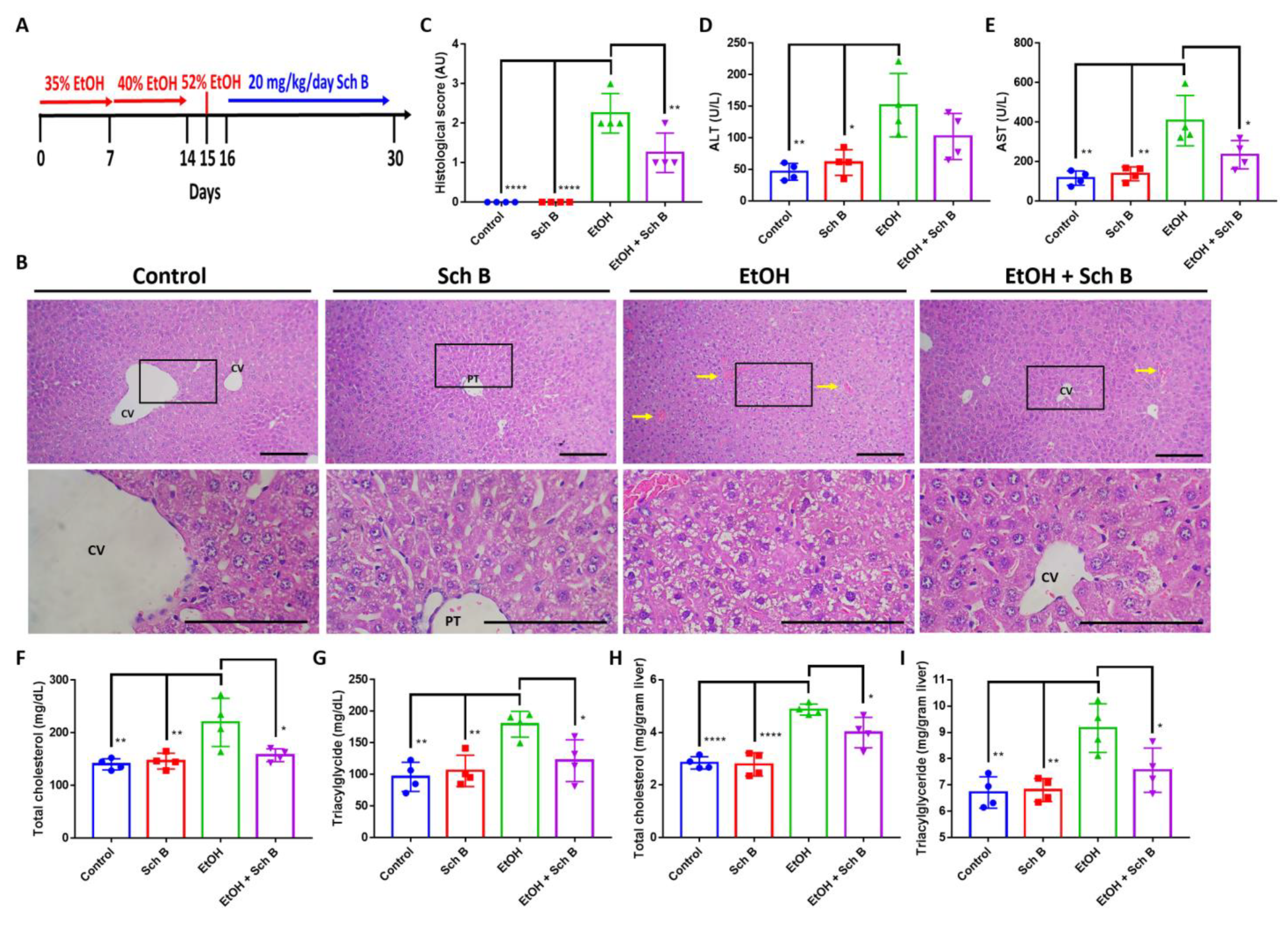
3.1. Schisandrin B Prevents Liver Injuries in Mice Consuming Ethanol
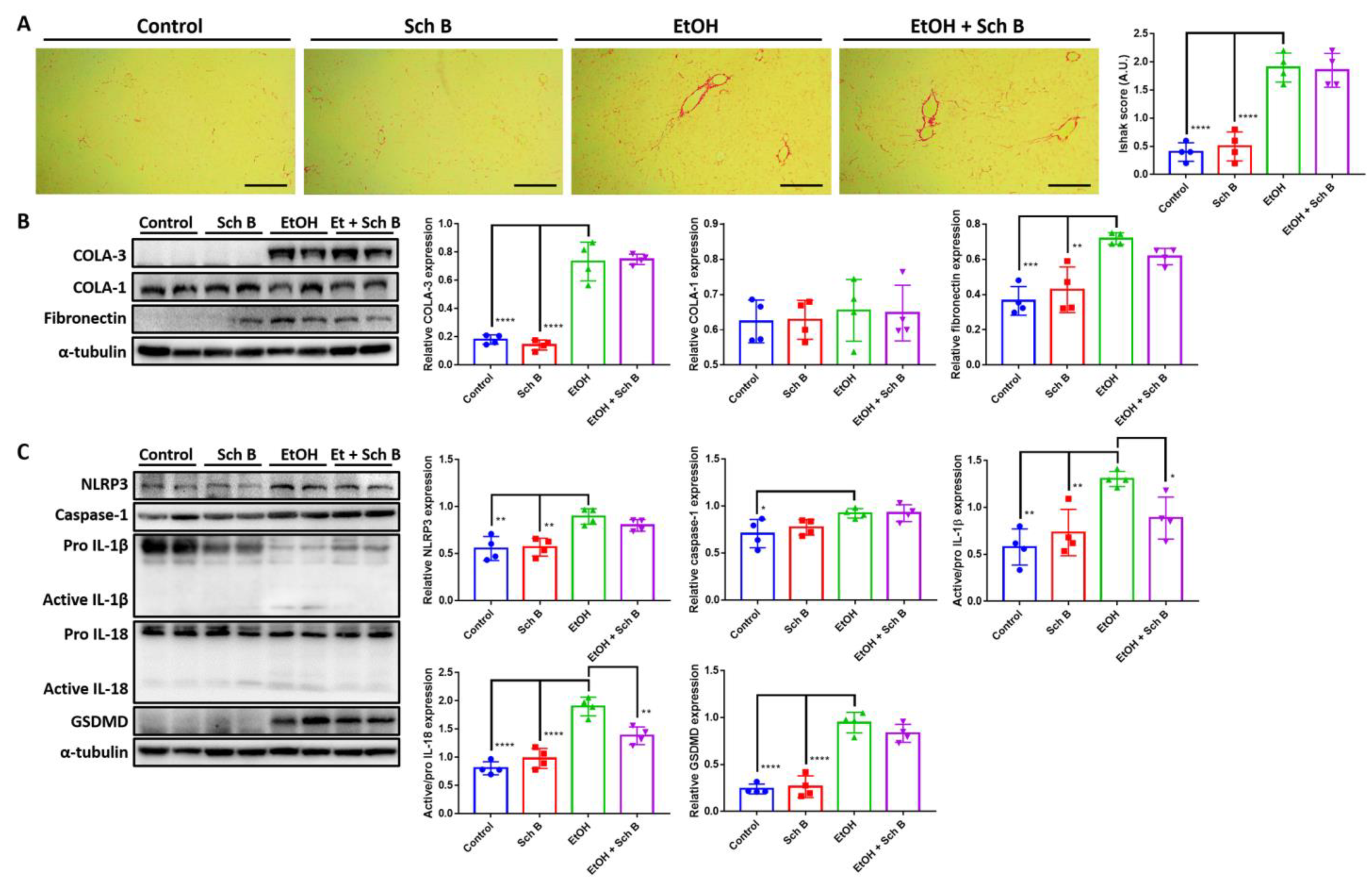
3.2. Schisandrin B Prevents Activation of Liver Inflammasomes in Mice Consuming Ethanol
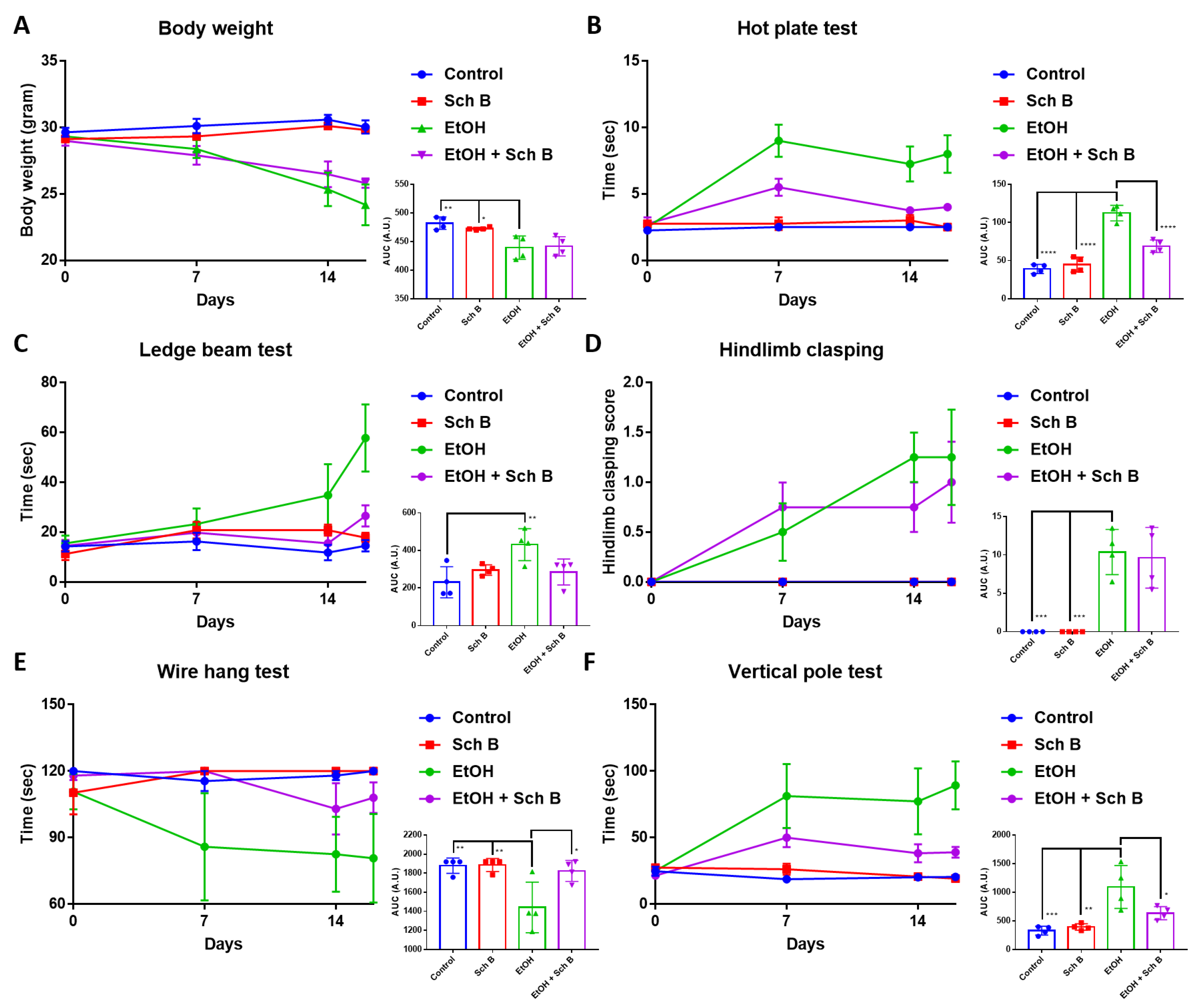
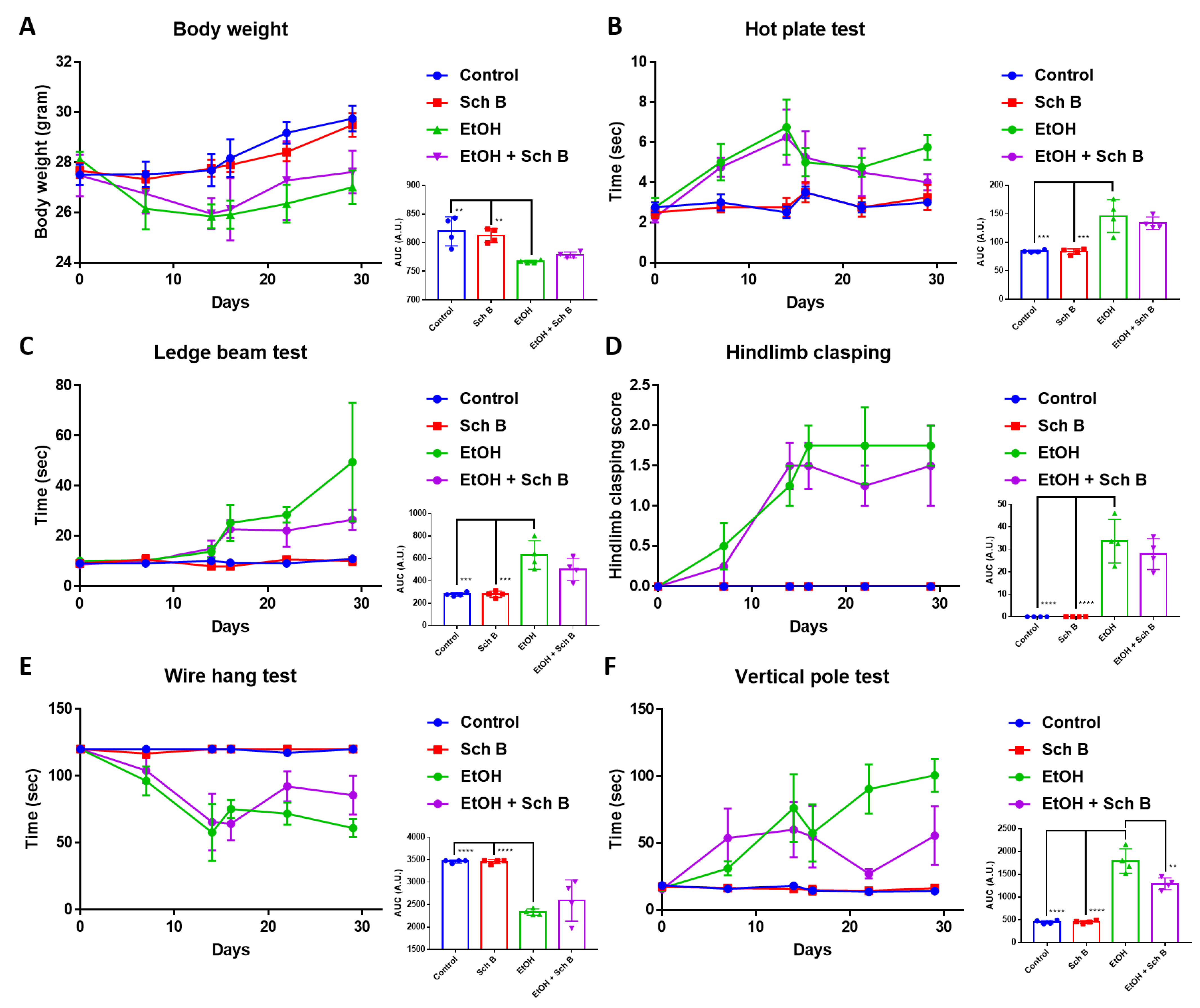
3.3. Schisandrin B Prevents Neurological Function Defects in Mice Consuming Ethanol
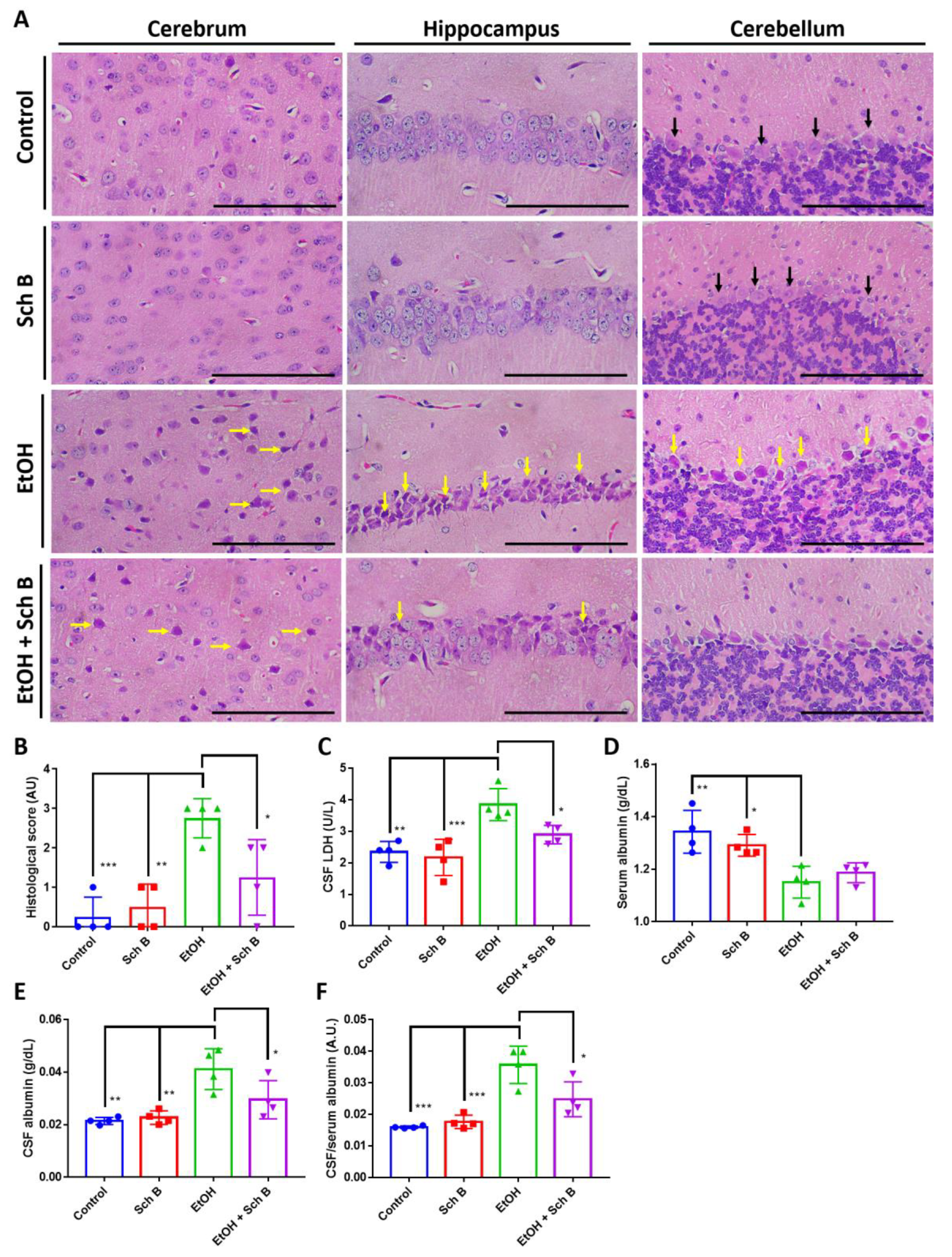
3.4. Schisandrin B Prevents Brain Injuries in Mice Consuming Ethanol
3.5. Schisandrin B Alleviates Ethanol-Induced Liver Injuries in Mice
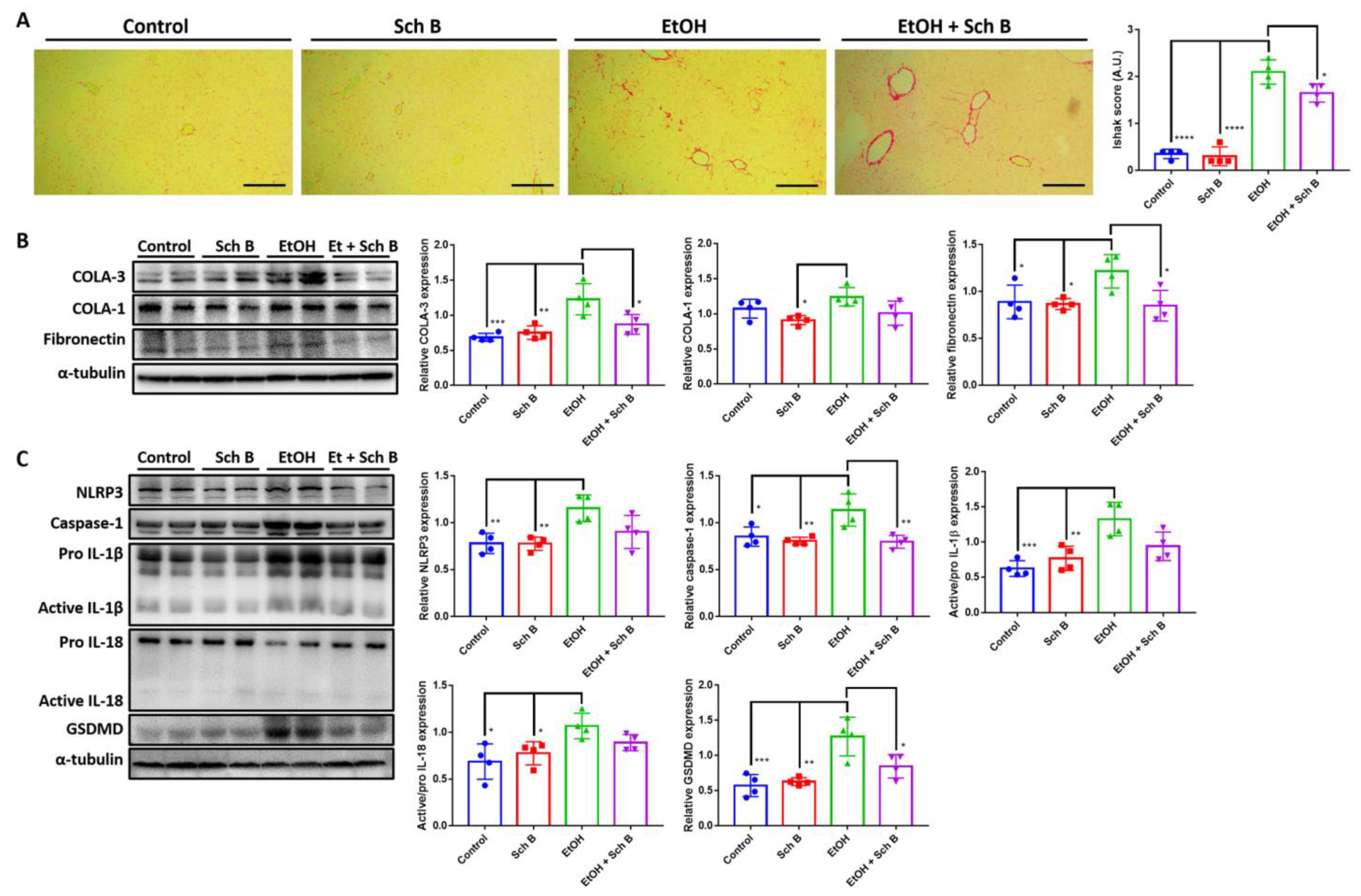
3.6. Schisandrin B Alleviates Ethanol-Induced Liver Fibrosis, Inflammasome Activation, and Pyroptosis
3.7. Schisandrin B Repairs Ethanol-Induced Neurofunctional Defects by Alleviation of Brain Injuries in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kärkkäinen, J.M.; Miilunpohja, S.; Rantanen, T.; Koskela, J.M.; Jyrkkä, J.; Hartikainen, J.; Paajanen, H. Alcohol Abuse Increases Rebleeding Risk and Mortality in Patients with Non-variceal Upper Gastrointestinal Bleeding. Dig. Dis. Sci. 2015, 60, 3707–3715. [Google Scholar] [CrossRef]
- MacMath, T.L. Alcohol and gastrointestinal bleeding. Emerg. Med. Clin. N. Am. 1990, 8, 859–872. [Google Scholar] [CrossRef]
- Clemens, D.L.; Schneider, K.J.; Arkfeld, C.K.; Grode, J.R.; Wells, M.A.; Singh, S. Alcoholic pancreatitis: New insights into the pathogenesis and treatment. World J. Gastrointest. Pathophysiol. 2016, 7, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Pervin, Z.; Stephen, J.M. Effect of alcohol on the central nervous system to develop neurological disorder: Pathophysiological and lifestyle modulation can be potential therapeutic options for alcohol-induced neurotoxication. AIMS Neurosci. 2021, 8, 390–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, D.J. Alcoholism and diabetes mellitus. Diabetes Metab. J. 2012, 36, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Tramacere, I.; Boffetta, P.; Negri, E.; La Vecchia, C. Alcohol consumption and cancer risk. Nutr. Cancer 2011, 63, 983–990. [Google Scholar] [CrossRef]
- Connor, J. Alcohol consumption as a cause of cancer. Addiction 2017, 112, 222–228. [Google Scholar] [CrossRef]
- Weerasinghe, A.; Schoueri-Mychasiw, N.; Vallance, K.; Stockwell, T.; Hammond, D.; McGavock, J.; Greenfield, T.K.; Paradis, C.; Hobin, E. Improving Knowledge that Alcohol Can Cause Cancer is Associated with Consumer Support for Alcohol Policies: Findings from a Real-World Alcohol Labelling Study. Int. J. Environ. Res. Public Health 2020, 17, 398. [Google Scholar] [CrossRef]
- Varma, V.; Webb, K.; Mirza, D.F. Liver transplantation for alcoholic liver disease. World J. Gastroenterol. 2010, 16, 4377–4393. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef]
- D’Mello, C.; Swain, M.G. Liver-brain interactions in inflammatory liver diseases: Implications for fatigue and mood disorders. Brain Behav. Immun. 2014, 35, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Hepatic encephalopathy. Gastroenterol. Rep. 2017, 5, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Ko, K.M. Schisandrin B: A Double-Edged Sword in Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell Longev. 2016, 2016, 6171658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, H.; Cao, Y.; Li, Y.; Sun, S.; Zhang, J.; Zhang, G. Schisandrin B attenuates CCl(4)-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug. Des. Dev. Ther. 2017, 11, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Cuiqiong, W.; Chao, X.; Xinling, F.; Yinyan, J. Schisandrin B suppresses liver fibrosis in rats by targeting miR-101-5p through the TGF-β signaling pathway. Artif. Cells Nanomed. Biotechnol. 2020, 48, 473–478. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Liang, T.-R.; Peng, S.-Y. Ameliorative effects of Schisandrin B on Schistosoma mansoni-induced hepatic fibrosis in vivo. PLOS Negl. Trop. Dis. 2021, 15, e0009554. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.; Mu, Y.; Zheng, Y. Schisandrin B inhibits cell proliferation and induces apoptosis in human cholangiocarcinoma cells. Oncol. Rep. 2016, 36, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zheng, J.X.; Zhuang, Y.S.; Zhou, Z.K.; Zhao, J.H.; Yang, L. Anti-Inflammatory Effects of Schisandrin B on LPS-Stimulated BV2 Microglia via Activating PPAR-γ. Inflammation 2017, 40, 1006–1011. [Google Scholar] [CrossRef]
- Xin, D.Q.; Hu, Z.M.; Huo, H.J.; Yang, X.J.; Han, D.; Xing, W.H.; Zhao, Y.; Qiu, Q.H. Schisandrin B attenuates the inflammatory response, oxidative stress and apoptosis induced by traumatic spinal cord injury via inhibition of p53 signaling in adult rats. Mol. Med. Rep. 2017, 16, 533–538. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, H.; Huang, L.; Xu, X.; Huang, Y.; Ma, L.; Li, L.; Yao, X.; Zhang, R.; Zhang, Y.; et al. Schisandrin B Protects against Acute Ethanol-Induced Cardiac Injury by Downregulating Autophagy via the NOX4/ROS Pathway. Pharmacology 2021, 106, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Chiu, P.Y.; Leung, H.Y.; Chen, N.; Leong, P.K.; Ko, K.M. Schisandrin B co-treatment ameliorates the impairment on mitochondrial antioxidant status in various tissues of long-term ethanol treated rats. Fitoterapia 2010, 81, 1239–1245. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, Y.-J.; Xu, D.-P.; Wang, F.; Zhou, Y.; Zheng, J.; Li, Y.; Zhang, J.-J.; Li, H.-B. Protective Effects of Lemon Juice on Alcohol-Induced Liver Injury in Mice. BioMed Res. Int. 2017, 2017, 7463571. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhou, T.; Wang, F.; Zhou, Y.; Li, Y.; Zhang, J.J.; Zheng, J.; Xu, D.P.; Li, H.B. The Effects of Syzygium samarangense, Passiflora edulis and Solanum muricatum on Alcohol-Induced Liver Injury. Int. J. Mol. Sci. 2016, 17, 1616. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Hung, M.Y.; Liang, T.R.; Peng, S.Y. An In-vivo Study into the Effects of Schisandrin B in the Liver, Spleen, Kidney, and Brain of Acute Thioacetamide-intoxicated Mice. Iran. J. Pharm. Res. 2021, 20, 300–314. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Chen, M.; Jin, J.; Qiu, Y.; Huang, M.; Huang, Z. Protective Effect of Schisandrin B Against Cyclosporine A-Induced Nephrotoxicity In Vitro and In Vivo. Am. J. Chin. Med. 2012, 40, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Jiang, X.; Francisco, C.; Christen, S.; Vexler, Z.S.; Täuber, M.G.; Ferriero, D.M. Manipulation of Antioxidant Pathways in Neonatal Murine Brain. Pediatr. Res. 2004, 56, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, A.; Tsipouras, M.G.; Tzallas, A.T.; Forlano, R.; Manousou, P.; Giannakeas, N. Quantification of Liver Fibrosis—A Comparative Study. Appl. Sci. 2020, 10, 447. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Chen, S.-J.; Zhou, S.-C.; Wu, S.-Z.; Wang, H. Inflammasomes and Fibrosis. Front. Immunol. 2021, 12, 643149. [Google Scholar] [CrossRef] [PubMed]
- He, W.-T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Tibbling, G.; Link, H.; Ohman, S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Invest. 1977, 37, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug. Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Poznanski, P.; Lesniak, A.; Korostynski, M.; Sacharczuk, M. Ethanol consumption following mild traumatic brain injury is related to blood-brain barrier permeability. Addict. Biol. 2020, 25, e12683. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Liang, T.R.; Jiang, S.J.; Peng, S.Y. Albendazole-Schisandrin B Co-Therapy on Angiostrongylus cantonensis-Induced Meningoencephalitis in Mice. Biomolecules 2020, 10, 1001. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Cheng, P.C.; Peng, S.Y. Resolution of systemic complications in Schistosoma mansoni-infected mice by concomitant treatment with praziquantel and Schisandrin B. Int. J. Parasitol. 2022, 52, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Weissenborn, K. Neurology and the liver. Neurol. Med. 1997, 63, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Jung, C.; Lee, D.-H. Neuroprotective effects of Schisandrin B against transient focal cerebral ischemia in Sprague-Dawley rats. Food Chem. Toxicol. 2012, 50, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Elkin, K.; Shi, Y.; Zhang, Z.; Cheng, Y.; Gu, J.; Liang, J.; Wang, C.; Ji, X. Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-κB signaling pathway inhibition. Neurol. Res. 2020, 42, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, Y.-H.; Shi, S.-S.; Tu, X.-K. Schisandrin B Inhibits NLRP3 Inflammasome Pathway and Attenuates Early Brain Injury in Rats of Subarachnoid Hemorrhage. Chin. J. Integr. Med. 2022, 28, 594–602. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, H.Y.P.; Liang, T.-R.; Peng, S.-Y. Prevention of the Pro-Aggressive Effects of Ethanol-Intoxicated Mice by Schisandrin B. Nutrients 2023, 15, 1909. https://doi.org/10.3390/nu15081909
Lam HYP, Liang T-R, Peng S-Y. Prevention of the Pro-Aggressive Effects of Ethanol-Intoxicated Mice by Schisandrin B. Nutrients. 2023; 15(8):1909. https://doi.org/10.3390/nu15081909
Chicago/Turabian StyleLam, Ho Yin Pekkle, Ting-Ruei Liang, and Shih-Yi Peng. 2023. "Prevention of the Pro-Aggressive Effects of Ethanol-Intoxicated Mice by Schisandrin B" Nutrients 15, no. 8: 1909. https://doi.org/10.3390/nu15081909
APA StyleLam, H. Y. P., Liang, T.-R., & Peng, S.-Y. (2023). Prevention of the Pro-Aggressive Effects of Ethanol-Intoxicated Mice by Schisandrin B. Nutrients, 15(8), 1909. https://doi.org/10.3390/nu15081909






