Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes
Abstract
1. Introduction
2. Materials and Methods
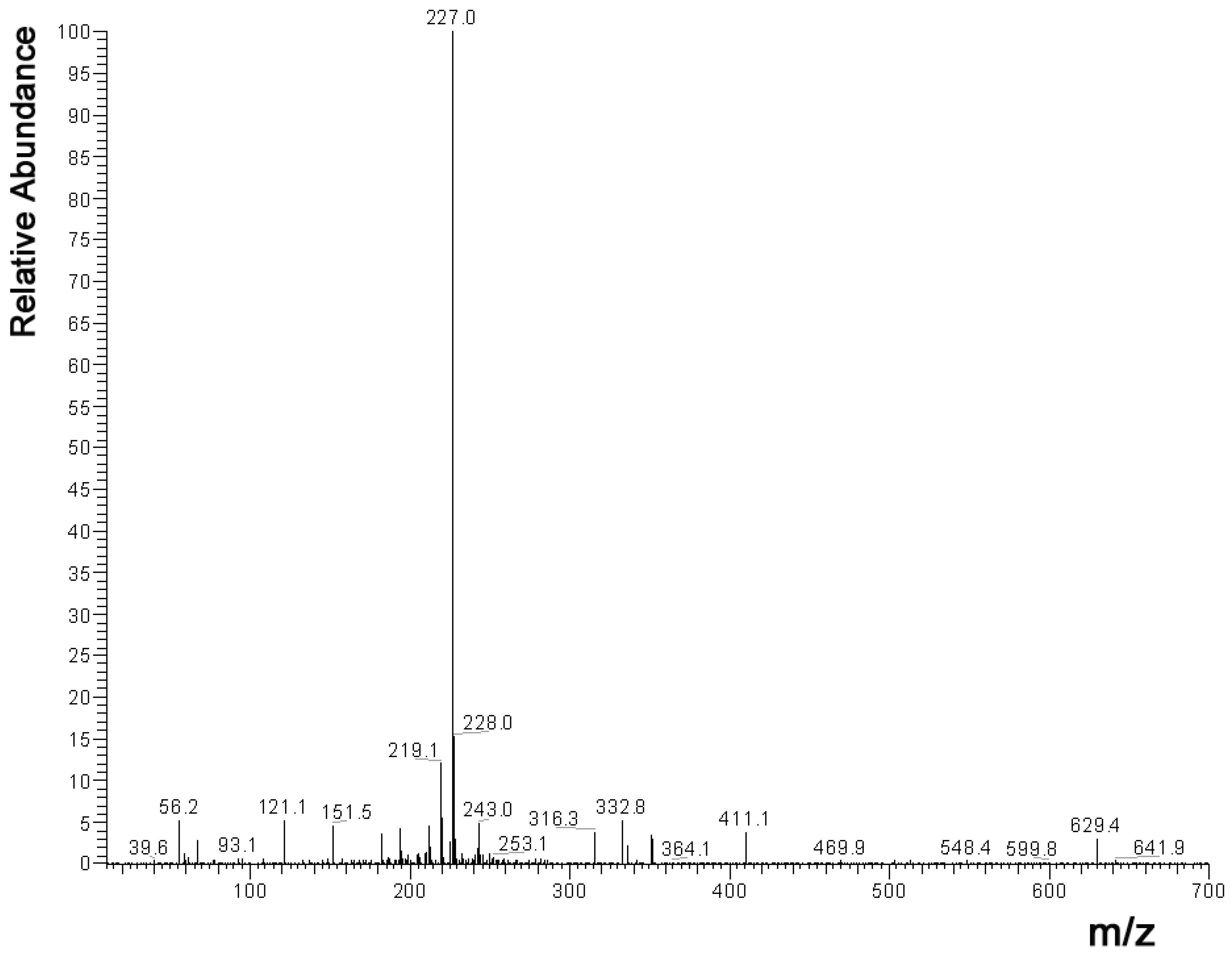
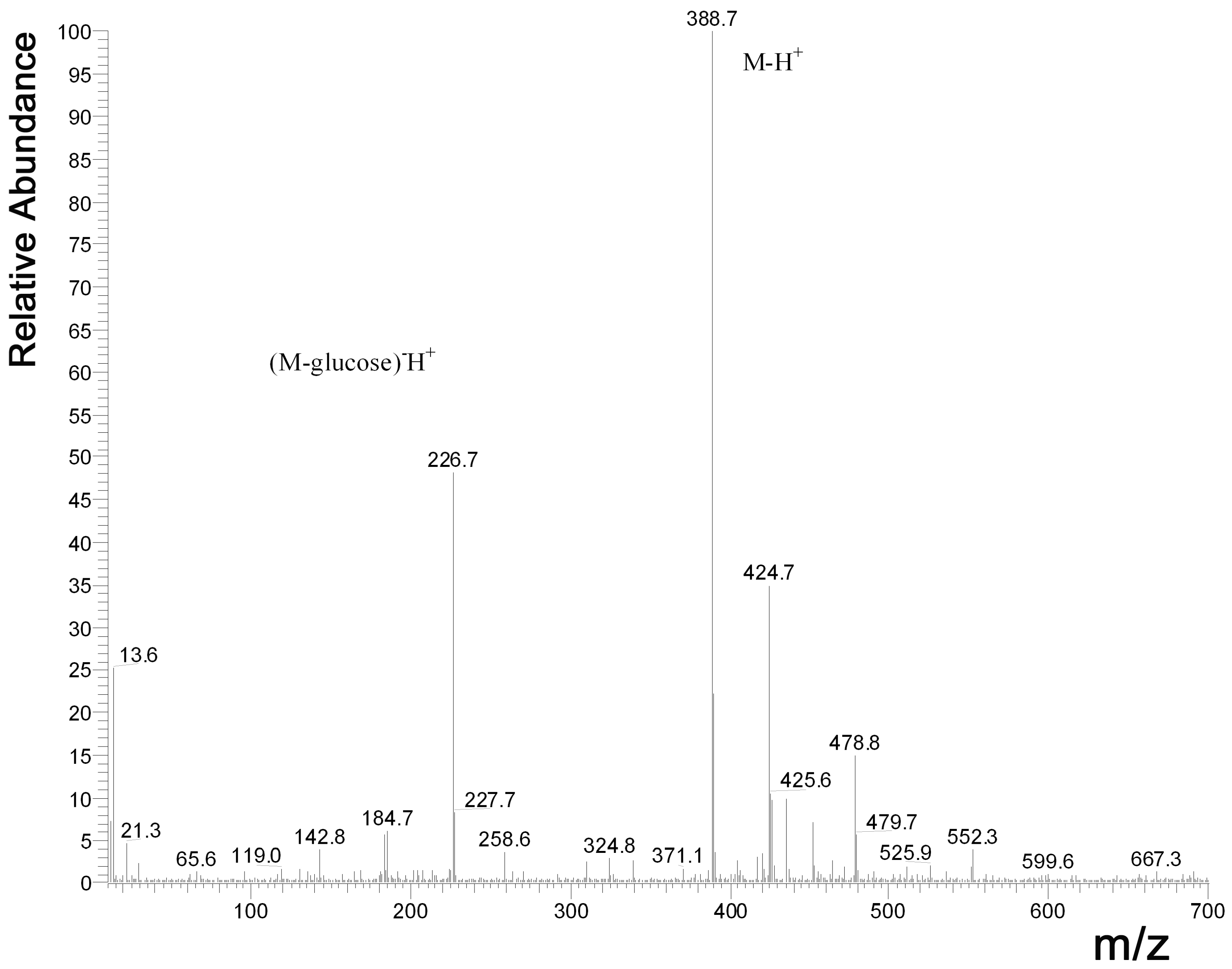
2.1. HPLC Measurements
2.2. Animals
2.3. Treatment of Animals
2.4. Total RNA Isolation
2.5. qRT-PCR
2.6. Statistical Analysis
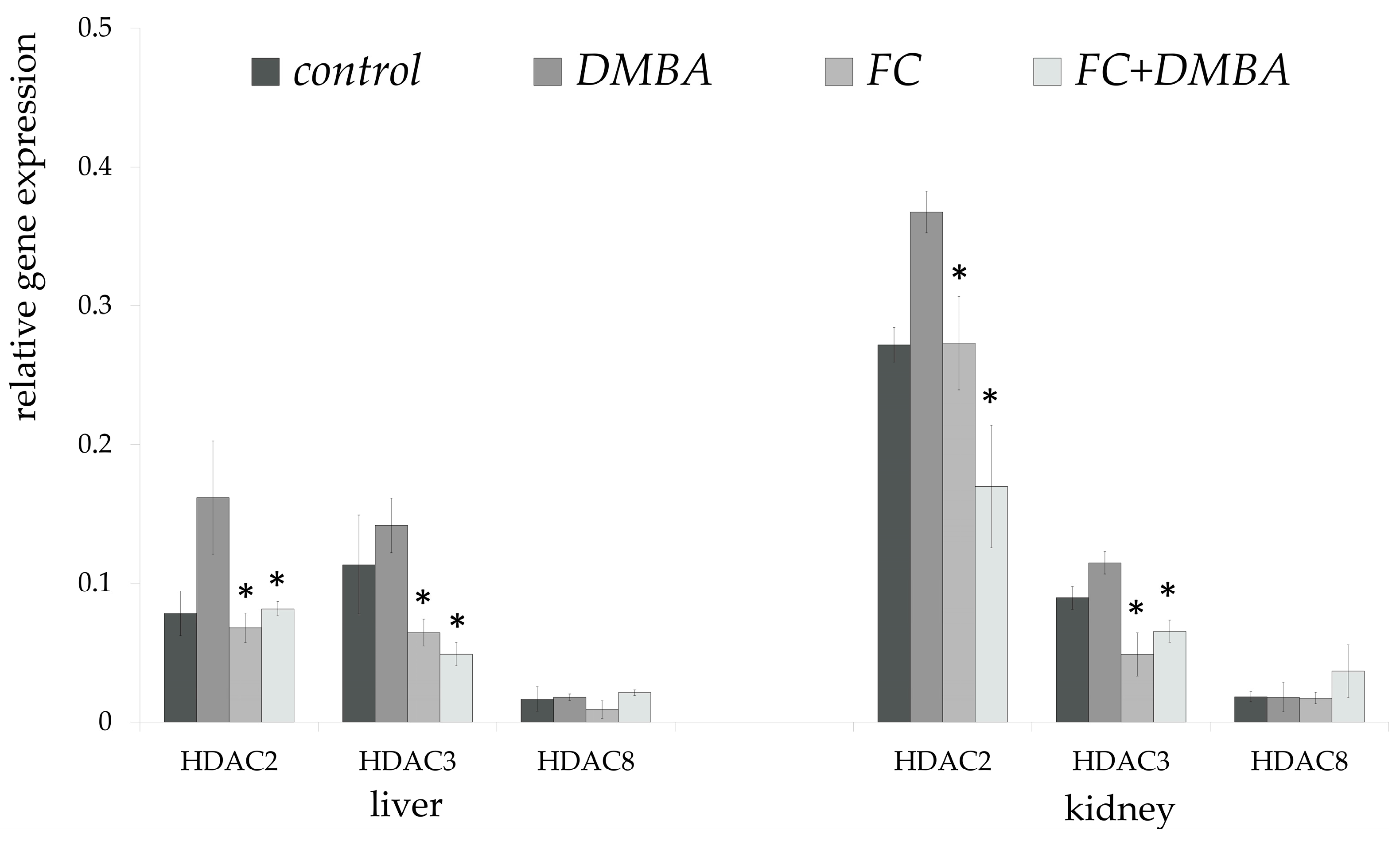
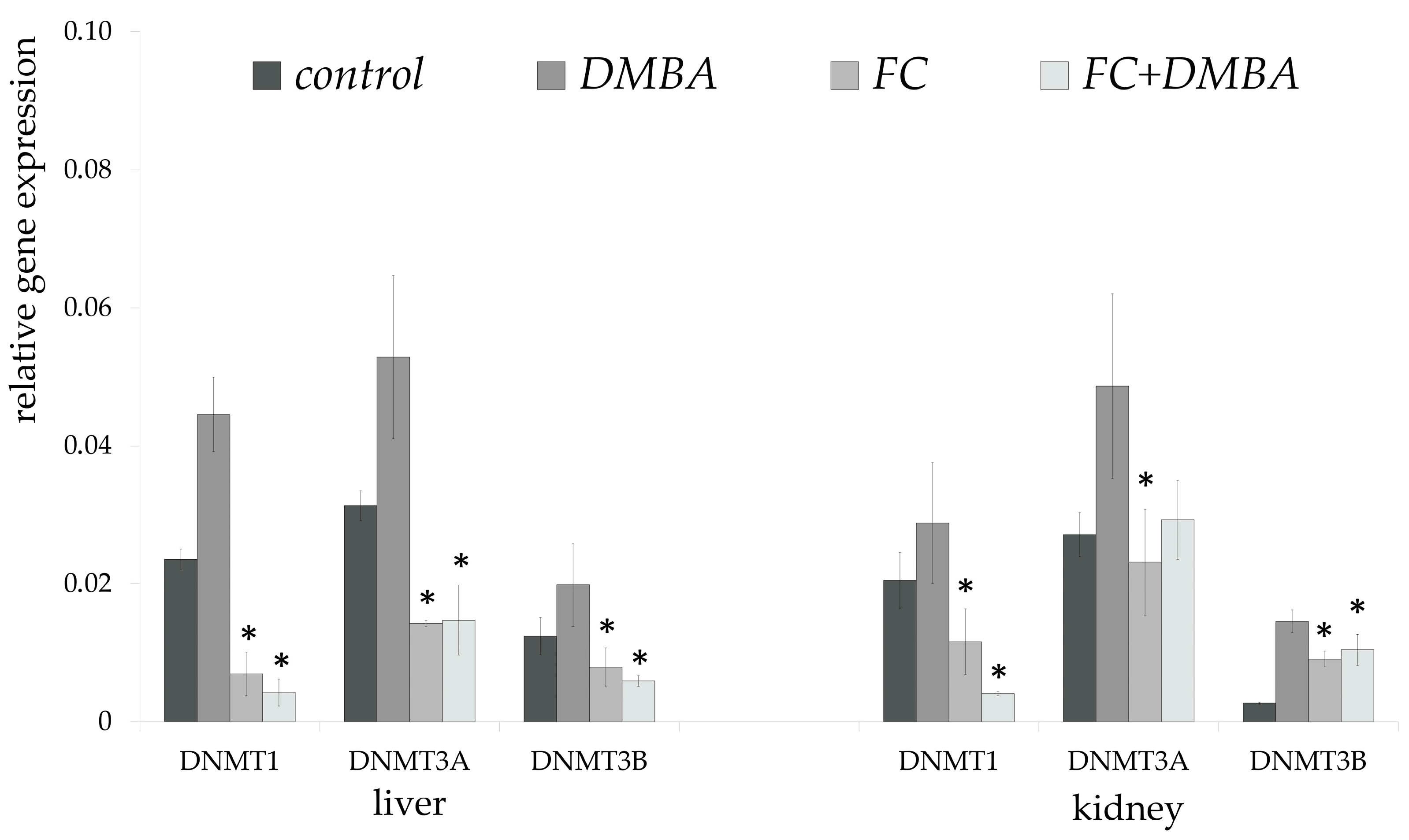
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, M.; Tollefsbol, T.O. Epigenetic linkage of aging, cancer and nutrition. J. Exp. Biol. 2015, 218, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Aguayo-Ortiz, R.; Kapilashrami, K.; Yoo, J.; Luo, M.; Medina-Franco, J.L.; Velázquez-Martínez, C.A. Resveratrol-salicylate derivatives as selective DNMT3 inhibitors and anticancer agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, Q.; Ji, L.; Wang, Y.; Qi, X.; Liu, L.; Liu, Z.; Lu, L. Epigenetic regulation of active Chinese herbal components for cancer prevention and treatment: A follow-up review. Pharmacol. Res. 2016, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Orillion, A.; Pili, R. Histone deacetylase inhibitors as immunomodulators in cancer therapeutics. Epigenomic 2016, 8, 415–428. [Google Scholar] [CrossRef]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting histone deacetylases with natural and synthetic agents: An emerging anticancer strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef]
- Arimoto-Kobayashi, S.; Sasaki, K.; Hida, R.; Miyake, N.; Fujii, N.; Saiki, Y.; Daimaru, K.; Nakashima, H.; Kubo, T.; Kiura, K. Chemopreventive effects and anti-tumorigenic mechanisms of 2, 6-dimethoxy-1, 4-benzoquinone, a constituent of Vitis coignetiae Pulliat (crimson glory vine, known as yamabudo in Japan), toward 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice. Food Chem. Toxicol. 2021, 154, 112319. [Google Scholar] [CrossRef]
- Kim, H.G.; Sung, N.Y.; Kim, J.H.; Cho, J.Y. In vitro anti-cancer effects of beauvericin through inhibition of actin polymerization and Src phosphorylation. Phytomedicine 2023, 109, 154573. [Google Scholar] [CrossRef]
- Huang, C.F.; Hung, T.W.; Yang, S.F.; Tsai, Y.L.; Yang, J.T.; Lin, C.L.; Hsieh, Y.H. Asiatic acid from centella asiatica exert anti-invasive ability in human renal cancer cells by modulation of ERK/p38MAPK-mediated MMP15 expression. Phytomedicine 2022, 100, 154036. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, S.; Bahetjan, Y.; Li, X.; Yang, X. Dehydrocostus lactone inhibits the proliferation of esophageal cancer cells in vivo and in vitro through ROS-mediated apoptosis and autophagy. Food Chem. Toxicol. 2022, 170, 113453. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Y.; Lv, H.; Zhang, H.; Liang, T.; Zhou, G.; Huang, L.; Tian, Y.; Liang, W. Apigenin in cancer therapy: From mechanism of action to nano-therapeutic agent. Food Chem. Toxicol. 2022, 168, 113385. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Lee, J.H.; Shu, L.; Zhang, C.; Su, Z.-Y.; Lu, Y.; Huang, M.-T.; Ramirez, C.; Pung, D.; Huang, Y.; et al. Genome-wide analysis of DNA methylation in UVB-and DMBA/TPA-induced mouse skin cancer models. Life Sci. 2014, 113, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tomesz, A.; Szabo, L.; Molnar, R.; Deutsch, A.; Darago, R.; Raposa, B.L.; Nowrasteh, G.; Varjas, T.; Nemeth, B.; Orsos, Z.; et al. Changes in miR-124-1, miR-212, miR-132, miR-134, and miR-155 expression patterns after 7, 12-dimethylbenz (a) anthracene treatment in CBA/Ca mice. Cells 2022, 11, 1020. [Google Scholar] [CrossRef]
- Molnar, R.; Szabo, L.; Tomesz, A.; Deutsch, A.; Darago, R.; Raposa, B.L.; Nowrasteh, G.; Varjas, T.; Nemeth, B.; Orsos, Z.; et al. The chemopreventive effects of polyphenols and coffee, based upon a DMBA mouse model with microRNA and mTOR gene expression biomarkers. Cells 2022, 11, 1300. [Google Scholar] [CrossRef]
- Chen, Y.K.; Hsue, S.S.; Lin, L.M. Survivin expression is regulated by an epigenetic mechanism for DMBA-induced hamster buccal-pouch squamous-cell carcinomas. Arch. Oral Biol. 2005, 50, 593–598. [Google Scholar] [CrossRef]
- Singhal, R.; Shankar, K.; Badger, T.M.; Ronis, M.J. Estrogenic status modulates aryl hydrocarbon receptor—Mediated hepatic gene expression and carcinogenicity. Carcinogenesis 2008, 29, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.C.; Almeldin, A.; Tong, T.; Rondelli, C.M.; Maguire, M.; Jaskula-Sztul, R.; Jefcoate, C.R. Cytochrome P4501B1 in bone marrow is co-expressed with key markers of mesenchymal stem cells. BMS2 cell line models PAH disruption of bone marrow niche development functions. Toxicol. Appl. Pharm. 2020, 401, 115111. [Google Scholar] [CrossRef]
- Szabó, L.; Kiss, I.; Orsós, Z.; Ember, I. Effect of Nano-Fruit-Café on the expression of oncogenes and tumor suppressor genes. Anticancer Res. 2008, 28, 3274. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Martínez, C.; Sánchez, M.J.; Navarro, C.; Chirlaque, M.D.; Tormo, M.-J.; et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Brit. J. Nutr. 2008, 100, 188–196. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, M.W.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yin, W.-J.; Lu, J.-S.; Wang, L.; Wu, J.; Wu, F.-Y.; Di, G.-H.; Shen, Z.-Z.; Shao, Z.-M. ERα negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J. Cancer Res. Clin. 2008, 134, 883–890. [Google Scholar] [CrossRef]
- Kim, H.; Ramirez, C.N.; Su, Z.-Y.; Kong, A.-N.T. Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef]
- Kuo, H.-C.D.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.-N. Anthocyanin Delphinidin Prevents Neoplastic Transformation of Mouse Skin JB6 P+ Cells: Epigenetic Re-activation of Nrf2-ARE Pathway. AAPS J. 2019, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, S.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Zhang, X.; Levenson, A.S. Epigenetic potential of resveratrol and analogs in preclinical models of prostate cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Basse, C.; Arock, M. The increasing roles of epigenetics in breast cancer: Implications for pathogenicity, biomarkers, prevention, and treatment. Int. J. Cancer 2015, 137, 2785–2794. [Google Scholar] [CrossRef]
- Olguín-Reyes, S.R.; Hernández-Ojeda, S.L.; Govezensky, T.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Sub-acute exposure effect of selected polycyclic aromatic hydrocarbons on protein levels of epigenetic modifiers in non-cancerous hepatic model. Biomed. Res. 2018, 29, 342. [Google Scholar] [CrossRef]
- Wang, L.-S.; Kuo, C.-T.; Cho, S.-J.; Seguin, C.; Siddiqui, J.; Stoner, K.; Weng, Y.-I.; Huang, T.H.-M.; Tichelaar, J.; Yearsley, M.; et al. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr. Cancer 2013, 65, 118–125. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, K.; Clarke, K.; Weiland, T.; Sauter, E.R. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr. Cancer 2014, 66, 270–277. [Google Scholar] [CrossRef]
- Gerhauser, C. Cancer chemoprevention and nutri-epigenetics: State of the art and future challenges. Top. Curr. Chem. 2013, 329, 73–132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, S.; Gao, C.; Dai, Q.; Zhang, C.; Sun, Q.; Lin, J.-S.; Guo, C.; Chen, Y.; Jiang, Y. Development of a versatile DNMT and HDAC inhibitor C02S modulating multiple cancer hallmarks for breast cancer therapy. Bioorg. Chem. 2019, 87, 200–208. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
| Fluid Consumption (for 28 Days) | Carcinogenic Exposure (on Day 27) ** | ||||
|---|---|---|---|---|---|
| Group 1 | Control | Tap water | ad libitum | Vehicle | |
| Group 2 | DMBA | Tap water | ad libitum | DMBA | 20 mg/kg bw |
| Group 3 | FC | Fruit seed and peel extract | Human-equivalent dose * | Vehicle | |
| Group 4 | FC + DMBA | Fruit seed and peel extract | Human-equivalent dose * | DMBA | 20 mg/kg bw |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| DNA methyltransferase 1 (DNMT1) | F-AAGAATGGTGTTGTCTACCGAC | R-CATCCAGGTTGCTCCCCTTG |
| DNA methyltransferase 3A (DNMT3A) | F-GAGGGAACTGAGACCCCAC | R-CTGGAAGGTGAGTCTTGGCA |
| DNA methyltransferase 3B (DNMT3B) | F-AGCGGGTATGAGGAGTGCAT | R-GGGAGCATCCTTCGTGTCTG |
| Histone deacetylase 2 (HDAC2) | F-GGAGGAGGCTACACAATCCG | R-TCTGGAGTGTTCTGGTTTGTCA |
| Histone deacetylase 3 (HDAC3) | F-GCCAAGACCGTGGCGTATT | R-GTCCAGCTCCATAGTGGAAGT |
| Histone deacetylase 8 (HDAC8) | F-ACTATTGCCGGAGATCCAATGT | R-CCTCCTAAAATCAGAGTTGCCAG |
| Hypoxanthine phosphoribo-syltransferase 1 (HPRT1) | F-TCAGTCAACGGGGGACATAAA | R-GGGGCTGTACTGCTTAACCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowrasteh, G.; Zand, A.; Raposa, L.B.; Szabó, L.; Tomesz, A.; Molnár, R.; Kiss, I.; Orsós, Z.; Gerencsér, G.; Gyöngyi, Z.; et al. Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes. Nutrients 2023, 15, 1867. https://doi.org/10.3390/nu15081867
Nowrasteh G, Zand A, Raposa LB, Szabó L, Tomesz A, Molnár R, Kiss I, Orsós Z, Gerencsér G, Gyöngyi Z, et al. Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes. Nutrients. 2023; 15(8):1867. https://doi.org/10.3390/nu15081867
Chicago/Turabian StyleNowrasteh, Ghodratollah, Afshin Zand, László Bence Raposa, László Szabó, András Tomesz, Richárd Molnár, István Kiss, Zsuzsa Orsós, Gellért Gerencsér, Zoltán Gyöngyi, and et al. 2023. "Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes" Nutrients 15, no. 8: 1867. https://doi.org/10.3390/nu15081867
APA StyleNowrasteh, G., Zand, A., Raposa, L. B., Szabó, L., Tomesz, A., Molnár, R., Kiss, I., Orsós, Z., Gerencsér, G., Gyöngyi, Z., & Varjas, T. (2023). Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes. Nutrients, 15(8), 1867. https://doi.org/10.3390/nu15081867










