Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action
Abstract
1. Introduction
2. Materials and Methods
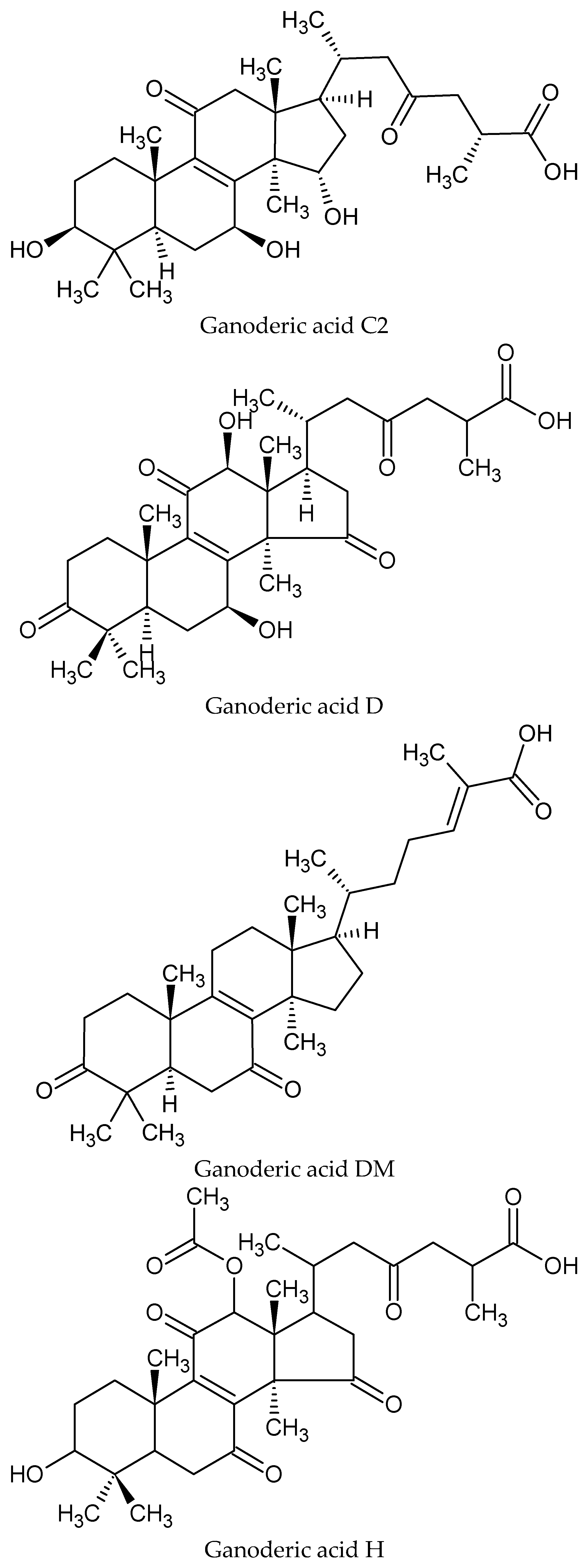
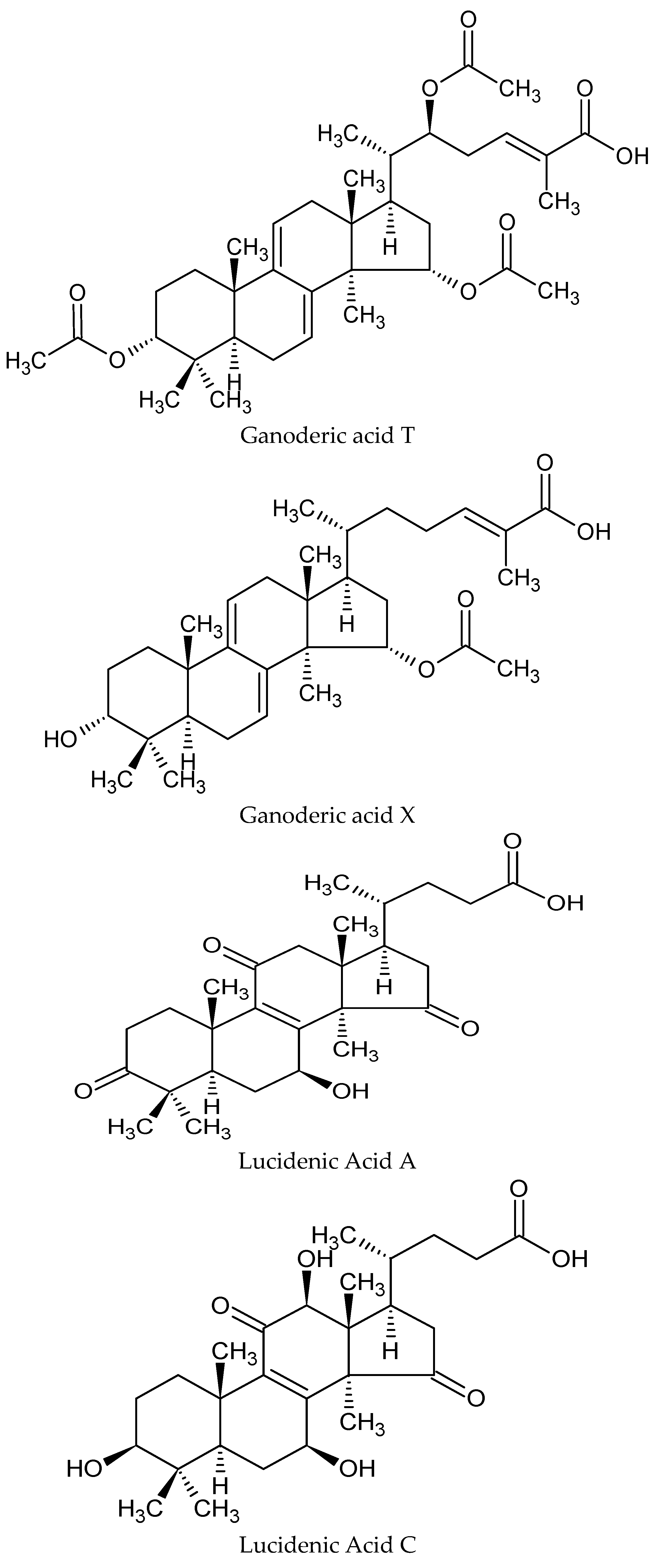
3. Nutritional and Bioactive Constituents
4. Potential Mechanisms of Action
5. Potential Hepatoprotective Effects
5.1. Protective Effects against Liver Fibrosis
5.2. Protective Effects against Alcohol-Induced Liver Injury
5.3. Protective Effects against Non-Alcoholic Fatty Liver Disease
5.4. Protective Effects against Hepatic Carcinoma
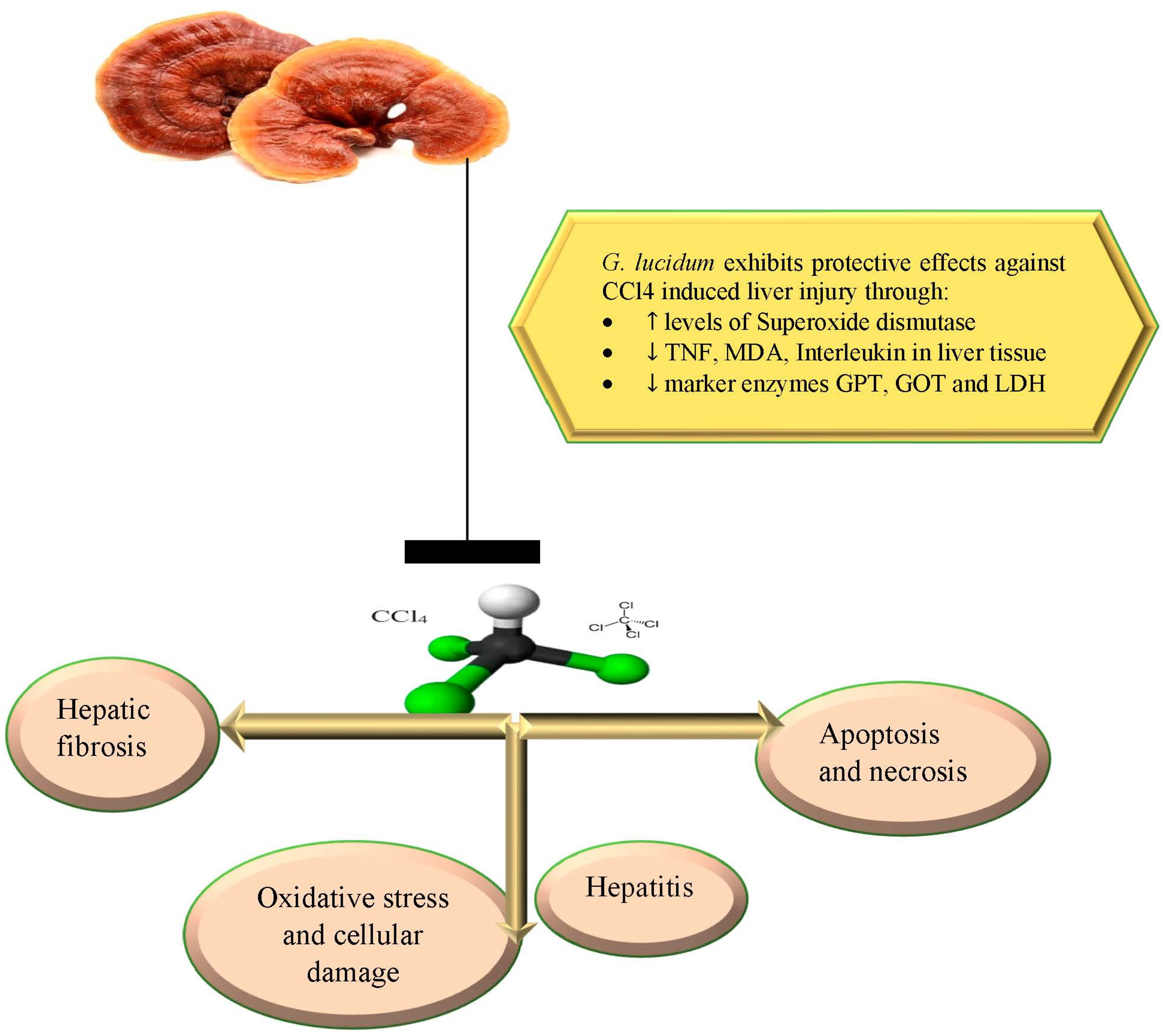
5.5. Protective Effect against Carbon Tetrachloride
5.6. Protective Effect against α-Amanitin-Induced Liver Injury
5.7. Protective Effects against Hepatitis B Virus
6. Effects on Microbiota and Latest Findings
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, G.; Yang, J.; Sheng, L.; Tang, X.; Zhang, X.; Hua, H. Deciphering the chemical composition of Ganoderma lucidum from different geographical origins by mass spectrometry molecular networking coupled with multivariate analysis. Biomed. Chromatogr. 2023, 37, e5506. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum—A comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wang, Y.; Fang, L.; Guo, C.; Sang, T.; Peng, H.; Zhao, Q.; Chen, S.; Lin, X. Ganoderma lucidum polysaccharide inhibits HSC activation and liver fibrosis via targeting inflammation, apoptosis, cell cycle, and ECM-receptor interaction mediated by TGF-β/Smad signaling. Phytomedicine 2023, 110, 154626. [Google Scholar] [CrossRef]
- Tiyah, S.W.; Ratnaningtyas, N.; Wibowo, E.; Mumpuni, A.; Ekowati, N. Ganoderma lucidum as Anti-Inflammatory Agent on The Level of Albumin and Globulin in Rat (Rattus Norvegicus) Rheumatoid Arthritis (RA) Model. Proc. ICMA-SURE 2023, 2, 85–93. [Google Scholar]
- de Mendonça, D.E.A.; de Godoy, M.A.F.; Lucredi, N.C.; Comar, J.F.; Almeida, I.V.; Vicentini, V.E.P. Toxicogenic effects of the mushroom Ganoderma lucidum on human liver and kidney tumor cells and peripheral blood lymphocytes. J. Ethnopharmacol. 2023, 307, 116226. [Google Scholar] [CrossRef]
- Hussein, A.; Ghonimy, A.; Jiang, H.; Qin, G.; El-Ashram, S.; Hussein, S.; Abd El-Razek, I.; El-Afifi, T.; Farouk, M.H. LC/MS analysis of mushrooms provided new insights into dietary management of diabetes mellitus in rats. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Veena, R.K.; Janardhanan, K.K. Bioactive total triterpenes extracted from fruiting bodies and mycelia of Ganoderma lucidum (Fr.) P. Karst ameliorate doxorubicin-induced myocardial injury in rats. Trans. R. Soc. S. Afr. 2023, 77, 237–245. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma lucidum: A potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef]
- Pavlović, M.O.; Stajić, M.; Gašić, U.; Duletić-Laušević, S.; Ćilerdžić, J. The chemical profiling and assessment of antioxidative, antidiabetic and antineurodegenerative potential of Kombucha fermented Camellia sinensis, Coffea arabica and Ganoderma lucidum extracts. Food Funct. 2023, 14, 262–276. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Zhang, J.; Guo, X.; Chen, Y.-Y.; Sun, J.-Y.; Miao, J.-L.; Carpena, M.; Prieto, M.; Li, N.-Y.; Zhou, Q.-X. Molecular mechanisms of the chemical constituents from anti-inflammatory and antioxidant active fractions of Ganoderma neo-japonicum Imazeki. Curr. Res. Food Sci. 2023, 6, 100441. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, P.; Sankaralingam, S.; Muniraj, I.K.; Perumal, M.; Pandurangan, N. Mass Multiplication, Economic Analysis, and Marketing of Ganoderma sp.(Reishi Mushroom). In Food Microbiology Based Entrepreneurship: Making Money From Microbes; Springer Nature: Singapore, 2023; pp. 89–113. [Google Scholar]
- Leng, Y.; Wang, F.; Chen, C.; Wan, X.; Li, X.; Wang, H.; Wang, S. Protective Effect of Ganoderma lucidum Spore Powder on Acute Liver Injury in Mice and its Regulation of Gut Microbiota. Front. Biosci.-Landmark 2023, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; GIRIJA, A.S.; Priyadharsini, J.V. Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from Ganoderma lucidum: A computational study. Indian J. Pharm. Sci. 2020, 82, 300–305. [Google Scholar] [CrossRef]
- Peng, H.; Zhong, L.; Cheng, L.; Chen, L.; Tong, R.; Shi, J.; Bai, L. Ganoderma lucidum: Current advancements of characteristic components and experimental progress in anti-liver fibrosis. Front. Pharmacol. 2022, 13, 1094405. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Du, J.-L.; Cao, L.-P.; Jia, R.; Shen, Y.-J.; Zhao, C.-Y.; Xu, P.; Yin, G.-J. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides on carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio L.). Int. Immunopharmacol. 2015, 25, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Tang, Q.; Yang, Y.; Xia, Y.; Zhou, S.; Wu, D.; Zhang, Z.; Dong, L.; Cui, S.W. Rheological properties of β-d-glucan from the fruiting bodies of Ganoderma lucidum. Food Hydrocoll. 2016, 58, 120–125. [Google Scholar] [CrossRef]
- Bulam, S.; Üstün, N.Ş.; Pekşen, A. Health benefits of Ganoderma lucidum as a medicinal mushroom. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 84–93. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2019, 121, 1160–1178. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Kim, S.-K. Anticancer and other therapeutic relevance of mushroom polysaccharides: A holistic appraisal. Biomed. Pharmacother. 2018, 105, 377–394. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Guo, Q.; Wang, M.; Li, Y.; Meng, Y.; Huang, L. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A macro fungus with phytochemicals and their pharmacological properties. In Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects; Springer: Cham, Switzerland, 2019; pp. 491–515. [Google Scholar]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. The potential of Ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Food Chem. Toxicol. 2017, 108, 139–147. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Panda, B.P.; Azad, Z.; Ahmad, A. Simultaneous bioprospecting of Ganoderma lucidum OE 52 with ganoderic acid B and C2 by submerged fermentation process. Adv. Sci. Focus 2013, 1, 258–261. [Google Scholar] [CrossRef]
- Heleno, S.A.; Stojković, D.; Barros, L.; Glamočlija, J.; Soković, M.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Res. Int. 2013, 51, 236–243. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Vyas, D.; Ganaie, M.A.; Dehariya, K.; Singh, V. HPLC determination of phenolics and free radical scavenging activity of ethanolic extracts of two polypore mushrooms. Int. J. Pharm. Pharm. Sci. 2014, 6, 679–684. [Google Scholar]
- Stojković, D.S.; Barros, L.; Calhelha, R.C.; Glamočlija, J.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Parepalli, Y.; Chavali, M.; Sami, R.; Khojah, E.; Elhakem, A.; El Askary, A.; Singh, M.; Sinha, S.; El-Chaghaby, G. Evaluation of Some Active Nutrients, Biological Compounds and Health Benefits of Reishi Mushroom (Ganoderma lucidum). Int. J. Pharmacol. 2021, 17, 243–250. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Fu, H.-Y.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017, 55, 1041–1046. [Google Scholar] [CrossRef]
- Wu, H.; Tang, S.; Huang, Z.; Zhou, Q.; Zhang, P.; Chen, Z. Hepatoprotective effects and mechanisms of action of triterpenoids from lingzhi or reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes) on α-amanitin-induced liver injury in mice. Int. J. Med. Mushrooms 2016, 18, 841–850. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Huang, Z. CD4+ CD25+ Treg derived from hepatocellular carcinoma mice inhibits tumor immunity. Immunol. Lett. 2012, 148, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Piao, R.; Cui, Y.; Xu, Q. Protective effects of the oil from spores of Ganoderma Lucidum on carbon tetrachloride-induced hepatic injury in mice. J. Med. Sci. Yanbian Univ. 2011, 1, 20–22. [Google Scholar]
- Susilo, R.J.K.; Winarni, D.; Husen, S.A.; Hayaza, S.; Punnapayak, H.; Wahyuningsih, S.P.A.; Sajidah, E.S.; Darmanto, W. Hepatoprotective effect of crude polysaccharides extracted from Ganoderma lucidum against carbon tetrachloride-induced liver injury in mice. Vet. World 2019, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, C.; Changlong, F.; Hua, H. Effect of broken Ganoderma Lucidum spore powder on serum ALT, AST levels and liver inflammation in mice with ConA-induced immune injury. Zhejiang J. Integr. Tradit. Chin. West. Med. 2017, 9, 760–764. [Google Scholar]
- Zhong, C.; Li, Y.; Li, W.; Li, Y.; Wu, C.; Zhang, K.; Zhou, G.; Wang, W.; Xu, H.; Huang, M. Ganoderma lucidum extract-mediated gasdermin E cleavage promotes tumor cell pyroptosis and inhibits metastasis in breast cancer. Food Chem. Toxicol. 2023, 174, 113654. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Ayeka, P.A. Potential of mushroom compounds as immunomodulators in cancer immunotherapy: A review. Evid.-Based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-B.; Zhang, H.-N. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sin. 2004, 25, 1387–1395. [Google Scholar] [PubMed]
- Cao, L.; Jin, H.; Liang, Q.; Yang, H.; Li, S.; Liu, Z.; Yuan, Z. A new anti-tumor cytotoxic triterpene from Ganoderma lucidum. Nat. Prod. Res. 2022, 36, 4125–4131. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, M.; Lin, Z.-B.; Zhou, S. Hepatoprotective activity and the mechanisms of action of Ganoderma lucidum (Curt.: Fr.) P. Karst. (Ling Zhi, Reishi mushroom) (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2003, 5, 22. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Gupta, G.K.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17, 100268. [Google Scholar] [CrossRef]
- Xu, J.; Li, P. Researches and application of Ganoderma spores powder. In Ganoderma and Health; Advances in Experimental Medicine and Biology Series; Springer: Singapore, 2019; Volume 1181, pp. 157–186. [Google Scholar]
- Guo, W.-L.; Cao, Y.-J.; You, S.-Z.; Wu, Q.; Zhang, F.; Han, J.-Z.; Lv, X.-C.; Rao, P.-F.; Ai, L.-Z.; Ni, L. Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 2022, 5, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Chen, Q.-Z.; Wang, Z.-J.; Hua, C. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides against carbon tetrachloride-induced liver injury in Kunming mice. Pharmacology 2019, 103, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.A.; de Sá-Nakanishi, A.B.; Bracht, A.; da Costa, S.M.G.; Koehnlein, E.A.; de Souza, C.G.M.; Peralta, R.M. Hepatoprotective effects of mushrooms. Molecules 2013, 18, 7609–7630. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, X.-R.; Dong, J.-R.; Lu, S.-Y.; Li, X.-N.; Zhou, L.; Qiu, M.-H. Rearranged lanostane-type triterpenoids with anti-hepatic fibrosis activities from Ganoderma applanatum. RSC Adv. 2018, 8, 31287–31295. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-J.; Chau, C.-F.; Yen, G.-C.; Liao, J.-W.; Chen, D.-H.; Chen, K.-D. Inhibitory effects of Ganoderma lucidum on tumorigenesis and metastasis of human hepatoma cells in cells and animal models. J. Agric. Food Chem. 2009, 57, 5049–5057. [Google Scholar] [CrossRef]
- Song, M.; Li, Z.-H.; Gu, H.-S.; Tang, R.-Y.; Zhang, R.; Zhu, Y.-L.; Liu, J.-L.; Zhang, J.-J.; Wang, L.-Y. Ganoderma lucidum spore polysaccharide inhibits the growth of hepatocellular carcinoma cells by altering macrophage polarity and induction of apoptosis. J. Immunol. Res. 2021, 2021, 6696606. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, J.; Hu, J.; Liao, Q.; Zhou, R.; Zhang, P.; Chen, Z. Hepatoprotective effects of aqueous extract from lingzhi or reishi medicinal mushroom Ganoderma lucidum (higher basidiomycetes) on α-Amanitin− induced liver injury in mice. Int. J. Med. Mushrooms 2013, 15, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Pan, Y.; Zhang, Z.; He, Y.; Teng, Y.; Liang, H.; Wu, X.; Yang, H.; Zhou, P. Amelioration of the lipogenesis, oxidative stress and apoptosis of hepatocytes by a novel proteoglycan from Ganoderma lucidum. Biol. Pharm. Bull. 2020, 43, 1542–1550. [Google Scholar] [CrossRef]
- Zhong, D.; Xie, Z.; Huang, B.; Zhu, S.; Wang, G.; Zhou, H.; Lin, S.; Lin, Z.; Yang, B. Ganoderma lucidum polysaccharide peptide alleviates hepatoteatosis via modulating bile acid metabolism dependent on FXR-SHP/FGF. Cell. Physiol. Biochem. 2018, 49, 1204–1220. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Wang, S.-F. Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum. Biotechnol. Lett. 2006, 28, 837–841. [Google Scholar] [CrossRef]
- Hassan, H.M.; Al-Wahaibi, L.H.; Elmorsy, M.A.; Mahran, Y.F. Suppression of cisplatin-induced hepatic injury in rats through alarmin high-mobility group box-1 pathway by Ganoderma lucidum: Theoretical and experimental study. Drug Des. Dev. Ther. 2020, 14, 2335–2353. [Google Scholar] [CrossRef]
- Oluwafemi Adetuyi, B.; Olamide Okeowo, T.; Adefunke Adetuyi, O.; Abraham Adebisi, O.; Ogunlana, O.O.; Janet Oretade, O.; Marraiki, N.; Beshbishy, A.M.; Welson, N.N.; Batiha, G.E.-S. Ganoderma lucidum from red mushroom attenuates formaldehyde-induced liver damage in experimental male rat model. Biology 2020, 9, 313. [Google Scholar] [CrossRef]
- Aydin, S.; Aytac, E.; Uzun, H.; Altug, T.; Mansur, B.; Saygili, S.; Buyukpinarbasili, N.; Sariyar, M. Effects of Ganoderma lucidum on obstructive jaundice-induced oxidative stress. Asian J. Surg. 2010, 33, 173–180. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Liu, Z.; Sun, X.; Wei, D.; Song, L.; Wu, C. Peptide mediated therapy in fibrosis: Mechanisms, advances and prospects. Biomed. Pharmacother. 2023, 157, 113978. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.S.; Joshi, U.M.; Young, R.A.; Meydrech, E.F.; Mehendale, H.M. Protection of hepatotoxic and lethal effects of CCl by partial hepatectomy. Toxicol. Pathol. 1989, 17, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Moreno, C.; Hampe, J.; Morgan, M.Y. The genetics of alcohol dependence and alcohol-related liver disease. J. Hepatol. 2017, 66, 195–211. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Huang, Z.-R.; You, S.-Z.; Guo, W.-L.; Zhang, F.; Liu, B.; Lv, X.-C.; Lin, Z.-X.; Liu, P.-H. The Protective Effects of Ganoderic Acids from Ganoderma lucidum Fruiting Body on Alcoholic Liver Injury and Intestinal Microflora Disturbance in Mice with Excessive Alcohol Intake. Foods 2022, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-C.; Wu, Q.; Cao, Y.-J.; Lin, Y.-C.; Guo, W.-L.; Rao, P.-F.; Zhang, Y.-Y.; Chen, Y.-T.; Ai, L.-Z.; Ni, L. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition. Food Funct. 2022, 13, 5820–5837. [Google Scholar] [CrossRef]
- Weiß, J.; Rau, M.; Geier, A. Non-alcoholic fatty liver disease: Epidemiology, clinical course, investigation, and treatment. Dtsch. Ärzteblatt Int. 2014, 111, 447. [Google Scholar]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Antonio, F.A.; Edavalath, M.; Mukherjee, A. Non-alcoholic fatty liver disease: A diabetologist’s perspective. Endocrine 2014, 45, 344–353. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, L.-L.; Li, W.; Zhang, Y.; Zhang, Y.; Liu, F.; Zou, L. Application of metabolomics for revealing the interventional effects of functional foods on metabolic diseases. Food Chem. 2022, 367, 130697. [Google Scholar] [CrossRef]
- Ren, F.; Chen, Q.; Meng, C.; Chen, H.; Zhou, Y.; Zhang, H.; Chen, W. Serum metabonomics revealed the mechanism of Ganoderma amboinense polysaccharides in preventing non-alcoholic fatty liver disease (NAFLD) induced by high-fat diet. J. Funct. Foods 2021, 82, 104496. [Google Scholar] [CrossRef]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Papandreou, C.; Furuse, J.; Ladrón de Guevara, L. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010, 15, 5–13. [Google Scholar] [CrossRef]
- Guo, C.-L.; Yang, H.-C.; Yang, X.-H.; Cheng, W.; Dong, T.-X.; Zhu, W.-J.; Xu, Z.; Zhao, L. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 5909–5913. [Google Scholar] [CrossRef]
- Beyer, M.; Schultze, J.L. Regulatory T cells in cancer. Blood 2006, 108, 804–811. [Google Scholar] [CrossRef]
- Li, A.; Shuai, X.; Jia, Z.; Li, H.; Liang, X.; Su, D.; Guo, W. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 2015, 13, 100. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, Q.; Yan, Z.; Chen, R.; Zeh Iii, H.; Kang, R.; Lotze, M.; Tang, D. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis. 2013, 4, e966. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Weng, C.J.; Chau, C.F.; Chen, K.D.; Chen, D.H.; Yen, G.C. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol. Nutr. Food Res. 2007, 51, 1472–1477. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Cederbaum, A.I. Metabolism of carbon tetrachloride to trichloromethyl radical: An ESR and HPLC-EC study. Chem. Res. Toxicol. 1999, 12, 730–736. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, I.J.; Kim, J.W.; Lee, M.K.; Ku, S.K.; Choi, J.S.; Lee, H.J. Hepatoprotective effects of blue honeysuckle on CCl4-induced acute liver damaged mice. Food Sci. Nutr. 2019, 7, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Patrick-Iwuanyanwu, K.; Wegwu, M.; Ayalogu, E. Prevention of CCI4-induced liver damage by ginger, garlic and vitamin E. Pak. J. Biol. Sci. 2007, 10, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Baldissera, M.D.; Guarda, N.S.; Bollick, Y.S.; Moresco, R.N.; Brusque, I.C.M.; Santos, R.C.; Baldisserotto, B. Melaleuca alternifolia essential oil nanoparticles ameliorate the hepatic antioxidant/oxidant status of silver catfish experimentally infected with Pseudomonas aeruginosa. Microb. Pathog. 2017, 108, 61–65. [Google Scholar] [CrossRef]
- Sancheti, S.; Sancheti, S.; Seo, S.-Y. Ameliorative effects of 7-methylcoumarin and 7-methoxycoumarin against CCl4-induced hepatotoxicity in rats. Drug Chem. Toxicol. 2013, 36, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Mahmoud, M.E. Therapeutic potential of morin against liver fibrosis in rats: Modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ. Toxicol. Pharmacol. 2014, 37, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Unal-Cevik, I.; Kılınç, M.; Can, A.; Gürsoy-Özdemir, Y.; Dalkara, T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 2004, 35, 2189–2194. [Google Scholar] [CrossRef]
- Sheena, N.; Ajith, T.; Janardhanan, K. Prevention of nephrotoxicity induced by the anticancer drug cisplatin, using Ganoderma lucidum, a medicinal mushroom occurring in South India. Curr. Sci. 2003, 85, 478–482. [Google Scholar]
- Gao, Z.; Yuan, F.; Li, H.; Feng, Y.; Zhang, Y.; Zhang, C.; Zhang, J.; Song, Z.; Jia, L. The ameliorations of Ganoderma applanatum residue polysaccharides against CCl4 induced liver injury. Int. J. Biol. Macromol. 2019, 137, 1130–1140. [Google Scholar] [CrossRef]
- Dabdoub, B.R.; Mohammed, R.H.; Abdulhadi, H.L. Ganoderma lucidum attenuates and prevents CCl4-induced hepatic and renal damage in Sprague–Dawley Rats. Syst. Rev. Pharm. 2020, 11, 1704–1709. [Google Scholar]
- Ye, H. Healthy benefits of Ganoderma lucidum as herb medicinal mushroom. CPQ Nutr. 2018, 1, 1–7. [Google Scholar]
- Cornberg, M.; Wong, V.W.-S.; Locarnini, S.; Brunetto, M.; Janssen, H.L.; Chan, H.L.-Y. The role of quantitative hepatitis B surface antigen revisited. J. Hepatol. 2017, 66, 398–411. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhong, D.; Yang, B. Preventive and therapeutic effect of Ganoderma (lingzhi) on liver injury. Ganoderma Health Pharmacol. Clin. Appl. 2019, 1182, 217–242. [Google Scholar]
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Ashraf, S.A.; Abullais, S.S.; Saad, H.H. Ganoderma lucidum: A potential pleiotropic approach of ganoderic acids in health reinforcement and factors influencing their production. Fungal Biol. Rev. 2022, 39, 100–125. [Google Scholar] [CrossRef]
- Caslin, B.; Maguire, C.; Karmakar, A.; Mohler, K.; Wylie, D.; Melamed, E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc. Natl. Acad. Sci. USA 2019, 116, 25808–25815. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.-E. Impact of drinking alcohol on gut microbiota: Recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci. 2021, 37, 91–97. [Google Scholar] [CrossRef]
- Guo, W.-L.; Pan, Y.-Y.; Li, L.; Li, T.-T.; Liu, B.; Lv, X.-C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Chen, S.; Guan, X.; Yong, T.; Gao, X.; Xiao, C.; Xie, Y.; Chen, D.; Hu, H.; Wu, Q. Structural characterization and hepatoprotective activity of an acidic polysaccharide from Ganoderma lucidum. Food Chem. X 2022, 13, 100204. [Google Scholar] [CrossRef]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef]





| Hepatic Injury | Different Form of Constituents | Hepatoprotective Effects | References |
|---|---|---|---|
| Hepatic injury induced by alcohol | G. lucidum ethanol extract |
| [51] |
| Carbon tetrachloride (CCl4)-induced liver injury | G. lucidum polysaccharides |
| [18,52] |
| Crude polysaccharides extract |
| [40] | |
| G. lucidum sporoderm-breaking spores |
| [50] | |
| G. lucidum Spores oil |
| [39] | |
| Concanavalin A (CON A)-induced immune liver injury | Broken G. lucidum spores powder |
| [41] |
| Galactosamine-induced liver fibrosis effects | G. lucidum triterpene extract |
| [53,54] |
| Hepatic fibrosis | Ganoderma applanatum (triterpenoids) Ganoapplanic acid A, C, F |
| [55] |
| G. lucidum spores powder |
| [50] | |
| Hepatic carcinoma | G. lucidum extract (GLE) |
| [8,56] |
| G. lucidum spore polysaccharide |
| [57] | |
| α-Amanitin Induced Liver Injury | G. lucidum aqueous extracts |
| [37,58] |
| Ganoderic acid C2 |
| [37] | |
| Non-alcoholic fatty liver disease | Fudan-Yueyang G. lucidum (FYGL) |
| [59,60] |
| Hepatitis B | Ganoderic acids |
| [61] |
| Drug induced liver injury (Cisplatin) | G. lucidum mushroom (GLM) |
| [62] |
| Formaldehyde (FA) induced liver fibrosis | G. lucidum ethanol extract |
| [17,63] |
| Obstructive jaundice | G. lucidum polysaccharide |
| [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.F.; Ahmad, F.A.; Zeyaullah, M.; Alsayegh, A.A.; Mahmood, S.E.; AlShahrani, A.M.; Khan, M.S.; Shama, E.; Hamouda, A.; Elbendary, E.Y.; et al. Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action. Nutrients 2023, 15, 1874. https://doi.org/10.3390/nu15081874
Ahmad MF, Ahmad FA, Zeyaullah M, Alsayegh AA, Mahmood SE, AlShahrani AM, Khan MS, Shama E, Hamouda A, Elbendary EY, et al. Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action. Nutrients. 2023; 15(8):1874. https://doi.org/10.3390/nu15081874
Chicago/Turabian StyleAhmad, Md Faruque, Fakhruddin Ali Ahmad, Md. Zeyaullah, Abdulrahman A. Alsayegh, Syed Esam Mahmood, Abdullah M. AlShahrani, Mohammad Suhail Khan, Eman Shama, Alshaimaa Hamouda, Ehab Y. Elbendary, and et al. 2023. "Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action" Nutrients 15, no. 8: 1874. https://doi.org/10.3390/nu15081874
APA StyleAhmad, M. F., Ahmad, F. A., Zeyaullah, M., Alsayegh, A. A., Mahmood, S. E., AlShahrani, A. M., Khan, M. S., Shama, E., Hamouda, A., Elbendary, E. Y., & Attia, K. A. H. A. (2023). Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action. Nutrients, 15(8), 1874. https://doi.org/10.3390/nu15081874








