Comparison of Three Low-Molecular-Weight Fluorescent Probes for Measuring Free Zinc Levels in Cultured Mammary Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Dye Loading and Fluorescence Measurements
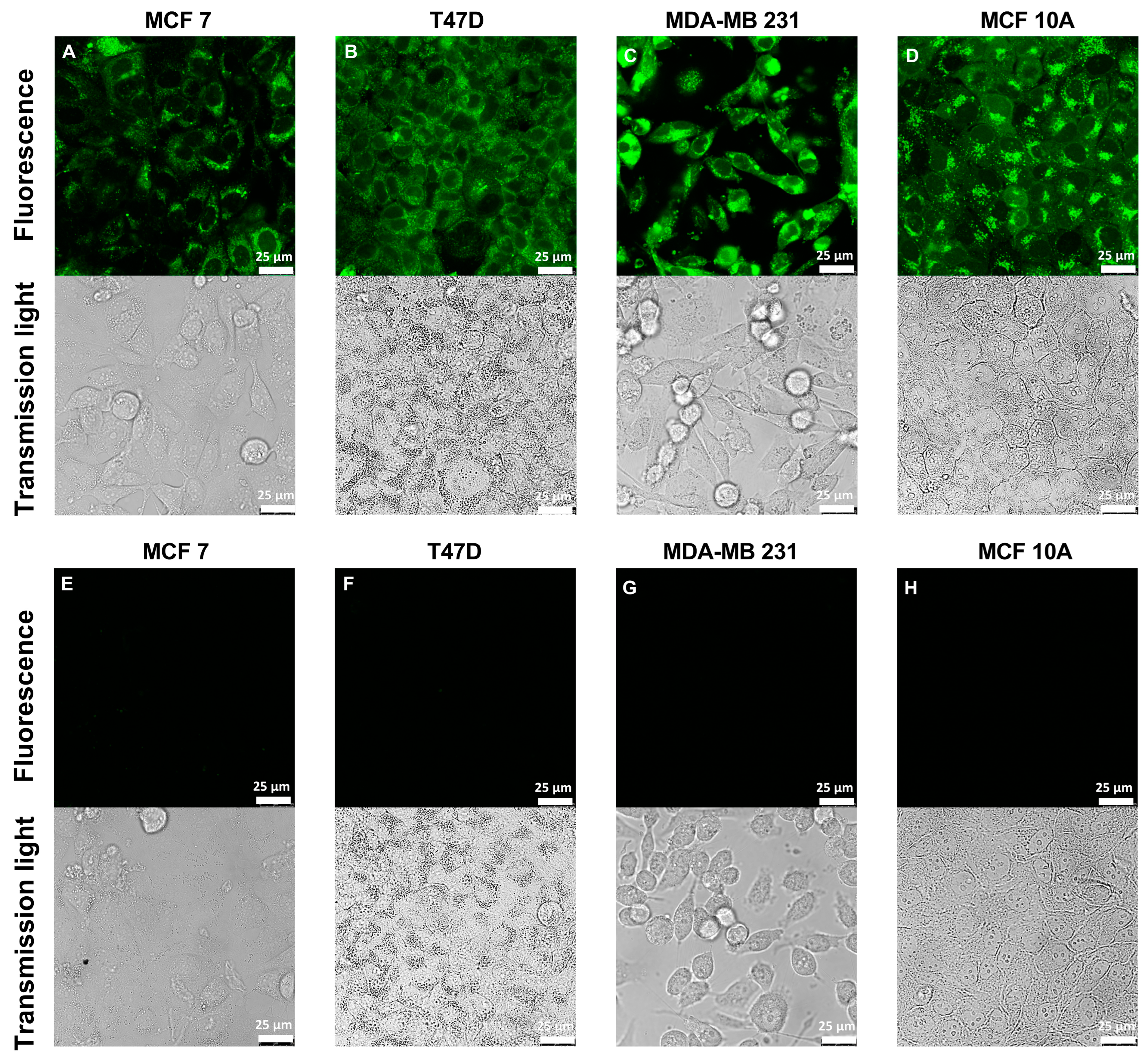
2.4. Confocal Laser Scanning Microscopy
2.5. Statistical Analysis
3. Results
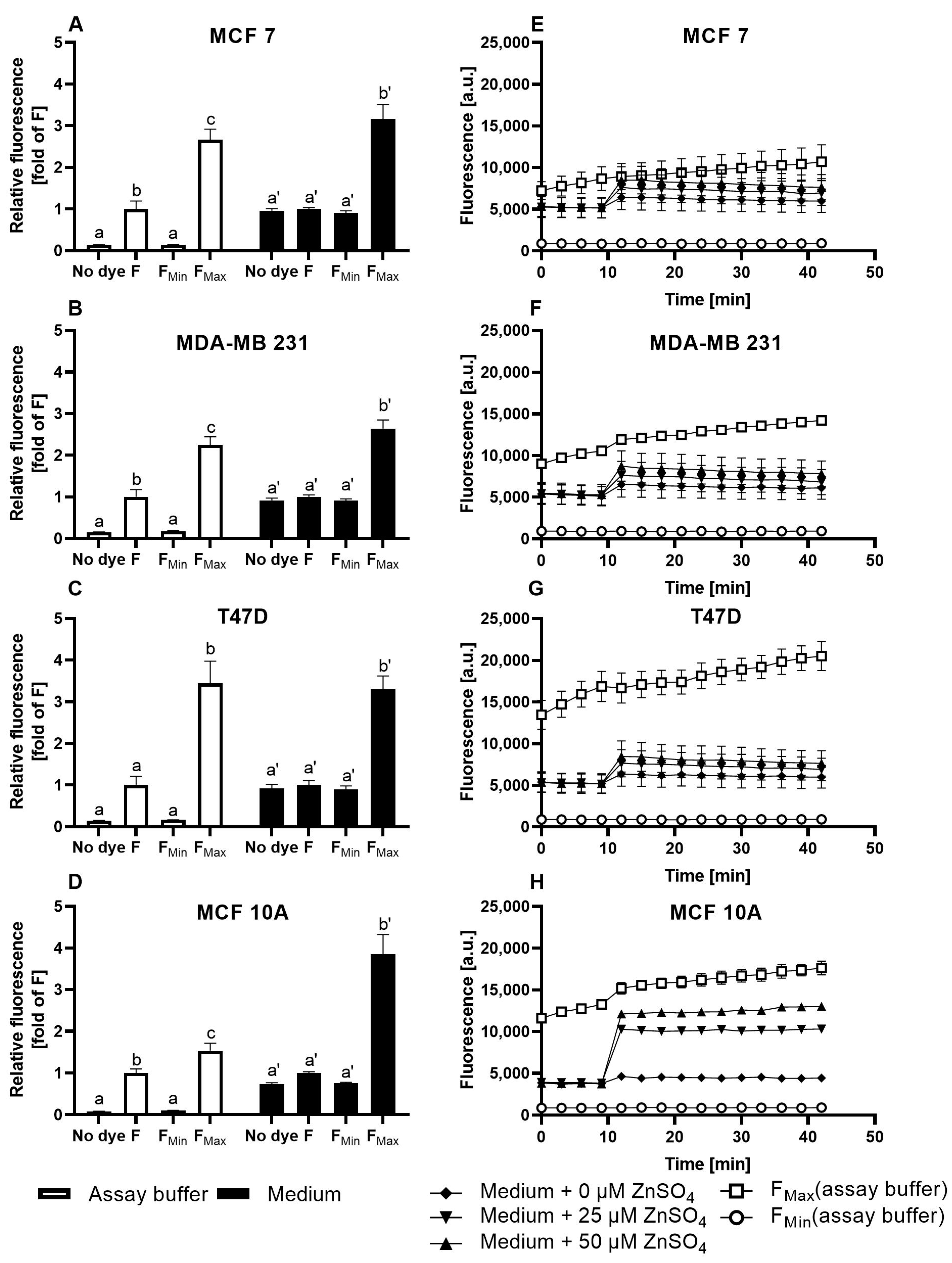
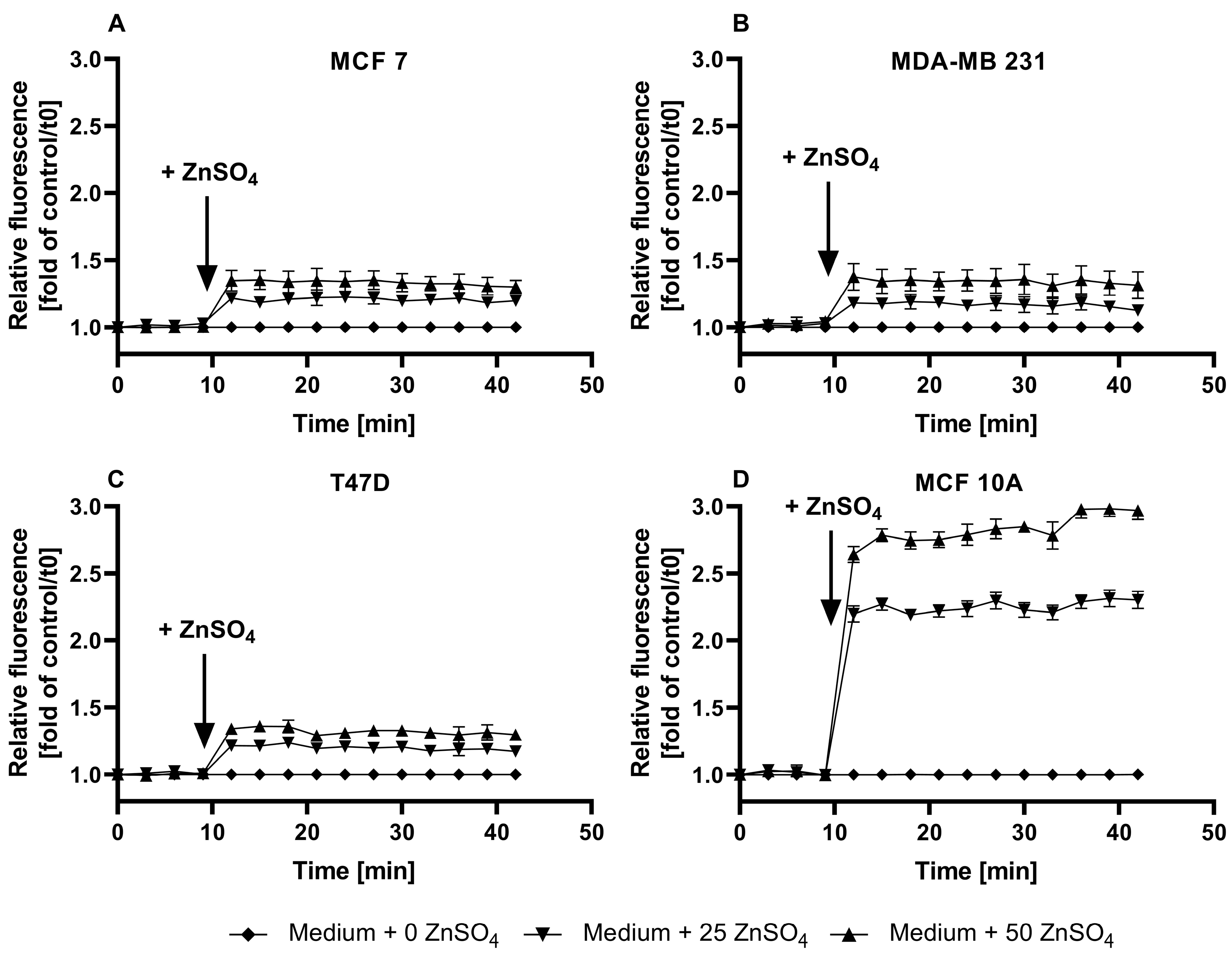
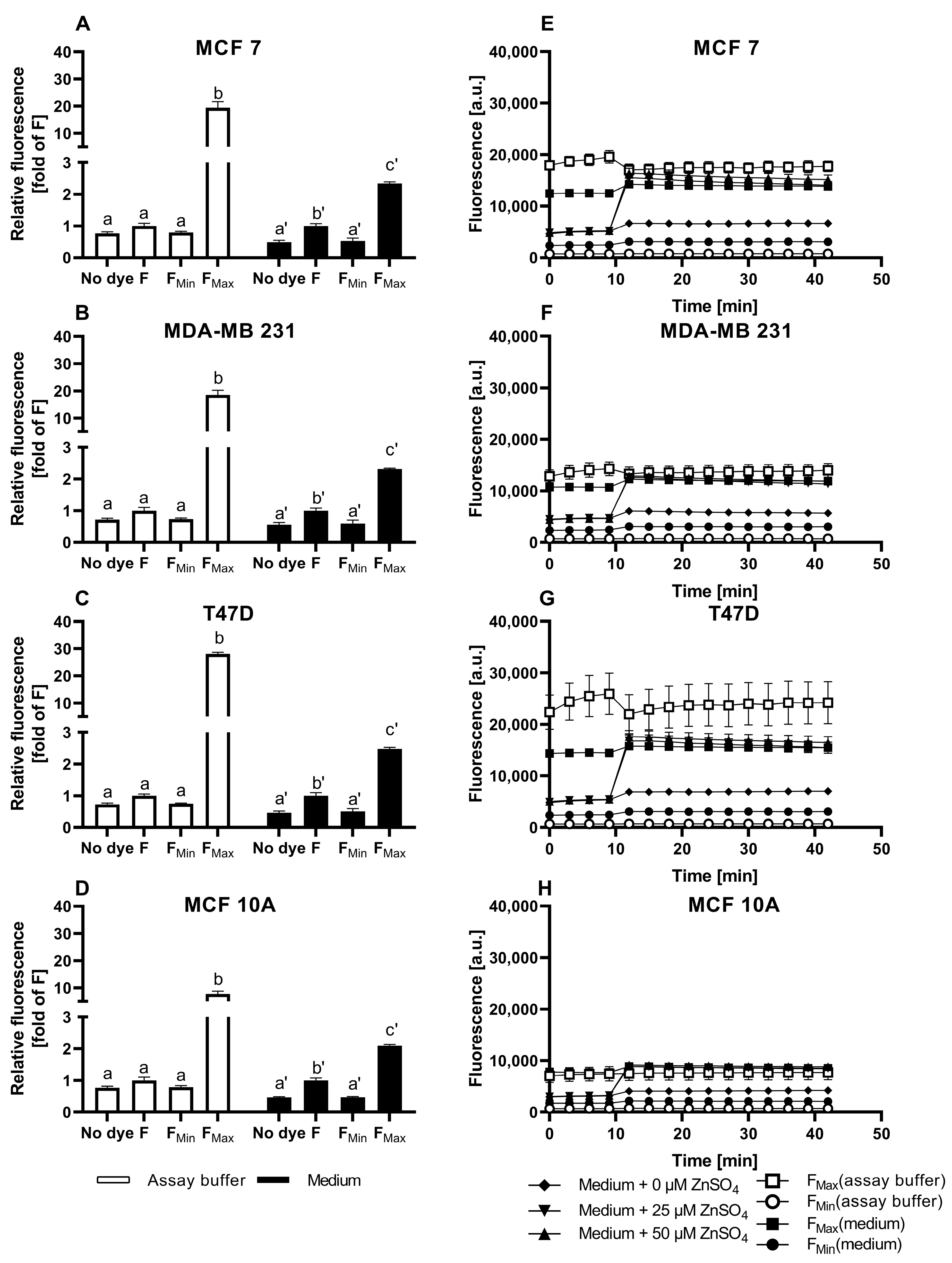
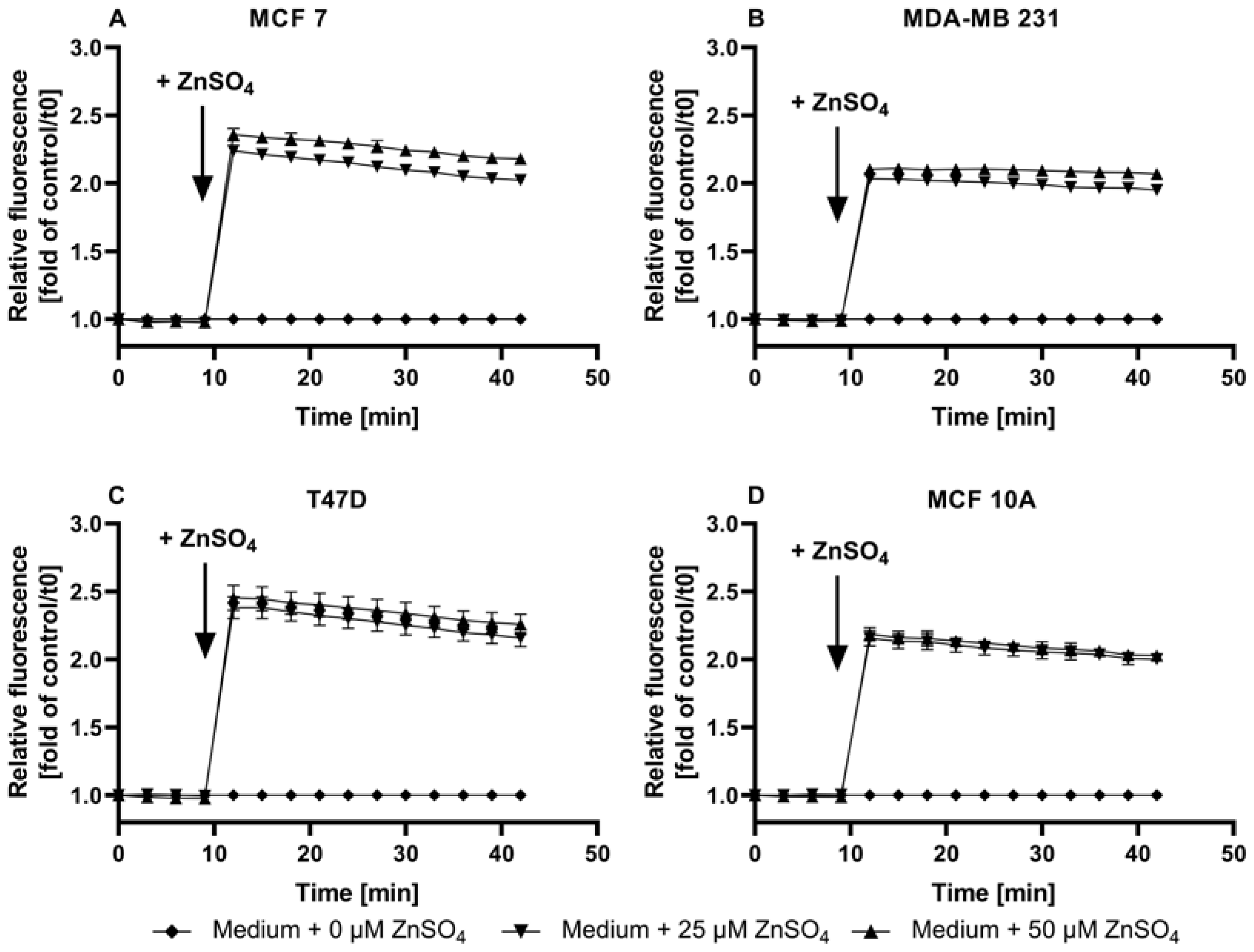
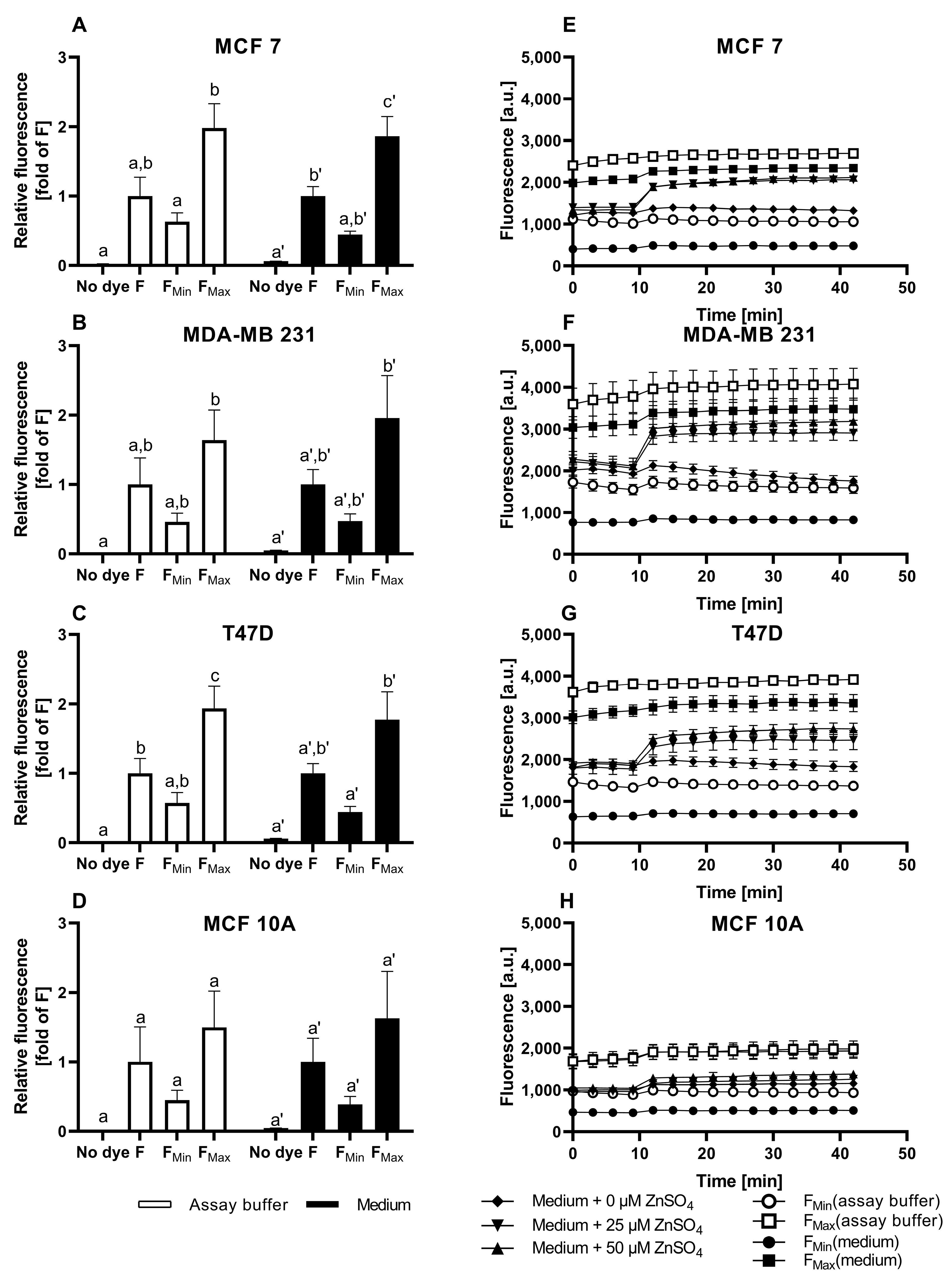
3.1. Characterization of FZ3 for the Detection of Free Intracellular Zinc
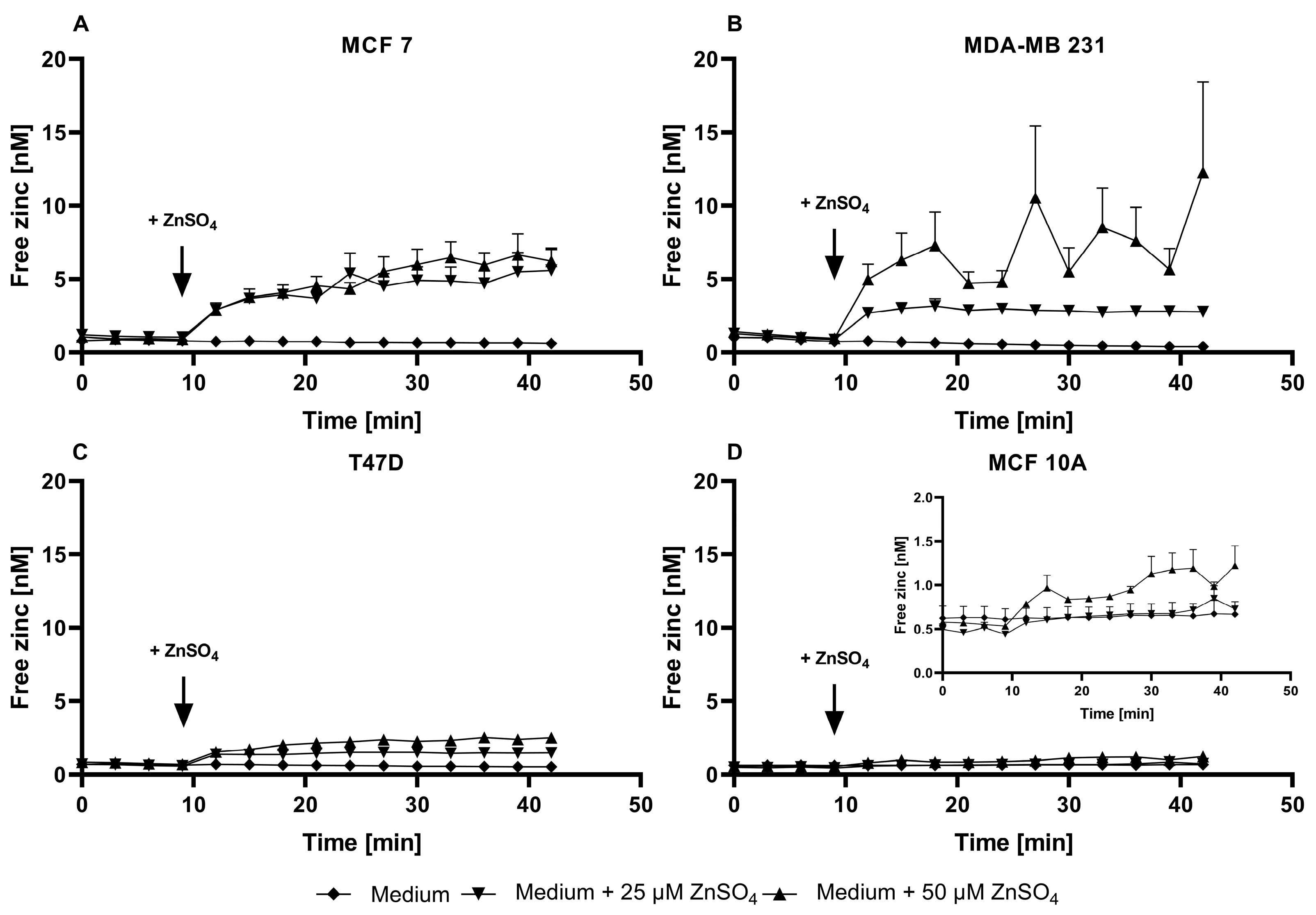
3.2. Evaluation of TSQ for the Measurement of Free Intracellular Zinc
3.3. Application of ZP1 for Detection and Quantification of Intracellular Free Zinc
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Larner, F.; Shousha, S.; Coombes, R.C. Zinc isotopes: A novel approach to biomarkers of breast cancer? Biomark. Med. 2015, 9, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Riesop, D.; Hirner, A.V.; Rusch, P.; Bankfalvi, A. Zinc distribution within breast cancer tissue: A possible marker for histological grading? J. Cancer Res. Clin. Oncol. 2015, 141, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Rusch, P.; Hirner, A.V.; Schmitz, O.; Kimmig, R.; Hoffmann, O.; Diel, M. Zinc distribution within breast cancer tissue of different intrinsic subtypes. Arch. Gynecol. Obstet. 2020, 303, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.; Kochupurakkal, B.S.; Alam, S.; Richardson, A.L.; Soybel, D.I.; Kelleher, S.L. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol. Cancer 2016, 15, 2. [Google Scholar] [CrossRef]
- Ionescu, J.G.; Novotny, J.; Stejskal, V.; Lätsch, A.; Blaurock-Busch, E.; Eisenmann-Klein, M. Increased levels of transition metals in breast cancer tissue. Neuro. Endocrinol. Lett. 2006, 27 (Suppl. S1), 36–39. [Google Scholar]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular Zn2+ and other metal ions are severely affected by their speciation in cell culture media. Metallomics 2015, 7, 102–111. [Google Scholar] [CrossRef]
- Haase, H.; Maret, W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp. Cell Res. 2003, 291, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Jacob, C.; Vallee, B.L.; Fischer, E.H. Inhibitory sites in enzymes: Zinc removal and reactivation by thionein. Proc. Natl. Acad. Sci. USA 1999, 96, 1936–1940. [Google Scholar] [CrossRef]
- Hübner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021, 41, 101916. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef]
- Taylor, K.M.; Vichova, P.; Jordan, N.; Hiscox, S.; Hendley, R.; Nicholson, R.I. ZIP7-Mediated Intracellular Zinc Transport Contributes to Aberrant Growth Factor Signaling in Antihormone-Resistant Breast Cancer Cells. Endocrinology 2008, 149, 4912–4920. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef]
- Lopez, V.; Kelleher, S.L. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp. Cell Res. 2010, 316, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Keydar, I.; Chen, L.; Karby, S.; Weiss, F.R.; Delarea, J.; Radu, M.; Chaitcik, S.; Brenner, H.J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur. J. Cancer 1979, 15, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cailleau, R.; Young, R.; Olivé, M.; Reeves, W.J., Jr. Breast tumor cell lines from pleural effusions. J. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef]
- Soule, H.D.; Maloney, T.M.; Wolman, S.R.; Peterson, W.D., Jr.; Brenz, R.; McGrath, C.M.; Russo, J.; Pauley, R.J.; Jones, R.F.; Brooks, S.C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6075–6086. [Google Scholar] [PubMed]
- Soule, H.D.; Vazquez, J.; Long, A.; Albert, S.; Brennan, M. A Human Cell Line From a Pleural Effusion Derived From a Breast Carcinoma 2. JNCI J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Han, B.; Yu, Y.; Yao, W.; Bose, S.; Karlan, B.Y.; Giuliano, A.E.; Cui, X. Evaluation of MCF10A as a Reliable Model for Normal Human Mammary Epithelial Cells. PLoS ONE 2015, 10, e0131285. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent Sensors for Zn2+ Based on a Fluorescein Platform: Synthesis, Properties and Intracellular Distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Kasarskis, E.J.; Ringo, D.; Frederickson, R.E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J. Neurosci. Methods 1987, 20, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.R.; Zhou, Z.-L.; Qian, W.-J.; Kennedy, R. Detection and Imaging of Zinc Secretion from Pancreatic β-Cells Using a New Fluorescent Zinc Indicator. J. Am. Chem. Soc. 2002, 124, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Duman, A.; Keil, C.; Schwerdtle, T.; Haase, H. The impact of apical and basolateral albumin on intestinal zinc resorption in the Caco-2/HT-29-MTX co-culture model. Metallomics 2018, 10, 979–991. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Pallasdies, L.; Schmacht, M.; Senz, M.; Nissen, J.; Kieserling, H.; Drusch, S.; Haase, H. Zinc availability from zinc-enriched yeast studied with an in vitro digestion/Caco-2 cell culture model. J. Trace Elem. Med. Biol. 2022, 71, 126934. [Google Scholar] [CrossRef]
- Alker, W.; Haase, H. Comparison of Free Zinc Levels Determined by Fluorescent Probes in THP1 Cells Using Microplate Reader and Flow Cytometer. Biol. Trace Elem. Res. 2020, 199, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Tomasiewicz, H.; Nowakowski, A.; Petering, D.H. TSQ (6-methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 2011, 50, 7563–7573. [Google Scholar] [CrossRef]
- Alam, S.; Kelleher, S.L. Cellular Mechanisms of Zinc Dysregulation: A Perspective on Zinc Homeostasis as an Etiological Factor in the Development and Progression of Breast Cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M. The LIV-1 Subfamily of Zinc Transporters: From Origins to Present Day Discoveries. Int. J. Mol. Sci. 2023, 24, 1255. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.C.; Lo, M.N.; Palmer, A.E. Techniques for measuring cellular zinc. Arch. Biochem. Biophys. 2016, 611, 20–29. [Google Scholar] [CrossRef]
- Huang, Z.; Lippard, S.J. Illuminating mobile zinc with fluorescence from cuvettes to live cells and tissues. Methods Enzym. 2012, 505, 445–468. [Google Scholar] [CrossRef]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal. Biochem. 2006, 352, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Keil, C.; Koza, J.; Straubing, S.; Schwerdtle, T.; Haase, H. In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins. Int. J. Mol. Sci. 2018, 19, 2662. [Google Scholar] [CrossRef]
- Lopez, V.; Foolad, F.; Kelleher, S.L. ZnT2-overexpression represses the cytotoxic effects of zinc hyper-accumulation in malignant metallothionein-null T47D breast tumor cells. Cancer Lett. 2011, 304, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Vogel-González, M.; Musa-Afaneh, D.; Rivera Gil, P.; Vicente, R. Zinc Favors Triple-Negative Breast Cancer’s Microenvironment Modulation and Cell Plasticity. Int. J. Mol. Sci. 2021, 22, 9188. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Hiscox, S.; Nicholson, R.I.; Hogstrand, C.; Kille, P. Protein Kinase CK2 Triggers Cytosolic Zinc Signaling Pathways by Phosphorylation of Zinc Channel ZIP7. Sci. Signal. 2012, 5, ra11. [Google Scholar] [CrossRef]
- Jones, S.; Farr, G.; Nimmanon, T.; Ziliotto, S.; Gee, J.M.W.; Taylor, K.M. The importance of targeting signalling mechanisms of the SLC39A family of zinc transporters to inhibit endocrine resistant breast cancer. Explor. Target Antitumor. Ther. 2022, 3, 224–239. [Google Scholar] [CrossRef]
- Mia, R.; Cragg, P.J.; Wallace, K.J. Low Molecular Weight Fluorescent probes for the detection of organophosphates. J. Lumin. 2021, 235, 118053. [Google Scholar] [CrossRef]
- Costanzo, V.; D’Apolito, L.; Sardella, D.; Iervolino, A.; La Manna, G.; Capasso, G.; Frische, S.; Trepiccione, F. Single nephron glomerular filtration rate measured by linescan multiphoton microscopy compared to conventional micropuncture. Pflügers Arch. —Eur. J. Physiol. 2022, 474, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Engbjerg, J.S.; Costanzo, V.; Sardella, D.; Bordoni, L.; Jakobsen, S.; D’Apolito, L.; Frøkiær, J.; Trepiccione, F.; Capasso, G.; Frische, S. The Probe for Renal Organic Cation Secretion (4-Dimethylaminostyryl)-N-Methylpyridinium (ASP+)) Shows Amplified Fluorescence by Binding to Albumin and Is Accumulated In Vivo. Mol. Imaging 2022, 2022, 7908357. [Google Scholar] [CrossRef]
- Han, Y.; Goldberg, J.M.; Lippard, S.J.; Palmer, A.E. Superiority of SpiroZin2 Versus FluoZin-3 for monitoring vesicular Zn2+ allows tracking of lysosomal Zn2+ pools. Sci. Rep. 2018, 8, 15034. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J.; et al. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. 2010, 235, 741–750. [Google Scholar] [CrossRef]
- Pratt, E.P.S.; Damon, L.J.; Anson, K.J.; Palmer, A.E. Tools and techniques for illuminating the cell biology of zinc. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2021, 1868, 118865. [Google Scholar] [CrossRef]
- Kochańczyk, T.; Drozd, A.; Krężel, A. Relationship between the architecture of zinc coordination and zinc binding affinity in proteins—Insights into zinc regulation. Metallomics 2015, 7, 244–257. [Google Scholar] [CrossRef]
- Hwang, J.J.; Kim, H.N.; Kim, J.; Cho, D.H.; Kim, M.J.; Kim, Y.S.; Kim, Y.; Park, S.J.; Koh, J.Y. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals 2010, 23, 997–1013. [Google Scholar] [CrossRef]
- Lo, M.N.; Damon, L.J.; Wei Tay, J.; Jia, S.; Palmer, A.E. Single cell analysis reveals multiple requirements for zinc in the mammalian cell cycle. Elife 2020, 9, e51107. [Google Scholar] [CrossRef]
- Hessels, A.M.; Taylor, K.M.; Merkx, M. Monitoring cytosolic and ER Zn2+ in stimulated breast cancer cells using genetically encoded FRET sensors. Metallomics 2016, 8, 211–217. [Google Scholar] [CrossRef]
- McCormick, N.; Velasquez, V.; Finney, L.; Vogt, S.; Kelleher, S.L. X-ray fluorescence microscopy reveals accumulation and secretion of discrete intracellular zinc pools in the lactating mouse mammary gland. PLoS ONE 2010, 5, e11078. [Google Scholar] [CrossRef]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35–43. [Google Scholar] [PubMed]
- Xu, H.; Zhu, C.; Chen, Y.; Bai, Y.; Han, Z.; Yao, S.; Jiao, Y.; Yuan, H.; He, W.; Guo, Z. A FRET-based fluorescent Zn2+ sensor: 3D ratiometric imaging, flow cytometric tracking and cisplatin-induced Zn2+ fluctuation monitoring. Chem. Sci. 2020, 11, 11037–11041. [Google Scholar] [CrossRef] [PubMed]
- Vaden, R.M.; Guillen, K.P.; Salvant, J.M.; Santiago, C.B.; Gibbons, J.B.; Pathi, S.S.; Arunachalam, S.; Sigman, M.S.; Looper, R.E.; Welm, B.E. A Cancer-Selective Zinc Ionophore Inspired by the Natural Product Naamidine A. ACS Chem. Biol. 2019, 14, 106–117. [Google Scholar] [CrossRef]
- Fang, L.; Watkinson, M. Subcellular localised small molecule fluorescent probes to image mobile Zn2+. Chem. Sci. 2020, 11, 11366–11379. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kowada, T.; Du, Y.; Amagai, Y.; Matsui, T.; Inaba, K.; Mizukami, S. Organelle-Level Labile Zn2+ Mapping Based on Targetable Fluorescent Sensors. ACS Sens. 2022, 7, 748–757. [Google Scholar] [CrossRef]
- Yue, D.; Wang, M.; Deng, F.; Yin, W.; Zhao, H.; Zhao, X.; Xu, Z. Biomarker-targeted fluorescent probes for breast cancer imaging. Chin. Chem. Lett. 2018, 29, 648–656. [Google Scholar] [CrossRef]

| Cell Line | Primary Tumor/Cell Type | Receptor Expression | Classification | ||
|---|---|---|---|---|---|
| Estrogen | Progesterone | HER2 Amplified | |||
| MCF7 | Invasive ductal carcinoma | + | + | − | Luminal A |
| T47D | Invasive ductal carcinoma | + | + | − | Luminal A |
| MDA-MB-231 | Adenocarcinoma | − | − | − | Basal |
| MCF10A | Mammary epithelial cells | − | − | − | Non-tumor mammary cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hübner, C.; Keil, C.; Jürgensen, A.; Barthel, L.; Haase, H. Comparison of Three Low-Molecular-Weight Fluorescent Probes for Measuring Free Zinc Levels in Cultured Mammary Cells. Nutrients 2023, 15, 1873. https://doi.org/10.3390/nu15081873
Hübner C, Keil C, Jürgensen A, Barthel L, Haase H. Comparison of Three Low-Molecular-Weight Fluorescent Probes for Measuring Free Zinc Levels in Cultured Mammary Cells. Nutrients. 2023; 15(8):1873. https://doi.org/10.3390/nu15081873
Chicago/Turabian StyleHübner, Christopher, Claudia Keil, Anton Jürgensen, Lars Barthel, and Hajo Haase. 2023. "Comparison of Three Low-Molecular-Weight Fluorescent Probes for Measuring Free Zinc Levels in Cultured Mammary Cells" Nutrients 15, no. 8: 1873. https://doi.org/10.3390/nu15081873
APA StyleHübner, C., Keil, C., Jürgensen, A., Barthel, L., & Haase, H. (2023). Comparison of Three Low-Molecular-Weight Fluorescent Probes for Measuring Free Zinc Levels in Cultured Mammary Cells. Nutrients, 15(8), 1873. https://doi.org/10.3390/nu15081873







