Lifestyle Intervention Randomized Controlled Trial for Age-Related Macular Degeneration (AMD-Life): Study Design
Abstract
1. Introduction
2. Materials and Methods
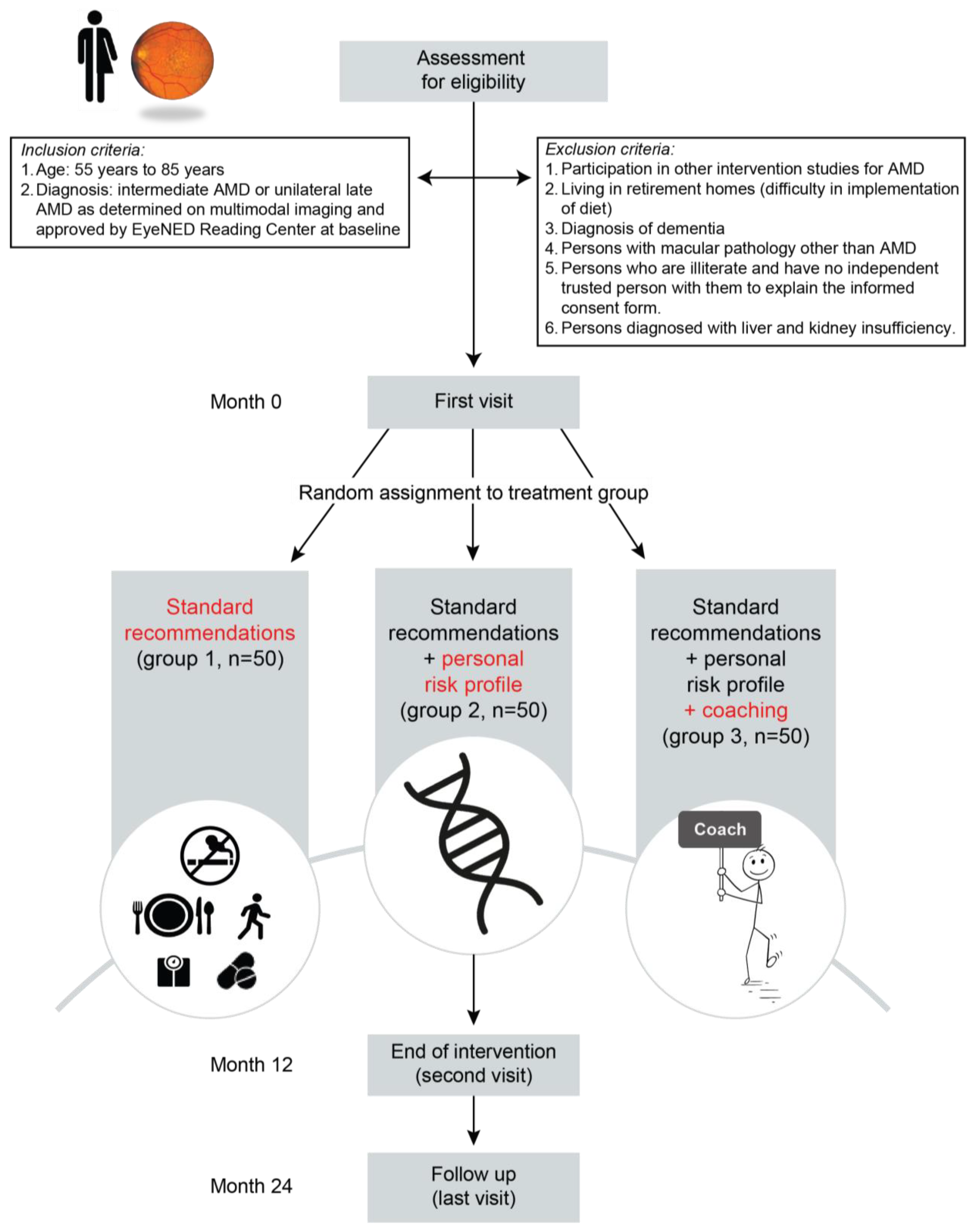
2.1. Study Design and Population
2.2. Interventions
2.2.1. Group A
2.2.2. Group B
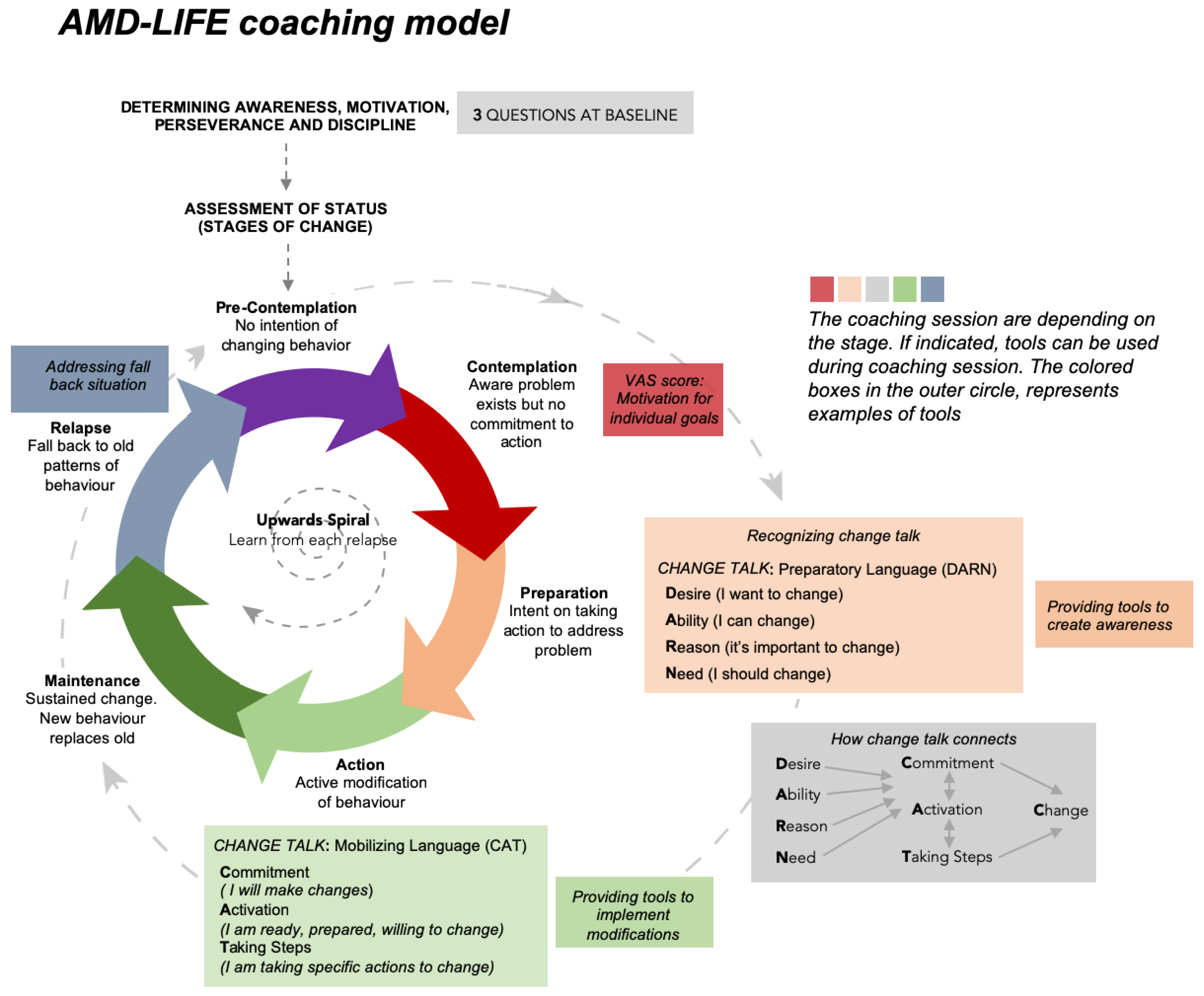
2.2.3. Group C
2.3. Avoiding Attrition Bias
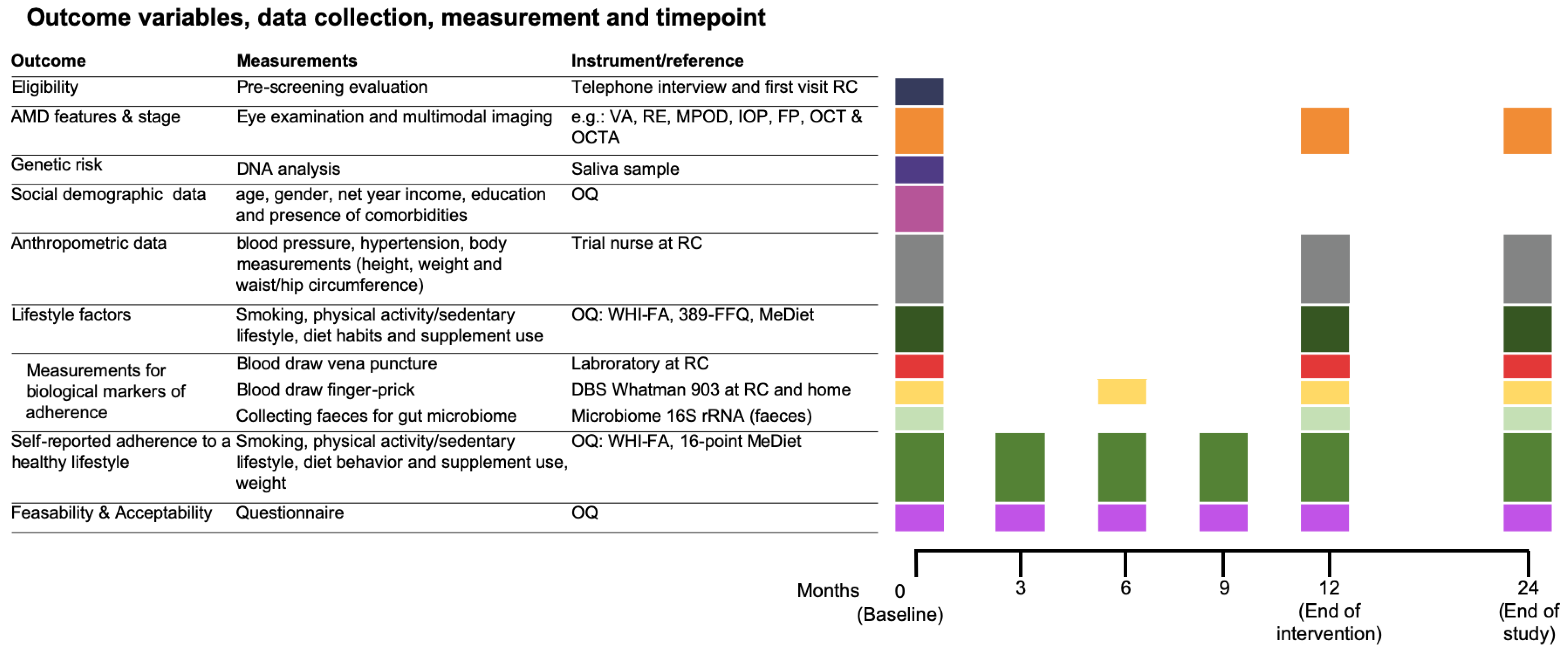
2.4. Data Collection and Measurement
2.4.1. Ophthalmologic Examination
2.4.2. Genetic Factors
2.4.3. Covariates
Social Demographics
Anthropometric Data
2.4.4. Biochemical Measurements
Vena Puncture
Finger-Prick
Collecting Feces
2.4.5. Lifestyle Factors
Smoking
Dietary Assessment and Supplement Use
Physical Activity
Feasibility and Acceptability
Assessment of Change in Habitual Behavior
3. Study Outcomes
3.1. Primary Study Outcome
3.2. Secondary Study Endpoints of AMD-Life
Other Study Parameters
3.3. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Owen, C.G.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.E.; Rudnicka, A.R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, R.; Klaver, C.C.; Vingerling, J.R.; Hofman, A.; de Jong, P.T. The risk and natural course of age-related maculopathy: Follow-up at 6 1/2 years in the Rotterdam study. Arch. Ophthalmol. 2003, 121, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Colijn, J.M.; Meester-Smoor, M.; Verzijden, T.; de Breuk, A.; Silva, R.; Merle, B.M.J.; Cougnard-Grégoire, A.; Hoyng, C.B.; Fauser, S.; Coolen, A.; et al. Genetic Risk, Lifestyle, and Age-Related Macular Degeneration in Europe: The EYE-RISK Consortium. Ophthalmology 2021, 128, 1039–1049. [Google Scholar] [CrossRef]
- Geerlings, M.J.; de Jong, E.K.; den Hollander, A.I. The complement system in age-related macular degeneration: A review of rare genetic variants and implications for personalized treatment. Mol. Immunol. 2017, 84, 65–76. [Google Scholar] [CrossRef]
- Corominas, J.; Colijn, J.M.; Geerlings, M.J.; Pauper, M.; Bakker, B.; Amin, N.; Lores Motta, L.; Kersten, E.; Garanto, A.; Verlouw, J.A.M.; et al. Whole-Exome Sequencing in Age-Related Macular Degeneration Identifies Rare Variants in COL8A1, a Component of Bruch’s Membrane. Ophthalmology 2018, 125, 1433–1443. [Google Scholar] [CrossRef]
- Huan, T.; Cheng, S.Y.; Tian, B.; Punzo, C.; Lin, H.; Daly, M.; Seddon, J.M. Identifying Novel Genes and Variants in Immune and Coagulation Pathways Associated with Macular Degeneration. Ophthalmol. Sci. 2023, 3, 100206. [Google Scholar] [CrossRef]
- Vingerling, J.R.; Hofman, A.; Grobbee, D.E.; de Jong, P.T. Age-related macular degeneration and smoking. The Rotterdam Study. Arch. Ophthalmol. 1996, 114, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Augood, C.; Bentham, G.C.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Vingerling, J.R.; et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology 2007, 114, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Weeks, D.E.; Conley, Y.P.; Tsai, H.J.; Mah, T.S.; Schmidt, S.; Postel, E.A.; Agarwal, A.; Haines, J.L.; Pericak-Vance, M.A.; Rosenfeld, P.J.; et al. Age-related maculopathy: A genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am. J. Hum. Genet. 2004, 75, 174–189. [Google Scholar] [CrossRef]
- Seddon, J.M.; Francis, P.J.; George, S.; Schultz, D.W.; Rosner, B.; Klein, M.L. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. Jama 2007, 297, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- de Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of Vegetables, Fruit, and Fish is Beneficial for Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Grégoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Féart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef]
- McGuinness, M.B.; Le, J.; Mitchell, P.; Gopinath, B.; Cerin, E.; Saksens, N.T.M.; Schick, T.; Hoyng, C.B.; Guymer, R.H.; Finger, R.P. Physical Activity and Age-related Macular Degeneration: A Systematic Literature Review and Meta-analysis. Am. J. Ophthalmol. 2017, 180, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Buaud, B.; Korobelnik, J.F.; Bron, A.; Delyfer, M.N.; Rougier, M.B.; Savel, H.; Vaysse, C.; Creuzot-Garcher, C.; Delcourt, C. Plasma long-chain omega-3 polyunsaturated fatty acids and macular pigment in subjects with family history of age-related macular degeneration: The Limpia Study. Acta Ophthalmol. 2017, 95, e763–e769. [Google Scholar] [CrossRef] [PubMed]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The Nutritional AMD Treatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations: Food-Based Dietary Guidelines. Available online: http://www.fao.org/nutrition/education/food-based-dietary-guidelines/regions/countries (accessed on 1 October 2022).
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ (Clin. Res. Ed.) 1995, 311, 1457–1460. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. New Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Organisation, W.H. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 1 October 2022).
- CBS and RIVM. Gezondheidsenquette/Leefstijlmonitor. Available online: https://www.cbs.nl/nl-nl/nieuws/2015/17/nederland-eet-onvoldoende-groente-fruit-en-vis (accessed on 1 January 2016).
- Laurenti, G. 1961–2003 Fish and Fishery Products: World Apparent Consumption Statistics Based on Food Balance Sheets; FAO Fisheries Circular. No. 821, Rev. 8; FAO: Rome, Italy, 2007; p. 429. [Google Scholar]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): Design implications. AREDS report no. 1. Control. Clin. Trials 1999, 20, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; Blodi, B.A.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; McClintic, S.M.; Nadeem, U.; Skondra, D. A Review of the Role of the Intestinal Microbiota in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 2072. [Google Scholar] [CrossRef]
- Hochstetler, B.S.; Scott, I.U.; Kunselman, A.R.; Thompson, K.; Zerfoss, E. Adherence to recommendations of the age-related eye disease study in patients with age-related macular degeneration. Retina 2010, 30, 1166–1170. [Google Scholar] [CrossRef]
- Ng, W.T.; Goggin, M. Awareness of and compliance with recommended dietary supplement among age-related macular degeneration patients. Clin. Exp. Ophthalmol. 2006, 34, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.U.; Pilli, S.; Telander, D.G.; Morse, L.S.; Park, S.S. Reprint of: Survey of patients with age-related macular degeneration: Knowledge and adherence to recommendations. Can. J. Ophthalmol. 2015, 50 (Suppl. S1), S23–S28. [Google Scholar] [CrossRef]
- Sahu, A.; Edwards, R.; Harrison, R.A.; Thornton, J.; Kelly, S.P. Attitudes and behaviour of ophthalmologists to smoking cessation. Eye 2008, 22, 246–250. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Evans, J.R. Advice about diet and smoking for people with or at risk of age-related macular degeneration: A cross-sectional survey of eye care professionals in the UK. BMC Public Health 2013, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Clark, E.C.; Lawson, P.J.; Casucci, B.A.; Flocke, S.A. Identifying teachable moments for health behavior counseling in primary care. Patient Educ. Couns. 2011, 85, e8–e15. [Google Scholar] [CrossRef]
- Brust, M.; Gebhardt, W.A.; Numans, M.E.; Kiefte-de Jong, J.C. Teachable moments: The right moment to make patients change their lifestyle. Ned. Tijdschr. Geneeskd. 2020, 164, D4835. [Google Scholar] [PubMed]
- Kwasnicka, D.; Dombrowski, S.U.; White, M.; Sniehotta, F. Theoretical explanations for maintenance of behaviour change: A systematic review of behaviour theories. Health Psychol. Rev. 2016, 10, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Diclemente, C.C. Toward a Comprehensive Model of Change. In Treating Addictive Behaviors: Processes of Change; Miller, W.R., Heather, N., Eds.; Springer: Boston, MA, USA, 1986; pp. 3–27. [Google Scholar]
- Miller, W.R.; Rollnick, S. Motivational Interviewing, Third Edition: Helping People Change, 3rd ed.; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- DiClemente, C.; Velasquez, M. Motivational interviewing and the stages of change. In Motivational Interviewing: Preparing People for Change; Miller, W., Rollnick, S., Eds.; Guilford Press: New York, NY, USA, 2002; pp. 201–216. [Google Scholar]
- Klein, R.; Davis, M.D.; Magli, Y.L.; Segal, P.; Klein, B.E.; Hubbard, L. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991, 98, 1128–1134. [Google Scholar] [CrossRef]
- EyeNED Reading Center. Available online: https://www.eyened.nl/ (accessed on 1 January 2023).
- Gattoussi, S.; Buitendijk, G.H.S.; Peto, T.; Leung, I.; Schmitz-Valckenberg, S.; Oishi, A.; Wolf, S.; Deák, G.; Delcourt, C.; Klaver, C.C.W.; et al. The European Eye Epidemiology spectral-domain optical coherence tomography classification of macular diseases for epidemiological studies. Acta Ophthalmol. 2019, 97, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Fallaize, R.; Livingstone, K.M.; Celis-Morales, C.; Macready, A.L.; San-Cristobal, R.; Navas-Carretero, S.; Marsaux, C.F.M.; O’Donovan, C.B.; Kolossa, S.; Moschonis, G.; et al. Association between Diet-Quality Scores, Adiposity, Total Cholesterol and Markers of Nutritional Status in European Adults: Findings from the Food4Me Study. Nutrients 2018, 10, 49. [Google Scholar] [CrossRef]
- Sakhi, A.K.; Bastani, N.E.; Ellingjord-Dale, M.; Gundersen, T.E.; Blomhoff, R.; Ursin, G. Feasibility of self-sampled dried blood spot and saliva samples sent by mail in a population-based study. BMC Cancer 2015, 15, 265. [Google Scholar] [CrossRef]
- Goldbohm, R.A.; van den Brandt, P.A.; Brants, H.A.; van’t Veer, P.; Al, M.; Sturmans, F.; Hermus, R.J. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J. Clin. Nutr. 1994, 48, 253–265. [Google Scholar] [PubMed]
- van Lee, L.; Geelen, A.; van Huysduynen, E.J.; de Vries, J.H.; van’t Veer, P.; Feskens, E.J. The Dutch Healthy Diet index (DHD-index): An instrument to measure adherence to the Dutch Guidelines for a Healthy Diet. Nutr. J. 2012, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- NEVO-Tabel; Dutch Food Composition Database 2011, R.V., Den Haag. 2011. Available online: https://nevo-online.rivm.nl/Home/En (accessed on 1 October 2022).
- Klipstein-Grobusch, K.; den Breeijen, J.H.; Goldbohm, R.A.; Geleijnse, J.M.; Hofman, A.; Grobbee, D.E.; Witteman, J.C. Dietary assessment in the elderly: Validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1998, 52, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Fernández-Jarne, E.; Serrano-Martínez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Evenson, K.R.; Morimoto, L.; Siscovick, D.; White, E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med. Sci. Sports Exerc. 2009, 41, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Efird, J. Blocked randomization with randomly selected block sizes. Int. J. Environ. Res. Public Health 2011, 8, 15–20. [Google Scholar] [CrossRef]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef]
- Lundahl, B.W.; Kunz, C.; Brownell, C.; Tollefson, D.; Burke, B.L. A Meta-Analysis of Motivational Interviewing: Twenty-Five Years of Empirical Studies. Res. Soc. Work. Pract. 2010, 20, 137–160. [Google Scholar] [CrossRef]
- Gray, E.; McCambridge, J.; Strang, J. The effectiveness of motivational interviewing delivered by youth workers in reducing drinking, cigarette and cannabis smoking among young people: Quasi-experimental pilot study. Alcohol Alcohol. 2005, 40, 535–539. [Google Scholar] [CrossRef]
- Fortier, M.S.; Duda, J.L.; Guerin, E.; Teixeira, P.J. Promoting physical activity: Development and testing of self-determination theory-based interventions. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Frost; Sullivan. The Economic Benefits of Using Lutein and Zeaxanthin Food Supplements in the European Union. Available online: https://www.frost.com/frost-perspectives/the-economic-benefits-of-using-lutein-and-zeaxanthin-food-supplements-in-the-european-union/ (accessed on 1 October 2022).
| Criteria for | Included in | |||
|---|---|---|---|---|
| Questions | 0.5 point | 1 Point | LF Scoring | |
| 1 | Do you use olive oil as the main culinary fat? | Yes | √ | |
| 2 | How much olive oil do you consume per day (incl. oil used for frying, salads, out-of-house meals, etc.)? | 2–3 tbsp | ≥4 tbsp | √ |
| 3 | How many grams of vegetable (incl. raw or as a salad) do you consume per day? | 200–400 g | ≥400 g | √ |
| 4 | How many fruit units (incl. natural fruit juices) do you consume per day? (units of 80 g each) | 2 | ≥3 | √ |
| 5 | How often do you eat meat? | <1 p/d | ||
| 6 | If you consume meat, what kind of meat do you consume most of the time? Do you preferentially consume lean meat: chicken, turkey, or rabbit meat, instead of red meat: veal, pork, hamburger, or sausage? | Both equally often | lean meat | √ |
| 7 | How many servings of meat do you consume per week? | <100–150 g | √ | |
| 8 | How many servings of butter, margarine, or cream do you consume per day? (1 serving: 12 g) | <12 g | √ | |
| 9 | How many sweetened and/or carbonated beverages do you drink per day? | <1 gl | √ | |
| 10 | How many glasses of red wine do you consume per week? (1 glass = 124 mL) | ≤3 gl | ||
| 11 | How many grams of legumes do you consume per week? (450 g per week = 65 g per day) | 300–450 g | ≥450 g | √ |
| 12 | How many servings of fish or shellfish do you consume per week? (1 fish = 100–150 g or 4–5 units of shellfish = 200 g) | 150–300 g | ≥300 g | √ |
| 13 | How often per week do you consume fatty fish? (Salmon, tuna, herring, eel, sardines, mackerel) | 1 p/w | ||
| 14 | How many times a week do you eat sweets or pastries such as cakes, cookies, and biscuits? | <3 | √ | |
| 15 | How many servings of nuts (including peanuts) do you consume per week? (1 serving = 30 g = about a handful) | 30–89 g | ≥90 g | √ |
| 16 | How many times per week do you consume vegetables, pasta, rice, or other dishes seasoned with sofrito (sauce made with tomato and onion, leek, or garlic and simmered with olive oil)? | 1 | ≥2 | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Koning-Backus, A.P.M.; Kiefte-de Jong, J.C.; van Rooij, J.G.J.; AMD-Life Team; Uitterlinden, A.G.; Voortman, T.G.; Meester-Smoor, M.A.; Klaver, C.C.W. Lifestyle Intervention Randomized Controlled Trial for Age-Related Macular Degeneration (AMD-Life): Study Design. Nutrients 2023, 15, 602. https://doi.org/10.3390/nu15030602
de Koning-Backus APM, Kiefte-de Jong JC, van Rooij JGJ, AMD-Life Team, Uitterlinden AG, Voortman TG, Meester-Smoor MA, Klaver CCW. Lifestyle Intervention Randomized Controlled Trial for Age-Related Macular Degeneration (AMD-Life): Study Design. Nutrients. 2023; 15(3):602. https://doi.org/10.3390/nu15030602
Chicago/Turabian Stylede Koning-Backus, Alexandra P. M., Jessica C. Kiefte-de Jong, Jeroen G. J. van Rooij, AMD-Life Team, André G. Uitterlinden, Trudy G. Voortman, Magda A. Meester-Smoor, and Caroline C. W. Klaver. 2023. "Lifestyle Intervention Randomized Controlled Trial for Age-Related Macular Degeneration (AMD-Life): Study Design" Nutrients 15, no. 3: 602. https://doi.org/10.3390/nu15030602
APA Stylede Koning-Backus, A. P. M., Kiefte-de Jong, J. C., van Rooij, J. G. J., AMD-Life Team, Uitterlinden, A. G., Voortman, T. G., Meester-Smoor, M. A., & Klaver, C. C. W. (2023). Lifestyle Intervention Randomized Controlled Trial for Age-Related Macular Degeneration (AMD-Life): Study Design. Nutrients, 15(3), 602. https://doi.org/10.3390/nu15030602







