Dietary Intake, Biological Status, and Barriers towards Omega-3 Intake in Elite Level (Tier 4), Female Athletes: Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Omega-3 Questionnaire
2.4. Omega-3 Biological Status
2.5. Statistical Analysis
3. Results
3.1. Participants
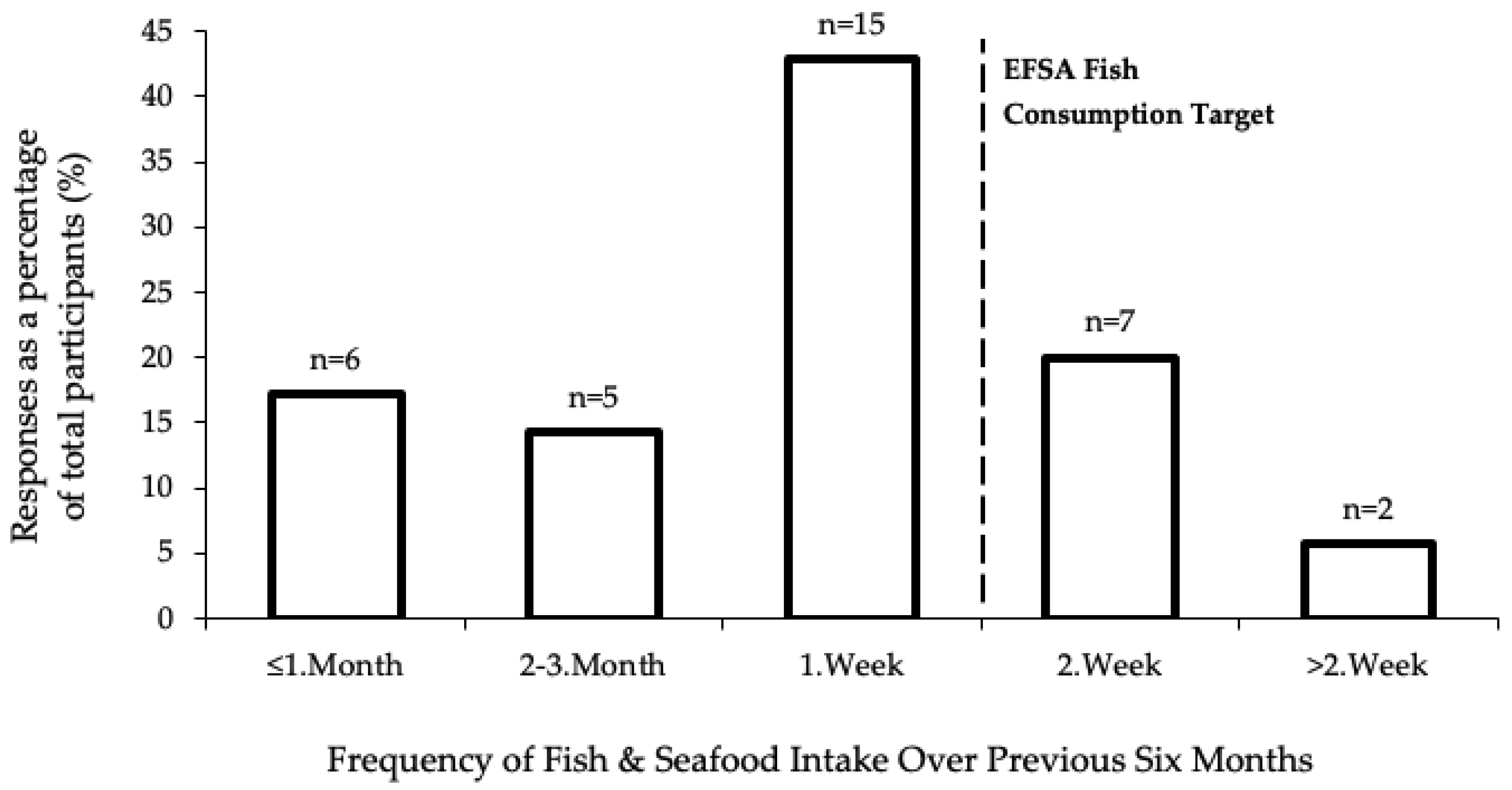
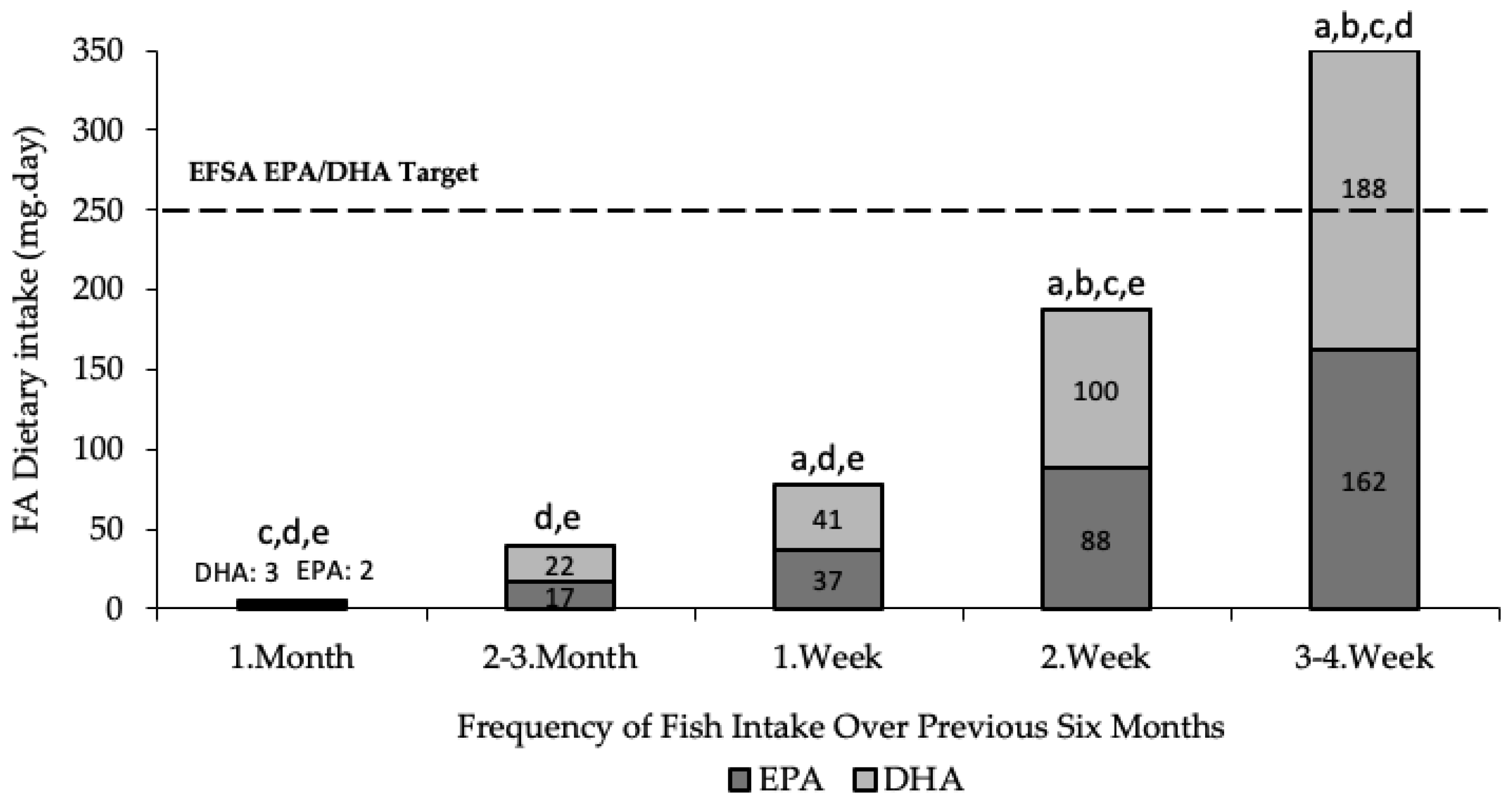
3.2. Diet
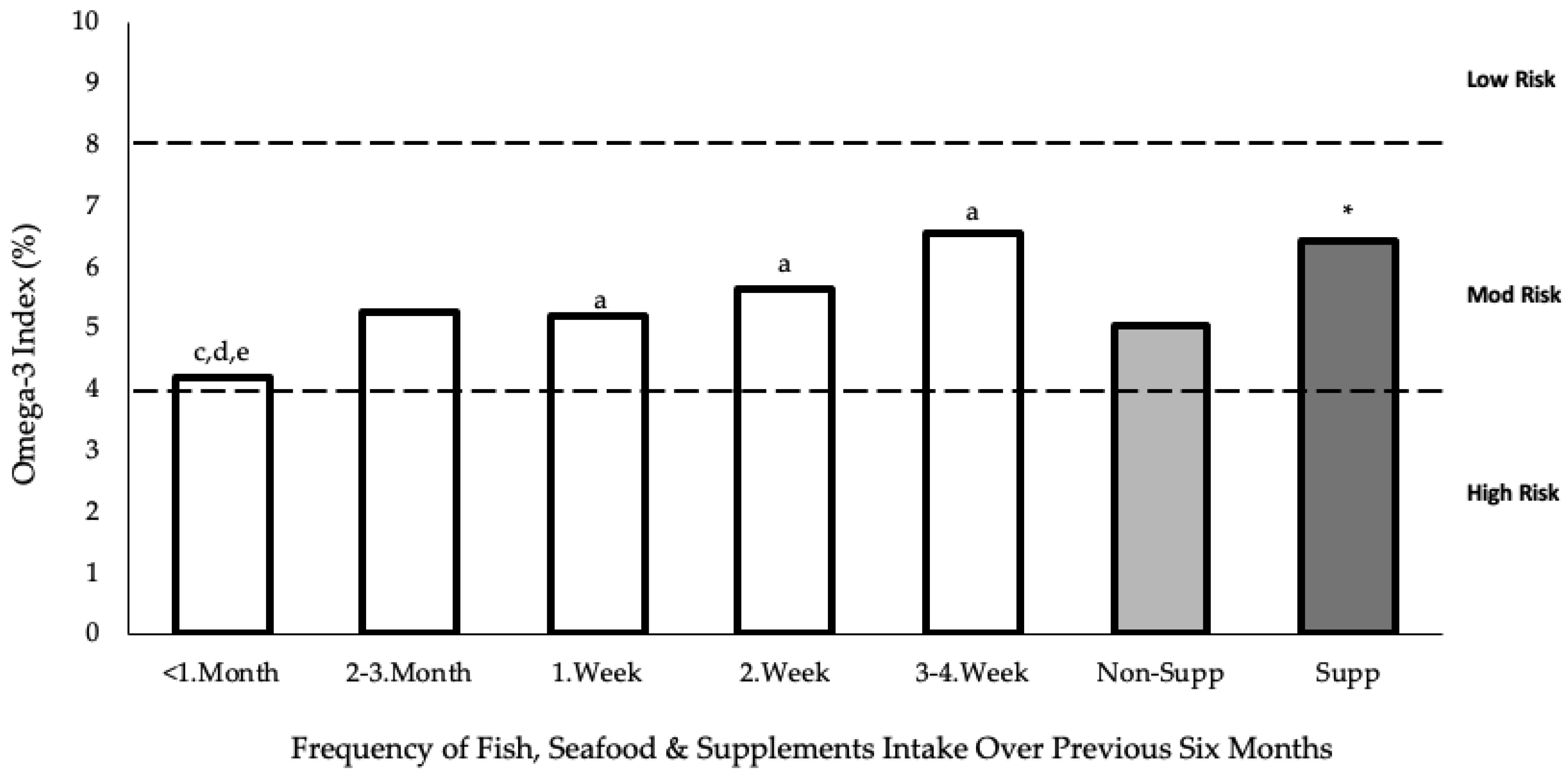
3.3. Blood
4. Discussion
4.1. Diet
4.2. Blood FA Profile
4.3. Barriers
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fink, J.S. Female athletes, women’s sport, and the sport media commercial complex: Have we really “come a long way, baby”? Sport Manag. Rev. 2013, 18, 331–342. [Google Scholar] [CrossRef]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex differences and considerations for female specific nutritional strategies: A narrative review. J. Int. Soc. Sports Nutr. 2021, 18, 27. [Google Scholar] [CrossRef]
- Hottenrott, L.; Ketelhut, S.; Schneider, C.; Wiewelhove, T.; Ferrauti, A. Age- and Sex-Related Differences in Recovery from High-Intensity and Endurance Exercise: A Brief Review. Int. J. Sports Physiol. Perform. 2021, 16, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Bosch, A.N. The Effect of Gender and Menstrual Phase on Serum Creatine Kinase Activity and Muscle Soreness Following Downhill Running. Antioxidants 2017, 6, 16. [Google Scholar] [CrossRef]
- Hackney, A.C.; Kallman, A.L.; Ağgön, E. Female sex hormones and the recovery from exercise: Menstrual cycle phase affects responses. Biomed. Hum. Kinet. 2019, 11, 87–89. [Google Scholar] [CrossRef]
- Bengtsson, H.; Ekstrand, J.; Hägglund, M. Muscle injury rates in professional football increase with fixture congestion: An 11-year follow-up of the UEFA Champions League injury study. Br. J. Sports Med. 2013, 47, 743–747. [Google Scholar] [CrossRef]
- Montgomery, P.G.; Pyne, D.; Hopkins, W.G.; Dorman, J.C.; Cook, K.; Minahan, C. The effect of recovery strategies on physical performance and cumulative fatigue in competitive basketball. J. Sports Sci. 2008, 26, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83 (Suppl. 6), 1467S. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar]
- Harris, W.S.; von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef]
- Armstrong, A.; Anzalone, A.J.; Pethick, W.; Murray, H.; Dahlquist, D.T.; Askow, A.T.; Heileson, J.L.; Hillyer, L.M.; Ma, D.W.L.; Oliver, J.M. An Evaluation of Omega-3 Status and Intake in Canadian Elite Rugby 7s Players. Nutrients 2021, 13, 3777. [Google Scholar] [CrossRef]
- Drobnic, F.; Rueda, F.; Pons, V.; Banquells, M.; Cordobilla, B.; Domingo, J.C. Erythrocyte Omega-3 Fatty Acid Content in Elite Athletes in Response to Omega-3 Supplementation: A Dose-Response Pilot Study. J. Lipids 2017, 2017, 1472719. [Google Scholar] [CrossRef]
- Ritz, P.P.; Rogers, M.B.; Zabinsky, J.S.; Hedrick, V.E.; Rockwell, J.A.; Rimer, E.G.; Kostelnik, S.B.; Hulver, M.W.; Rockwell, M.S. Dietary and Biological Assessment of the Omega-3 Status of Collegiate Athletes: A Cross-Sectional Analysis. PLoS ONE 2020, 15, e0228834. [Google Scholar] [CrossRef]
- von Schacky, C.; Kemper, M.; Haslbauer, R.; Halle, M. Low Omega-3 Index in 106 German elite winter endurance athletes: A pilot study. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Harris, W.S.; Pottala, J.V. Clinical Investigation: Determinants of Blood Cell Omega-3 Fatty Acid Content. Open Biomark. J. 2008, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Etherton, T.D.; Fleming, J.A.; Kris-Etherton, P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose–response randomized controlled trial. J. Am. Heart Assoc. 2013, 2, e000513. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Martin, D.T.; Telford, R.; Ballas, S.K. Greater erythrocyte deformability in world-class endurance athletes. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H2188–H2193. [Google Scholar] [CrossRef]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Herter-Aeberli, I.; Graf, C.; Vollenweider, A.; Häberling, I.; Srikanthan, P.; Hersberger, M.; Berger, G.; Mathis, D. Validation of a Food Frequency Questionnaire to Assess Intake of n-3 Polyunsaturated Fatty Acids in Switzerland. Nutrients 2019, 11, 1863. [Google Scholar] [CrossRef]
- Li, K.; McNulty, B.A.; Tiernery, A.M.; Devlin, N.F.C.; Joyce, T.; Leite, J.C.; Flynn, A.; Walton, J.; Brennan, J.; Gibney, M.J.; et al. Dietary fat intakes in Irish adults in 2011: How much has changed in 10 years? Br. J. Nutr. 2016, 115, 1798–1809. [Google Scholar] [CrossRef]
- Irish Universities Nutrition Alliance. National Adult Nutrition Survey; Irish Universities Nutrition Alliance: Irish, UK, 2011. [Google Scholar]
- Public Health England. McCance and Widdowson’s Composition of Foods Integrated Dataset; PHE: London, UK, 2021. [Google Scholar]
- Govzman, S.; Looby, S.; Wang, X.; Butler, F.; Gibney, E.R.; Timon, C.M. A systematic review of the determinants of seafood consumption. Br. J. Nutr. 2021, 126, 66–80. [Google Scholar] [CrossRef]
- Harris, W.S.; Polreis, J. Measurement of the Omega-3 Index in Dried Blood Spots. Ann. Clin. Lab. Res. 2016, 4, 137. [Google Scholar] [CrossRef]
- National Institutes of Health. Omega-3 Fatty Acids. 2022. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer/ (accessed on 5 May 2022).
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the European Parliament Related to the Safety Assessment of Wild and Farmed Fish. EFSA J. 2005, 1–118. [Google Scholar] [CrossRef]
- Schlechtriem, C.; Bron, E.J.; Tocher, D.R. Inter-individual variation in total fatty acid compositions of flesh of Atlantic salmon smolts-fed diets containing fish oil or vegetable oil. Aquac. Res. 2007, 38, 1045–1055. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.M.; Dehghan Nayeri, N.; Mashhadi, M.; Varaei, S. Effect of omega-3 fatty acids on premenstrual syndrome: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2022, 48, 1293–1305. [Google Scholar] [CrossRef]
- Buckley, J.D.; Burgess, S.; Murphy, K.J.; Howe, P.R. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J. Sci. Med. Sport 2009, 12, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D. Omega-3 Polyunsaturated Fatty Acids in Physical Performance Optimization. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Pottala, J.V.; Yaffe, K.; Robinson, J.G.; Espeland, M.A.; Wallace, R.; Harris, W.S. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI Study. Neurology 2014, 82, 435–442. [Google Scholar] [CrossRef] [PubMed]
- von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Stanton, A.V.; James, K.; Brennan, M.M.; O’donovan, F.; Buskandar, F.; Shortall, K.; El-Sayed, T.; Kennedy, J.; Hayes, H.; Fahey, A.G.; et al. Omega-3 index and blood pressure responses to eating foods naturally enriched with omega-3 polyunsaturated fatty acids: A randomized controlled trial. Sci. Rep. 2020, 10, 15444. [Google Scholar] [CrossRef]
- Thuppal, S.V.; Von Schacky, C.; Harris, W.S.; Sherif, K.D.; Denby, N.; Steinbaum, S.R.; Haycock, B.; Bailey, R.L. Discrepancy between Knowledge and Perceptions of Dietary Omega-3 Fatty Acid Intake Compared with the Omega-3 Index. Nutrients 2017, 9, 930. [Google Scholar] [CrossRef]
- McDonnell, S.L.; French, C.B.; Baggerly, C.A.; Harris, W.S. Cross-sectional study of the combined associations of dietary and supplemental eicosapentaenoic acid + docosahexaenoic acid on Omega-3 Index. Nutr. Res. 2019, 71, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Hirota, S.; Hirayama, T.; Adachi, N.; Hagiwara, C.; Iwama, N.; Kamachi, K.; Araki, E.; Kawashima, H.; Kiso, Y. Age-related changes of dietary intake and blood eicosapentaenoic acid, docosahexaenoic acid, and arachidonic acid levels in Japanese men and women. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Reksten, A.M.; Ho, Q.T.; Nøstbakken, O.J.; Markhus, M.W.; Kjellevold, M.; Bøkevoll, A.; Hannisdal, R.; Frøyland, L.; Madsen, L.; Dahl, L. Temporal variations in the nutrient content of Norwegian farmed Atlantic salmon (Salmo salar), 2005–2020. Food Chem. 2022, 373 Pt B, 131445. [Google Scholar] [CrossRef]
- Jackson, K.; Polreis, J.; Tintle, N.; Kris-Etherton, P.; Harris, W. Association of reported fish intake and supplementation status with the omega-3 index. Prostaglandins Leukot. Essent. Fat. Acids 2019, 142, 4–10. [Google Scholar] [CrossRef]
- Kohler, A.; Bittner, D.; Low, A.; von Schacky, C. Effects of a convenience drink fortified with n-3 fatty acids on the n-3 index. Br. J. Nutr. 2010, 104, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, F.; Spence, M.; Hollywood, L.; McGowan, L.; Surgenor, D.; McCloat, A.; Mooney, E.; Caraher, M.; Raats, M.; Dean, M. Learning cooking skills at different ages: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.R.; Mitchell, N.; Sutton, L.; Backhouse, S.H. Sports nutritionists’ perspectives on enablers and barriers to nutritional adherence in high performance sport: A qualitative analysis informed by the COM-B model and theoretical domains framework. J. Sports Sci. 2019, 37, 2075–2085. [Google Scholar] [CrossRef]
- Close, G.L.; Kasper, A.M.; Walsh, N.P.; Maughan, R.J. “Food First but Not Always Food Only”: Recommendations for Using Dietary Supplements in Sport. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 371–386. [Google Scholar] [CrossRef]
- Lust, C.A.; Burns, J.; Jones, M.T.; Smith, S.B.; Choi, S.H.; Krk, M.; Gable, D.A.; Oliver, J.M.; Ma, D.W. The Dose-Response Effect of Docosahexaenoic Acid on the Omega-3 Index in American Football Athletes. Med. Sci. Sports Exerc. 2023, 55, 865–872. [Google Scholar] [CrossRef]
- Tomczyk, M.; Jost, Z.; Chroboczek, M.; Urbański, R.; Calder, P.C.; Fisk, H.L.; Sprengel, M.; Antosiewicz, J. Effects of 12 Wk of Omega-3 Fatty Acid Supplementation in Long-Distance Runners. Med. Sci. Sports Exerc. 2023, 55, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mathews, N.M. Prohibited Contaminants in Dietary Supplements. Sports Health 2018, 10, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, A.C.; Cladis, D.P.; Santerre, C.R. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J. Sci. Food Agric. 2015, 95, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Derraik, J.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci. Rep. 2015, 5, 7928. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Harris, W.S.; Vors, C.; Charest, A.; Marin, J.; Jackson, K.H.; Tchernof, A.; Couture, P.; Lamarche, B. Supplementation with high-dose docosahexaenoic acid increases the Omega-3 Index more than high-dose eicosapentaenoic acid. Prostaglandins, Leukot. Essent. Fat. Acids 2017, 120, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; Jackson, K.H.; Tintle, N.L.; Shearer, G.C.; Bernasconi, A.; Masson, S.; Latini, R.; Heydari, B.; Kwong, R.Y.; Flock, M.; et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 2019, 110, 1034–1040. [Google Scholar] [CrossRef]
- Wilson, P.B.; Madrigal, L.A. Associations Between Whole Blood and Dietary Omega-3 Polyunsaturated Fatty Acid Levels in Collegiate Athletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 497–505. [Google Scholar] [CrossRef]
- McCartney, D.M.A.; Younger, K.M.; Walsh, J.; O’Neill, M.; Sheridan, C.; Kearney, J.M. Socio-economic differences in food group and nutrient intakes among young women in Ireland. Br. J. Nutr. 2013, 110, 2084–2097. [Google Scholar] [CrossRef]
- Heikkinen, A.; Alaranta, A.; Helenius, I.; Vasankari, T. Use of dietary supplements in Olympic athletes is decreasing: A follow-up study between 2002 and 2009. J. Int. Soc. Sports Nutr. 2011, 8, 1. [Google Scholar] [CrossRef]
| Blood Sample (% w/w) | Total Intake (g·day) | Spearman’s Rho | ||
|---|---|---|---|---|
| ALA | 0.44 ±0.15 | 0.81 ± 1.28 | r = 0.31 | |
| EPA ** | 0.66 ± 0.25 | 0.15 ± 0.29 | r = 0.60 | |
| DHA ** | 2.73 ± 0.62 | 0.10 ± 0.15 | r = 0.59 | |
| O3I | EPA + DHA ** | 5.19 ± 0.86 | 0.25 ± 0.44 | r = 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooks, M.P.; Madigan, S.M.; Woodside, J.V.; Nugent, A.P. Dietary Intake, Biological Status, and Barriers towards Omega-3 Intake in Elite Level (Tier 4), Female Athletes: Pilot Study. Nutrients 2023, 15, 2821. https://doi.org/10.3390/nu15132821
Hooks MP, Madigan SM, Woodside JV, Nugent AP. Dietary Intake, Biological Status, and Barriers towards Omega-3 Intake in Elite Level (Tier 4), Female Athletes: Pilot Study. Nutrients. 2023; 15(13):2821. https://doi.org/10.3390/nu15132821
Chicago/Turabian StyleHooks, Matthew P., Sharon M. Madigan, Jayne V. Woodside, and Anne P. Nugent. 2023. "Dietary Intake, Biological Status, and Barriers towards Omega-3 Intake in Elite Level (Tier 4), Female Athletes: Pilot Study" Nutrients 15, no. 13: 2821. https://doi.org/10.3390/nu15132821
APA StyleHooks, M. P., Madigan, S. M., Woodside, J. V., & Nugent, A. P. (2023). Dietary Intake, Biological Status, and Barriers towards Omega-3 Intake in Elite Level (Tier 4), Female Athletes: Pilot Study. Nutrients, 15(13), 2821. https://doi.org/10.3390/nu15132821







