A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Declaration
2.2. Pharmacokinetic Experiment
2.3. Survival Experiment
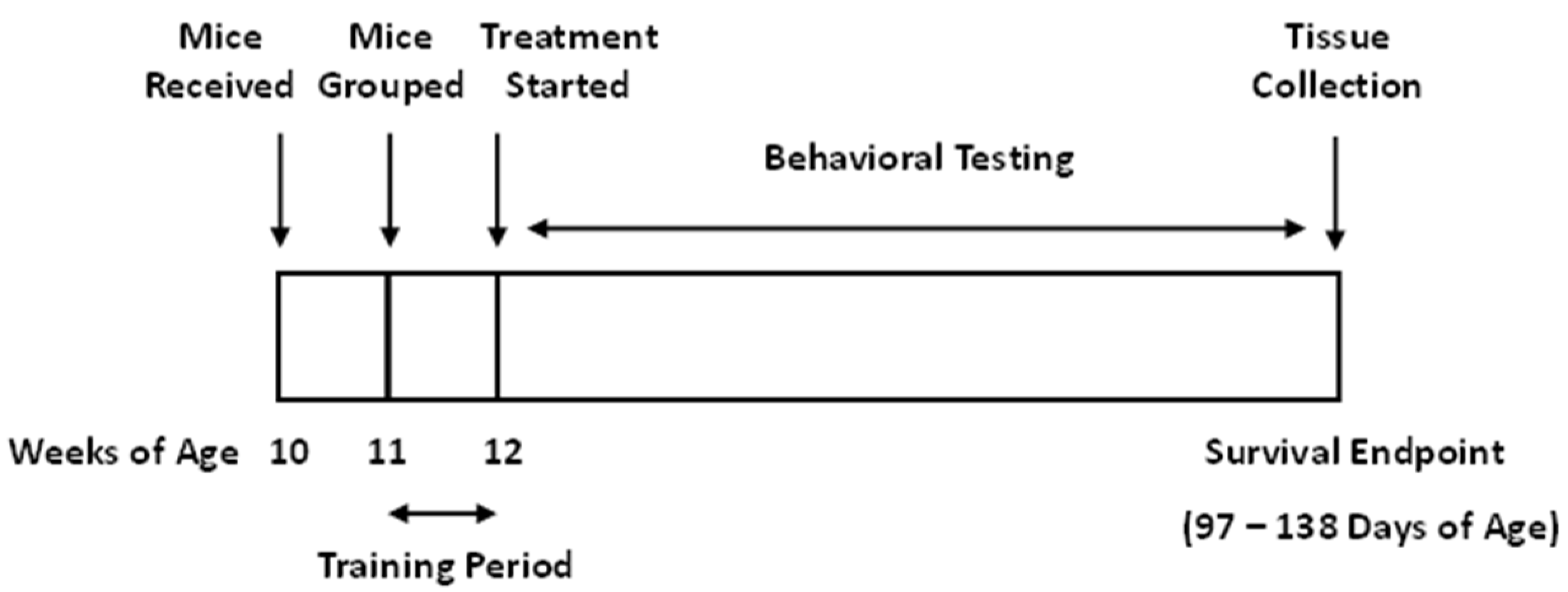
2.3.1. Animals
2.3.2. Treatment Groups and Diets
2.3.3. Assessment of Motor Function
2.3.4. Assessment of Neurological Function
2.3.5. Survival Endpoint
2.4. Statistical Analysis
3. Results
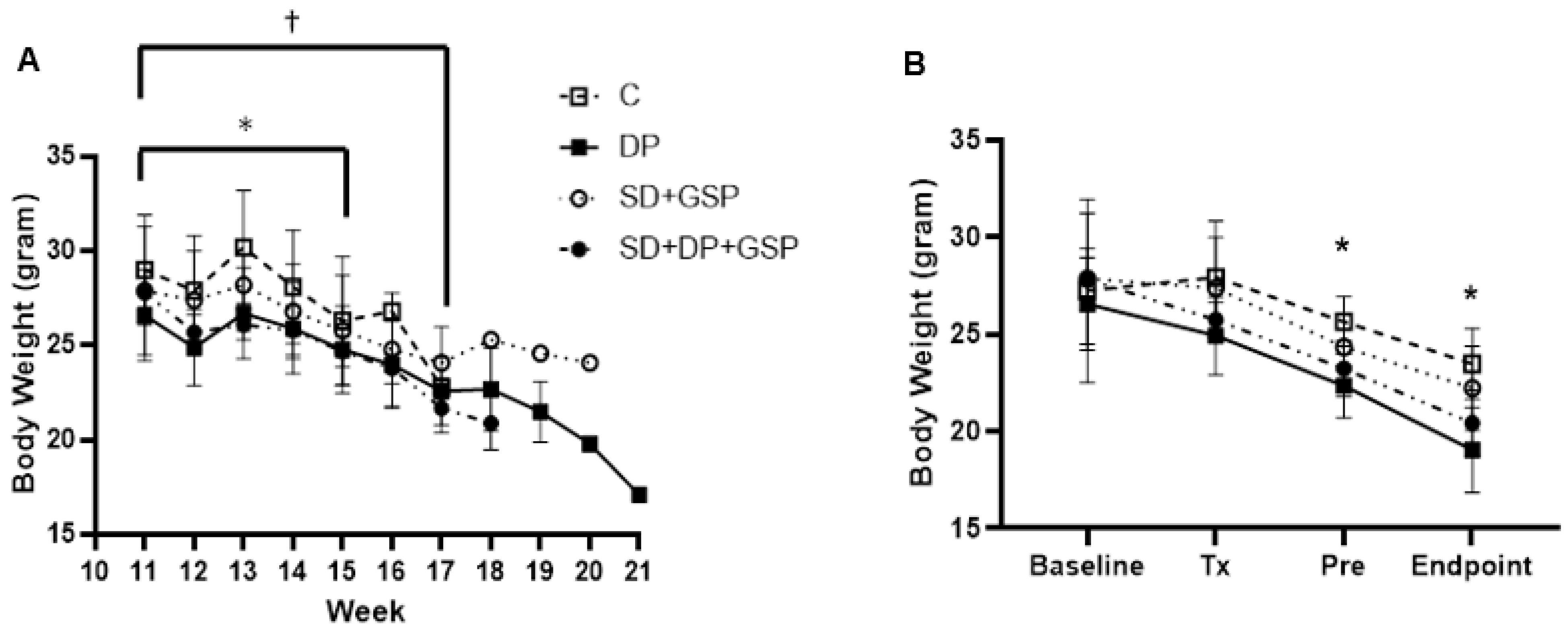
3.1. Effect of Treatment on Body and Organ Weight
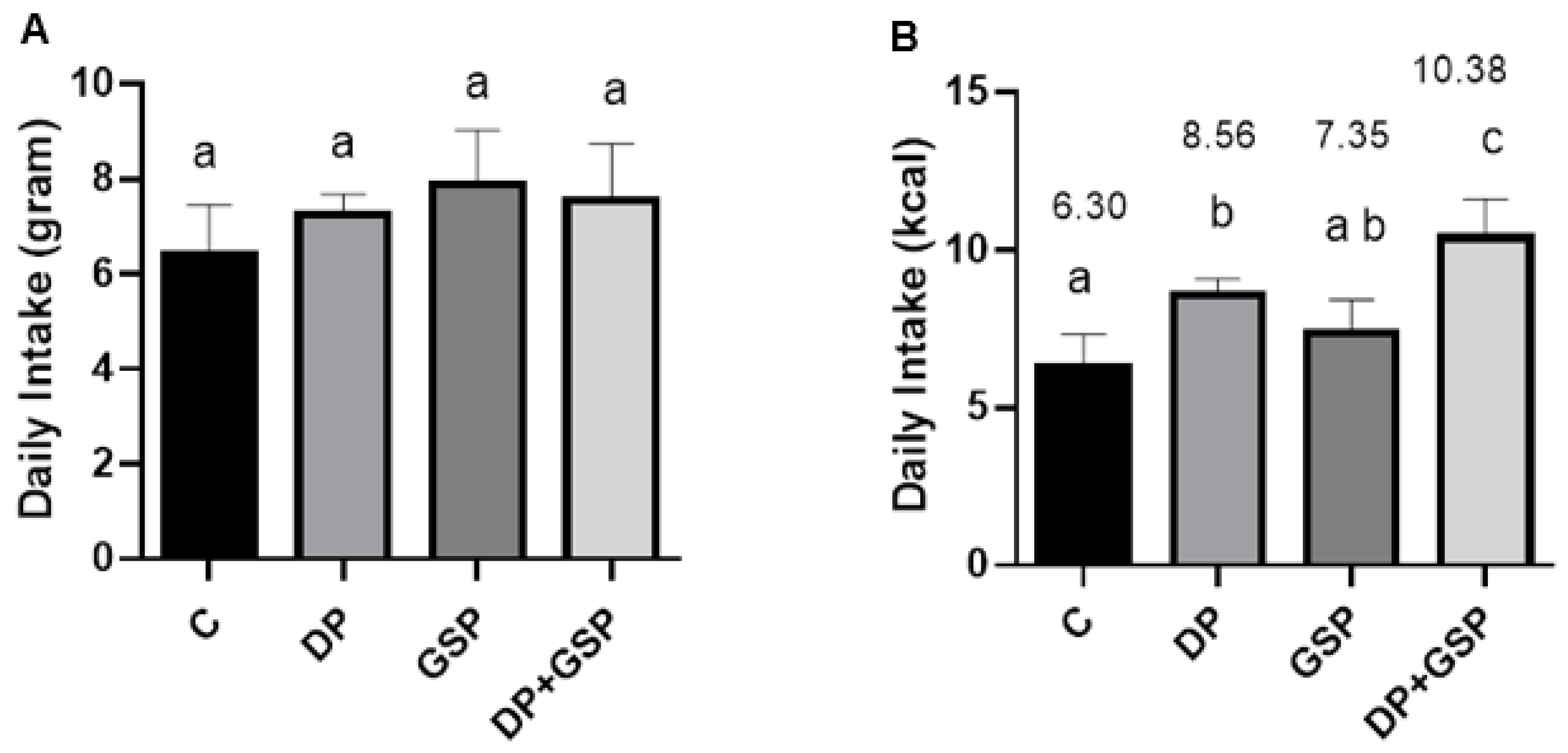
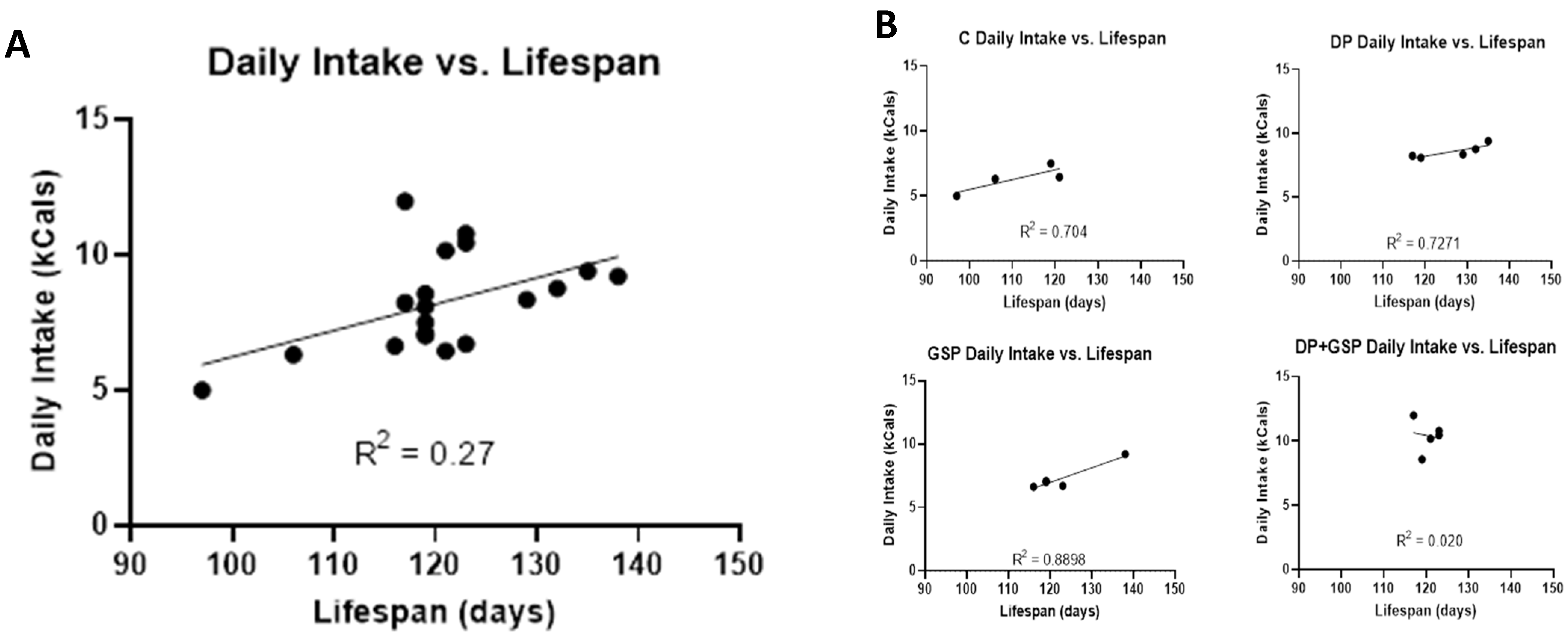
3.2. Effect of Treatment on Food Consumption
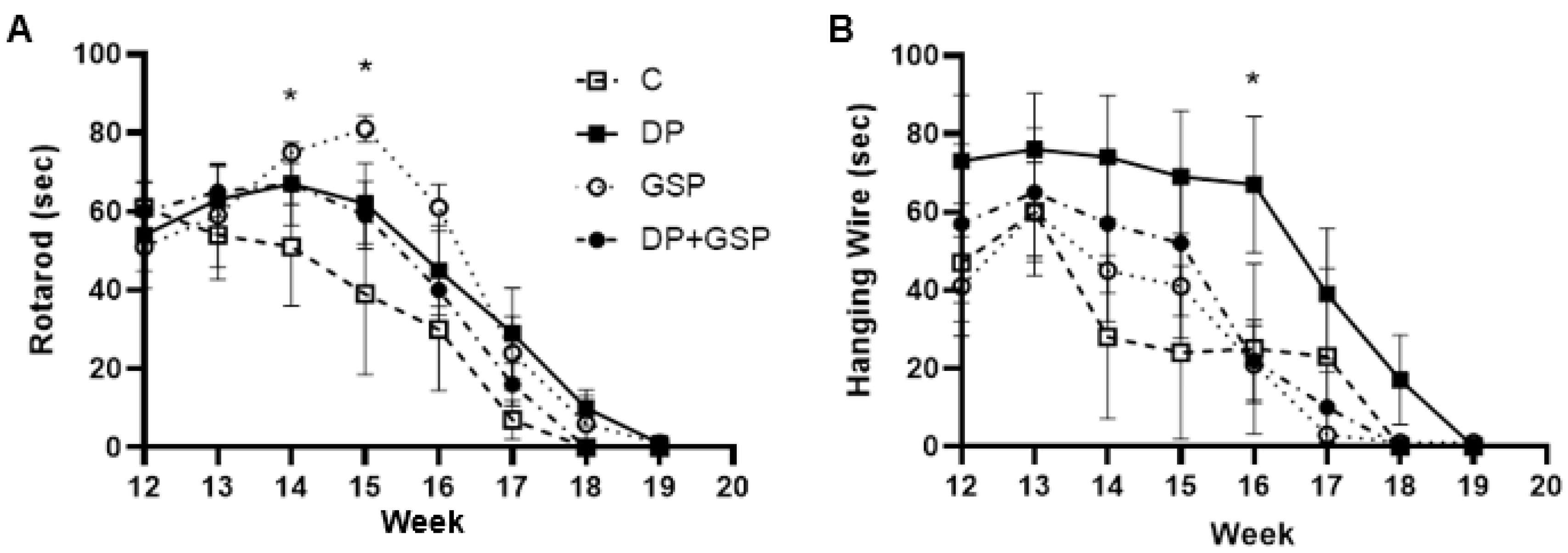
3.3. Effect of Treatment on Motor Function
3.4. Effect of Treatment on Neurological Function
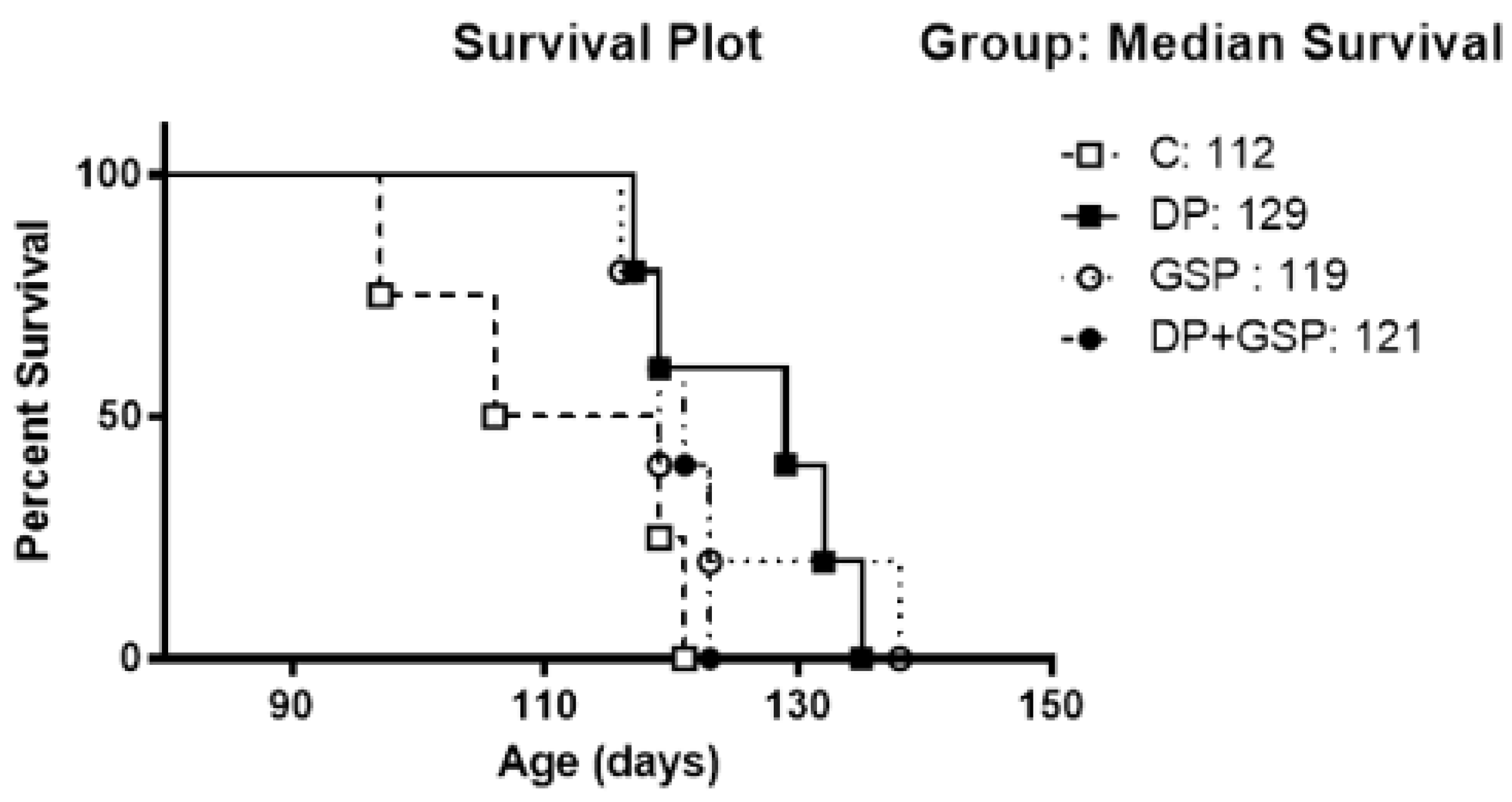
3.5. Effect of Treatment on Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talbott, E.O.; Malek, A.M.; Lacomis, D. The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 2016, 138, 225–238. [Google Scholar] [CrossRef]
- Ngo, S.T.; Mi, J.D.; Henderson, R.D.; McCombe, P.A.; Steyn, F.J. Exploring targets and therapies for amyotrophic lateral sclerosis: Current insights into dietary interventions. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, B.; Horton, D.K.; Mitsumoto, H. Potential Environmental Factors in Amyotrophic Lateral Sclerosis. Neurol. Clin. 2015, 33, 877–888. [Google Scholar] [CrossRef]
- Halperin, J.J.; Kaplan, G.P.; Brazinsky, S.; Tsai, T.F.; Cheng, T.; Ironside, A.; Wu, P.; Delfiner, J.; Golightly, M.; Brown, R.H.; et al. Immunologic reactivity against Borrelia burgdorferi in patients with motor neuron disease. Arch. Neurol. 1990, 47, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.C.; Feuer, R.; Cashman, N.; Luo, H. Enteroviral Infection: The Forgotten Link to Amyotrophic Lateral Sclerosis? Front. Mol. Neurosci. 2018, 11, 63. [Google Scholar] [CrossRef]
- Ruffo, P.; Perrone, B.; Conforti, F.L. SOD-1 Variants in Amyotrophic Lateral Sclerosis: Systematic Re-Evaluation According to ACMG-AMP Guidelines. Genes 2022, 13, 537. [Google Scholar] [CrossRef]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C.A. Animal models of amyotrophic lateral sclerosis: A comparison of model validity. Neural. Regen. Res. 2018, 13, 2050–2054. [Google Scholar] [CrossRef]
- Bruijn, L.I.; Miller, T.M.; Cleveland, D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004, 27, 723–749. [Google Scholar] [CrossRef]
- Pansarasa, O.; Bordoni, M.; Diamanti, L.; Sproviero, D.; Gagliardi, S.; Cereda, C. SOD1 in Amyotrophic Lateral Sclerosis: "Ambivalent" Behavior Connected to the Disease. Int. J. Mol. Sci. 2018, 19, 1345. [Google Scholar] [CrossRef]
- Boillee, S.; Vande Velde, C.; Cleveland, D.W. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron 2006, 52, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Bouchard, J.P.; Duquette, P.; Eisen, A.; Gelinas, D.; Harati, Y.; Munsat, T.L.; Powe, L.; Rothstein, J.; Salzman, P.; et al. Clinical trials of riluzole in patients with ALS. ALS/Riluzole Study Group-II. Neurology 1996, 47, S86–S90, discussion S90–S92. [Google Scholar] [CrossRef]
- Doble, A. The pharmacology and mechanism of action of riluzole. Neurology 1996, 47, S233–S241. [Google Scholar] [CrossRef]
- Koh, J.Y.; Kim, D.K.; Hwang, J.Y.; Kim, Y.H.; Seo, J.H. Antioxidative and proapoptotic effects of riluzole on cultured cortical neurons. J. Neurochem. 1999, 72, 716–723. [Google Scholar] [CrossRef]
- Leibowitz, A.; Boyko, M.; Shapira, Y.; Zlotnik, A. Blood glutamate scavenging: Insight into neuroprotection. Int. J. Mol. Sci. 2012, 13, 10041–10066. [Google Scholar] [CrossRef]
- Boyko, M.; Stepensky, D.; Gruenbaum, B.F.; Gruenbaum, S.E.; Melamed, I.; Ohayon, S.; Glazer, M.; Shapira, Y.; Zlotnik, A. Pharmacokinetics of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase and their blood glutamate-lowering activity in naive rats. Neurochem. Res. 2012, 37, 2198–2205. [Google Scholar] [CrossRef]
- Perez-Mato, M.; Ramos-Cabrer, P.; Sobrino, T.; Blanco, M.; Ruban, A.; Mirelman, D.; Menendez, P.; Castillo, J.; Campos, F. Human recombinant glutamate oxaloacetate transaminase 1 (GOT1) supplemented with oxaloacetate induces a protective effect after cerebral ischemia. Cell Death Dis. 2014, 5, e992. [Google Scholar] [CrossRef]
- Ruban, A.; Berkutzki, T.; Cooper, I.; Mohar, B.; Teichberg, V.I. Blood glutamate scavengers prolong the survival of rats and mice with brain-implanted gliomas. Invest. New Drugs. 2012, 30, 2226–2235. [Google Scholar] [CrossRef]
- Ruban, A.; Malina, K.C.; Cooper, I.; Graubardt, N.; Babakin, L.; Jona, G.; Teichberg, V.I. Combined Treatment of an Amyotrophic Lateral Sclerosis Rat Model with Recombinant GOT1 and Oxaloacetic Acid: A Novel Neuroprotective Treatment. Neurodegener. Dis. 2015, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; Cash, A.; Hamadani, L.; Diemer, T. Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell 2009, 8, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Poff, A.M.; Held, H.E.; Landon, C.S.; Goldhagen, C.R.; Mavromates, N.; D’Agostino, D.P. Metabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS ONE 2014, 9, e103526. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Canfield, C.E.; Copes, N.; Poff, A.M.; Fiorelli, T.N.; Landon, C.S.; Goldhagen, C.R.; Mavromates, N.; D’Agostino, D.P. Biochemical alterations in Amyotrophic Lateral Sclerosis (ALS) Mouse Model resulted from the Deanna Protocol Supplement Complex. Metabolomics 2017, 13, 55. [Google Scholar] [CrossRef]
- Lavado, A.; Guo, X.; Smith, A.S.; Akanda, N.; Martin, C.; Cai, Y.; Elbrecht, D.; Tran, M.; Bryant, J.P.; Colon, A.; et al. Evaluation of Holistic Treatment for ALS Reveals Possible Mechanism and Therapeutic Potential. Int. J. Pharm. Pharm. Res. 2017, 11, 348–374. [Google Scholar]
- Ludolph, A.C.; Bendotti, C.; Blaugrund, E.; Chio, A.; Greensmith, L.; Loeffler, J.P.; Mead, R.; Niessen, H.G.; Petri, S.; Pradat, P.F.; et al. Guidelines for preclinical animal research in ALS/MND: A consensus meeting. Amyotroph. Lateral. Scler. 2010, 11, 38–45. [Google Scholar] [CrossRef]
- Desport, J.C.; Preux, P.M.; Truong, T.C.; Vallat, J.M.; Sautereau, D.; Couratier, P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology 1999, 53, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.; Pradat, P.F.; Ludolph, A.C.; Loeffler, J.P. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011, 10, 75–82. [Google Scholar] [CrossRef]
- Crivello, M.; Hogg, M.C.; Jirstrom, E.; Halang, L.; Woods, I.; Rayner, M.; Coughlan, K.S.; Lewandowski, S.A.; Prehn, J.H.M. Vascular regression precedes motor neuron loss in the FUS (1-359) ALS mouse model. Dis. Model. Mech. 2019, 12, dmm040238. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, H.; Kowall, N.W. Motor neuronal protection by L-arginine prolongs survival of mutant SOD1 (G93A) ALS mice. Biochem. Biophys. Res. Commun. 2009, 384, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, I.; Stuehr, D.J.; Gross, S.S.; Nathan, C.; Levi, R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc. Natl. Acad. Sci. USA 1988, 85, 8664–8667. [Google Scholar] [CrossRef]
- Boger, R.H.; Bode-Boger, S.M.; Thiele, W.; Creutzig, A.; Alexander, K.; Frolich, J.C. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J. Am. Coll. Cardiol. 1998, 32, 1336–1344. [Google Scholar] [CrossRef]
- Morris, C.R.; Kuypers, F.A.; Lavrisha, L.; Ansari, M.; Sweeters, N.; Stewart, M.; Gildengorin, G.; Neumayr, L.; Vichinsky, E.P. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 2013, 98, 1375–1382. [Google Scholar] [CrossRef]
- Andrade, F.; Rodriguez-Soriano, J.; Prieto, J.A.; Elorz, J.; Aguirre, M.; Ariceta, G.; Martin, S.; Sanjurjo, P.; Aldamiz-Echevarria, L. The arginine-creatine pathway is disturbed in children and adolescents with renal transplants. Pediatr. Res. 2008, 64, 218–222. [Google Scholar] [CrossRef]
- Vandoorne, T.; De Bock, K.; Van Den Bosch, L. Energy metabolism in ALS: An underappreciated opportunity? Acta Neuropathol. 2018, 135, 489–509. [Google Scholar] [CrossRef]
- Dupuis, L.; Oudart, H.; Rene, F.; Gonzalez de Aguilar, J.L.; Loeffler, J.P. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: Benefit of a high-energy diet in a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2004, 101, 11159–11164. [Google Scholar] [CrossRef]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef]
- Crugnola, V.; Lamperti, C.; Lucchini, V.; Ronchi, D.; Peverelli, L.; Prelle, A.; Sciacco, M.; Bordoni, A.; Fassone, E.; Fortunato, F.; et al. Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Yang, L.; Browne, S.; Baik, M.; Beal, M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA 1998, 95, 8892–8897. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Thompson, J.L.; Levy, G.; Buchsbaum, R.; Shefner, J.; Krivickas, L.S.; Katz, J.; Rollins, Y.; Barohn, R.J.; Jackson, C.E.; et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 2009, 66, 235–244. [Google Scholar] [CrossRef]
- Lucchetti, J.; Marino, M.; Papa, S.; Tortarolo, M.; Guiso, G.; Pozzi, S.; Bonetto, V.; Caccia, S.; Beghi, E.; Bendotti, C.; et al. A mouse model of familial ALS has increased CNS levels of endogenous ubiquinol9/10 and does not benefit from exogenous administration of ubiquinol10. PLoS ONE 2013, 8, e69540. [Google Scholar] [CrossRef]
- de Carvalho, M.; Kiernan, M.C.; Swash, M. Fasciculation in amyotrophic lateral sclerosis: Origin and pathophysiological relevance. J. Neurol. Neurosurg. Psychiatry 2017, 88, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lapin, I. Phenibut (beta-phenyl-GABA): A tranquilizer and nootropic drug. CNS Drug Rev. 2001, 7, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers. Arch. 2010, 460, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Gruenbaum, S.E.; Gruenbaum, B.F.; Shapira, Y.; Zlotnik, A. Brain to blood glutamate scavenging as a novel therapeutic modality: A review. J. Neural. Transm. 2014, 121, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Mohar, B.; Jona, G.; Teichberg, V.I. Blood glutamate scavenging as a novel neuroprotective treatment for paraoxon intoxication. J. Cereb. Blood Flow Metab. 2014, 34, 221–227. [Google Scholar] [CrossRef]
- Group, A.U.; Fournier, C.; Bedlack, B.; Hardiman, O.; Heiman-Patterson, T.; Gutmann, L.; Bromberg, M.; Ostrow, L.; Carter, G.; Kabashi, E.; et al. ALS Untangled No. 20: The Deanna protocol. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2013, 14, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Harris, J.L.; Carl, S.M.; E, L.; Lu, J.; Eva Selfridge, J.; Roy, N.; Hutfles, L.; Koppel, S.; Morris, J.; et al. Oxaloacetate activates brain mitochondrial biogenesis, enhances the insulin pathway, reduces inflammation and stimulates neurogenesis. Hum. Mol. Genet. 2014, 23, 6528–6541. [Google Scholar] [CrossRef]
- Yamamoto, H.A.; Mohanan, P.V. Effect of alpha-ketoglutarate and oxaloacetate on brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Toxicol. Lett. 2003, 143, 115–122. [Google Scholar] [CrossRef]
- Liu, S.; He, L.; Yao, K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. Biomed. Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Genton, L.; Viatte, V.; Janssens, J.P.; Heritier, A.C.; Pichard, C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clin. Nutr. 2011, 30, 553–559. [Google Scholar] [CrossRef]
- Holm, T.; Maier, A.; Wicks, P.; Lang, D.; Linke, P.; Munch, C.; Steinfurth, L.; Meyer, R.; Meyer, T. Severe loss of appetite in amyotrophic lateral sclerosis patients: Online self-assessment study. Interact. J. Med. Res. 2013, 2, e8. [Google Scholar] [CrossRef]
- Jawaid, A.; Murthy, S.B.; Wilson, A.M.; Qureshi, S.U.; Amro, M.J.; Wheaton, M.; Simpson, E.; Harati, Y.; Strutt, A.M.; York, M.K.; et al. A decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALS. Amyotroph. Lateral. Scler. 2010, 11, 542–548. [Google Scholar] [CrossRef]
- Paganoni, S.; Deng, J.; Jaffa, M.; Cudkowicz, M.E.; Wills, A.M. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011, 44, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lange, D.J.; Voustianiouk, A.; MacGrogan, D.; Ho, L.; Suh, J.; Humala, N.; Thiyagarajan, M.; Wang, J.; Pasinetti, G.M. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006, 7, 29. [Google Scholar] [CrossRef]
- Dupuis, L.; Loeffler, J.P. Neuromuscular junction destruction during amyotrophic lateral sclerosis: Insights from transgenic models. Curr. Opin. Pharmacol. 2009, 9, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, F.P.; Carrasco, M.A.; Siao, M.C.; Maniatis, T.; Eggan, K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 2007, 10, 608–614. [Google Scholar] [CrossRef]
- Nagai, M.; Re, D.B.; Nagata, T.; Chalazonitis, A.; Jessell, T.M.; Wichterle, H.; Przedborski, S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007, 10, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Chun, S.J.; Boillee, S.; Fujimori-Tonou, N.; Yamashita, H.; Gutmann, D.H.; Takahashi, R.; Misawa, H.; Cleveland, D.W. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008, 11, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolny, G.; Aucello, M.; Rizzuto, E.; Beccafico, S.; Mammucari, C.; Boncompagni, S.; Belia, S.; Wannenes, F.; Nicoletti, C.; Del Prete, Z.; et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008, 8, 425–436. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Giacinti, C.; Pelosi, L.; Nicoletti, C.; Winn, N.; Barberi, L.; Molinaro, M.; Rosenthal, N.; Musaro, A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J. Cell Biol. 2005, 168, 193–199. [Google Scholar] [CrossRef]
- Benatar, M. Lost in translation: Treatment trials in the SOD1 mouse and in human ALS. Neurobiol. Dis. 2007, 26, 1–13. [Google Scholar] [CrossRef]


| Treatment | Group Name | Sample Size |
|---|---|---|
| Control | C | 4 |
| Deanna Protocol | DP | 5 |
| Glutamate Scavenging Protocol | GSP | 5 |
| Deanna Protocol + Glutamate Scavenging Protocol | DP+GSP | 5 |
| Component | C | DP | GSP | DP+GSP |
|---|---|---|---|---|
| Std. Chow Mash | 53.8 | 42.4 | 51.1 | 40.3 |
| Water | 46.1 | 36.1 | 43.8 | 34.3 |
| Saccharin | <0.1 | <0.1 | <0.1 | <0.1 |
| AAKG | 0 | 10.2 | 0 | 9.7 |
| MCT | 0 | 10.2 | 0 | 9.7 |
| β-phenyl-GABA | 0 | 1.0 | 0 | 1.0 |
| Co-Q10 | 0 | 0.1 | 0 | 0.1 |
| Oxaloacetic Acid | 0 | 0 | 5.0 | 5.0 |
| Score | Description of Movement Behavior |
|---|---|
| 0 | Hind legs are fully extended during tail elevation |
| 1 | Hind legs are not fully extended during tail elevation |
| 1.5 | Abnormal gait |
| 2 | Toes curl under while walking |
| 2.5 | Difficulty walking but still using all four legs |
| 3 | Dragging hind legs while walking, partial leg paralysis |
| 3.5 | Single hind leg paralysis |
| 4 | Complete hind limb paralysis or unable to return to upright position within 10 s |
| Weights | C | DP | GSP | DP+GSP |
|---|---|---|---|---|
| Body (g) | 24 +/− 0.92 | 19 +/− 0.98 ** | 22 +/− 0.97 | 20 +/− 1.74 * |
| Brain (mg) | 443 +/− 23.00 | 388 +/− 14.85 | 398 +/− 10.13 | 386 +/− 14.49 |
| Heart | 138 +/− 10.90 | 130 +/− 13.61 | 142 +/− 4.36 | 137 +/− 5.97 |
| Lungs | 280 +/− 6.33 | 197 +/− 26.18 | 257 +/− 40.68 | 196 +/− 14.15 |
| Liver | 1084 +/− 70.61 | 639 +/− 224.61 * | 1138 +/− 21.14 | 1034 +/− 38.53 |
| Kidneys | 366 +/− 110.55 | 388 +/− 19.68 | 495 +/− 32.7 | 534 +/− 17.28 |
| Spleen | 93 +/− 20.43 | 70 +/− 8.21 | 68 +/− 7.94 | 65 +/− 7.66 |
| Muscle | 111 +/− 8.74 | 63 +/− 12.66 * | 90 +/− 14.25 | 84 +/− 7.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, C.Q.; Ramirez, M.; Landon, C.S.; DeBlasi, J.M.; Koutnik, A.P.; Ari, C.; D’Agostino, D.P. A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS. Nutrients 2023, 15, 1821. https://doi.org/10.3390/nu15081821
Rogers CQ, Ramirez M, Landon CS, DeBlasi JM, Koutnik AP, Ari C, D’Agostino DP. A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS. Nutrients. 2023; 15(8):1821. https://doi.org/10.3390/nu15081821
Chicago/Turabian StyleRogers, Christopher Q., Melissa Ramirez, Carol S. Landon, Janine M. DeBlasi, Andrew P. Koutnik, Csilla Ari, and Dominic P. D’Agostino. 2023. "A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS" Nutrients 15, no. 8: 1821. https://doi.org/10.3390/nu15081821
APA StyleRogers, C. Q., Ramirez, M., Landon, C. S., DeBlasi, J. M., Koutnik, A. P., Ari, C., & D’Agostino, D. P. (2023). A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS. Nutrients, 15(8), 1821. https://doi.org/10.3390/nu15081821








