Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Treatment
2.2. Probiotics Viability, Identity and Purity Testing
2.3. Animals
2.4. Wound Induction
2.5. Study Design
2.6. RNA Studies
2.6.1. Total RNA Extraction and Purification
2.6.2. cDNA Synthesis and Real-Time Reverse Transcription Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. 1st Experiment
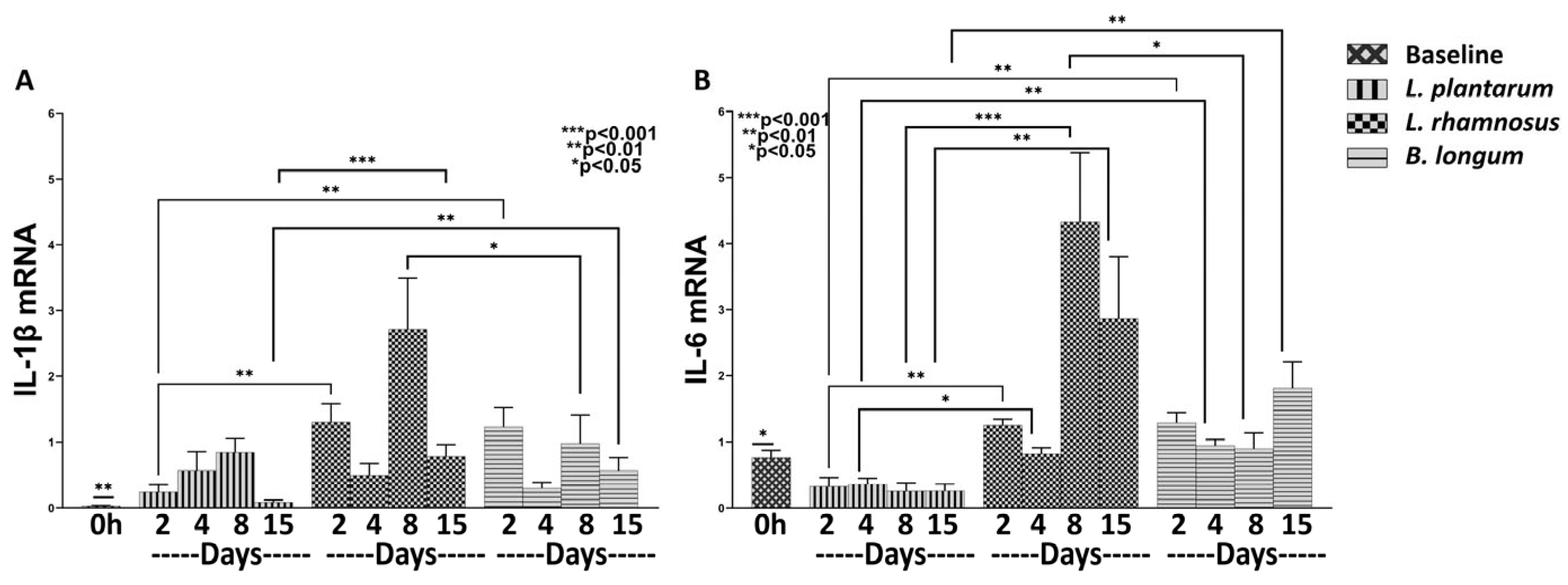
3.1.1. L. plantarum Reduces Inflammation More Efficiently than the Combo L. rhamnosus–B. longum
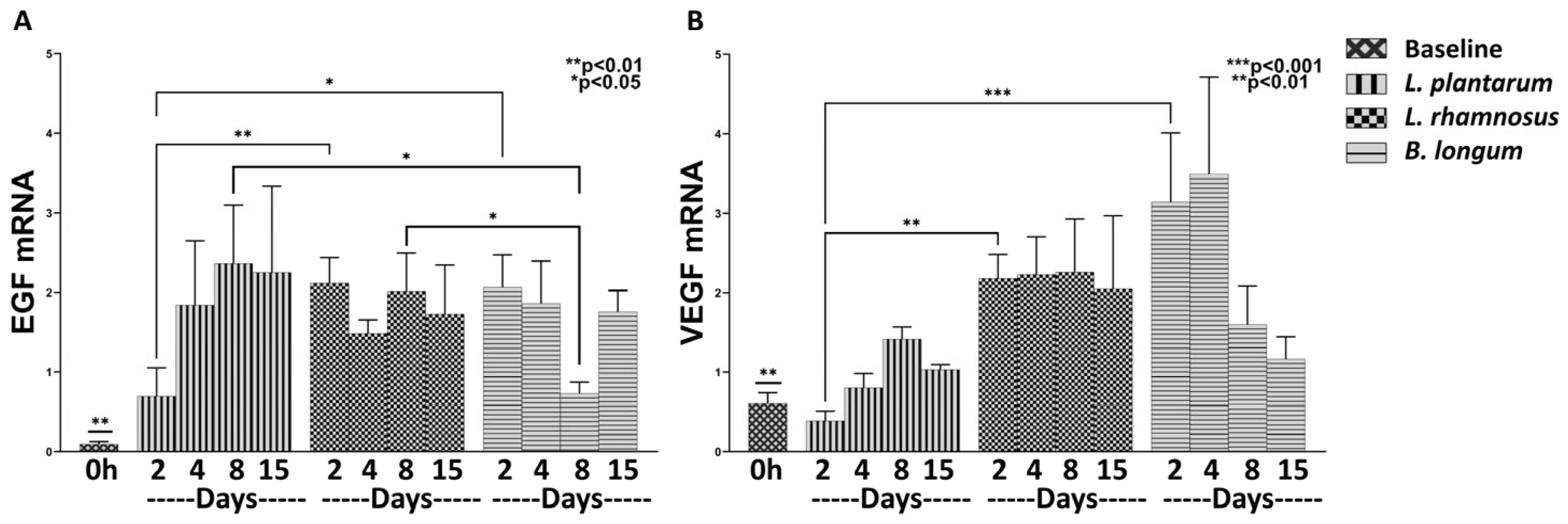
3.1.2. The Combo Regime Works Better than L. plantarum in Promoting the Expression of Healing Factors
3.1.3. The Combo Regime L. rhamnosus–B. longum, but Not the L. plantarum Strongly Enhance the Angiogenesis Process
3.2. 2nd Experiment
3.2.1. L. plantarum Exerts a Strong Anti-Inflammatory Effect Compared to Both L. rhamnosus and B. longum
3.2.2. L. rhamnosus Works Better than B. longum in Promoting Expression of Healing Factors
3.2.3. B. longum Seems Stronger in Promoting the Expression of Angiogenic Factors Compared to L. rhamnosus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Poret, A.J.; Hashemi, D.; Eseonu, A.; Yu, S.H.; D’Gama, J.; Neel, V.A.; Lieberman, T.D. Cutaneous Surgical Wounds Have Distinct Microbiomes from Intact Skin. Microbiol. Spectr. 2022, 11, e0330022. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef]
- Togo, C.; Zidorio, A.P.; Gonçalves, V.; Botelho, P.; de Carvalho, K.; Dutra, E. Does Probiotic Consumption Enhance Wound Healing? A Systematic Review. Nutrients 2021, 14, 111. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Huang, T.Y.; Wang, H.L.; Chien, P.J.; Chang, W.W.; Lee, H.T. Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells 2021, 10, 3264. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef]
- Tavaria, F.K. Topical use of probiotics: The natural balance. Porto Biomed. J. 2017, 2, 69–70. [Google Scholar] [CrossRef]
- Moysidis, M.; Stavrou, G.; Cheva, A.; Abba Deka, I.; Tsetis, J.K.; Birba, V.; Kapoukranidou, D.; Ioannidis, A.; Tsaousi, G.; Kotzampassi, K. The 3-D configuration of excisional skin wound healing after topical probiotic application. Injury 2022, 53, 1385–1393. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidοbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals 2022, 15, 1293. [Google Scholar] [CrossRef]
- Filidou, E.; Kandilogiannakis, L.; Tarapatzi, G.; Su, C.; Po, E.N.F.; Paspaliaris, V.; Kolios, G. Conditioned medium from a human adipose-derived stem cell line ameliorates inflammation and fibrosis in a lung experimental model of idiopathic pulmonary fibrosis. Life Sci. 2021, 287, 120123. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- França, K. Topical Probiotics in Dermatological Therapy and Skincare: A Concise Review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Foligné, B.; Deutsch, S.-M.; Breton, J.; Cousin, F.J.; Dewulf, J.; Samson, M.; Pot, B.; Jan, G. Promising Immunomodulatory Effects of Selected Strains of Dairy Propionibacteria as Evidenced In Vitro and In Vivo. Appl. Environ. Microbiol. 2010, 76, 8259–8264. [Google Scholar] [CrossRef]
- Filidou, E.; Kolios, G. Probiotics in Intestinal Mucosal Healing: A New Therapy or an Old Friend? Pharmaceuticals 2021, 14, 1181. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Shah, J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhou, M.; Chen, X.; Wang, J.; Zhao, X.; Zhu, Y.; Liu, T. Development and Characterization of Multifunctional Wound Dressing with the Property of Anti-bacteria and Angiogenesis. Probiotics Antimicrob. Proteins 2022. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug. Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2014, 4, 213–224. [Google Scholar] [CrossRef]
- O’Toole, E.A. Extracellular matrix and keratinocyte migration. Clin. Exp. Dermatol. 2001, 26, 525–530. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Argañaraz Aybar, J.N.; Ortiz Mayor, S.; Olea, L.; Garcia, J.J.; Nisoria, S.; Kolling, Y.; Melian, C.; Rachid, M.; Torres Dimani, R.; Werenitzky, C.; et al. Topical Administration of Lactiplantibacillus plantarum Accelerates the Healing of Chronic Diabetic Foot Ulcers through Modifications of Infection, Angiogenesis, Macrophage Phenotype and Neutrophil Response. Microorganisms 2022, 10, 634. [Google Scholar] [CrossRef]
- Moreira, C.F.; Cassini-Vieira, P.; Canesso, M.C.C.; Felipetto, M.; Ranfley, H.; Teixeira, M.M.; Nicoli, J.R.; Martins, F.S.; Barcelos, L.S. Lactobacillus rhamnosus CGMCC 1.3724 (LPR) Improves Skin Wound Healing and Reduces Scar Formation in Mice. Probiotics Antimicrob. Proteins 2021, 13, 709–719. [Google Scholar] [CrossRef]
- Lam, E.K.; Yu, L.; Wong, H.P.; Wu, W.K.; Shin, V.Y.; Tai, E.K.; So, W.H.; Woo, P.C.; Cho, C.H. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur. J. Pharmacol. 2007, 565, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Corydon, T.J.; Pantalone, D.; Sahana, J.; Wise, P.; Wehland, M.; Krüger, M.; Melnik, D.; Kopp, S.; Infanger, M.; et al. Role of Apoptosis in Wound Healing and Apoptosis Alterations in Microgravity. Front. Bioeng. Biotechnol. 2021, 9, 679650. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Mu, L.; Zhou, Y.; Chen, Y.; Wu, J.; Wang, Y.; Yang, H.; Li, M.; Xu, W.; et al. A Frog-Derived Immunomodulatory Peptide Promotes Cutaneous Wound Healing by Regulating Cellular Response. Front. Immunol. 2019, 10, 2421. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Yildiz, S.C.; Demir, C.; Ayhanci, A. Examination of the effects of kefir on healing factors in a mice burn model infected with E. coli, S. aureus and P. aeruginosa using qRT-PCR. Burns 2023, 49, 425–431. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Eskandari, M.H. Kefir Accelerates Burn Wound Healing through Inducing Fibroblast Cell Migration In Vitro and Modulating the Expression of IL-1ß, TGF-ß1, and bFGF Genes In Vivo. Probiotics Antimicrob. Proteins 2019, 11, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. Lactobacillus plantarum USM8613 Aids in Wound Healing and Suppresses Staphylococcus aureus Infection at Wound Sites. Probiotics Antimicrob. Proteins 2020, 12, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, T.; Lu, J.; Wei, K.; Tian, H.; Liu, W.; Xu, T.; Wang, X.; Wang, S.; Yang, R.; et al. Adjuvant treatment and molecular mechanism of probiotic compounds in patients with gastric cancer after gastrectomy. Food Funct. 2021, 12, 6294–6308. [Google Scholar] [CrossRef]
- Tagliari, E.; Campos, L.F.; Campos, A.C.; Costa-Casagrande, T.A.; Noronha, L. Effect of Probiotic Oral Administration on Skin wound healing in rats. Arq. Bras. Cir. Dig. 2019, 32, e1457. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zheng, C.; Wang, S.; Yang, R.; Liu, Z.; Chen, T. Treatment with a probiotic combination reduces abdominal adhesion in rats by decreasing intestinal inflammation and restoring microbial composition. Oncol. Rep. 2020, 43, 986–998. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R. Probiotics. Indian J. Med. Microbiol. 2009, 27, 202–209. [Google Scholar] [CrossRef]




| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | TGGGCTCCCTCTCATCAGTT | CTTGGTGGTTTGCTACGACG |
| Gapdh | AGTGCCAGCCTCGTCTCATA | GGTAACCAGGCGTCCGATA |
| IL-1b | GCAATGGTCGGGACATAGTT | AGACCTGACTTGGCAGAGGA |
| Col-1 | ATCAGCCCAAACCCCAAGGAGA | CGCAGGAAGGTCAGCTGGATAG |
| Col-3 | TGATGGGATCCAATGAGGGAGA | GAGTCTCATGGCCTTGCGTGTTT |
| α-SMA | TGACCCAGATTATGTTTGAG | AGATAGGCACGTTGTGAGTC |
| TGF-β1 | CATTTGGAGCCTGGACACACA | GCTTGCGACCCACGTAGTAGAC |
| CTGF | CAGCATGGACGTTCGTCTG | AACCACGGTT TGGTCCTTGG |
| EGFR | TACCTGAGAGACCGCCATA | TGCTTCTTCTGCTTCCCTA |
| VEGF | GCAATGATGAAGCCCTGGAGT | CTGAACAAGGCTCACAGTGATTTT |
| IL-6 | GCCCTTCAGGAACAGCTATGA | TGTCAACAACATCAGTCCCAAGA |
| IL-17 | ACTTTCCGGGTGGAGAAGAT | CTTAGGGGCTAGCCTCAGGT |
| IL-10 | GGCCATTCCATCCGGGGTGA | AAGGCAGCCCTCAGCTCTCG |
| EGF | TGTGGGCTGAGAAGAAGCTG | GAGTACCAGATCTGCCGCT |
| PDGF | TTCTTGATCTGGCCCCCAT | TTGACGCTGCTGGTGTTACAG |
| IL-8 | CTTTGTCCATTCCCACTTCTGA | TCCCTAACGGTTGCCTTTGTAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotou, D.; Filidou, E.; Gaitanidou, M.; Tarapatzi, G.; Spathakis, M.; Kandilogiannakis, L.; Stavrou, G.; Arvanitidis, K.; Tsetis, J.K.; Gionga, P.; et al. Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients 2023, 15, 1822. https://doi.org/10.3390/nu15081822
Panagiotou D, Filidou E, Gaitanidou M, Tarapatzi G, Spathakis M, Kandilogiannakis L, Stavrou G, Arvanitidis K, Tsetis JK, Gionga P, et al. Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients. 2023; 15(8):1822. https://doi.org/10.3390/nu15081822
Chicago/Turabian StylePanagiotou, Dimitrios, Eirini Filidou, Maria Gaitanidou, Gesthimani Tarapatzi, Michail Spathakis, Leonidas Kandilogiannakis, George Stavrou, Konstantinos Arvanitidis, Joulia K. Tsetis, Persefoni Gionga, and et al. 2023. "Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin" Nutrients 15, no. 8: 1822. https://doi.org/10.3390/nu15081822
APA StylePanagiotou, D., Filidou, E., Gaitanidou, M., Tarapatzi, G., Spathakis, M., Kandilogiannakis, L., Stavrou, G., Arvanitidis, K., Tsetis, J. K., Gionga, P., Shrewsbury, A. D., Manolopoulos, V. G., Kapoukranidou, D., Lasithiotakis, K., Kolios, G., & Kotzampassi, K. (2023). Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients, 15(8), 1822. https://doi.org/10.3390/nu15081822









