Synbiotics as Supplemental Therapy for the Alleviation of Chemotherapy-Associated Symptoms in Patients with Solid Tumours
Abstract
1. Introduction
2. Cancer Chemotherapy
Chemotherapeutic Agents and Side Effects
3. Gut Microbiota
4. Modulation of Gut Microbiota
4.1. Probiotics
4.2. Prebiotics
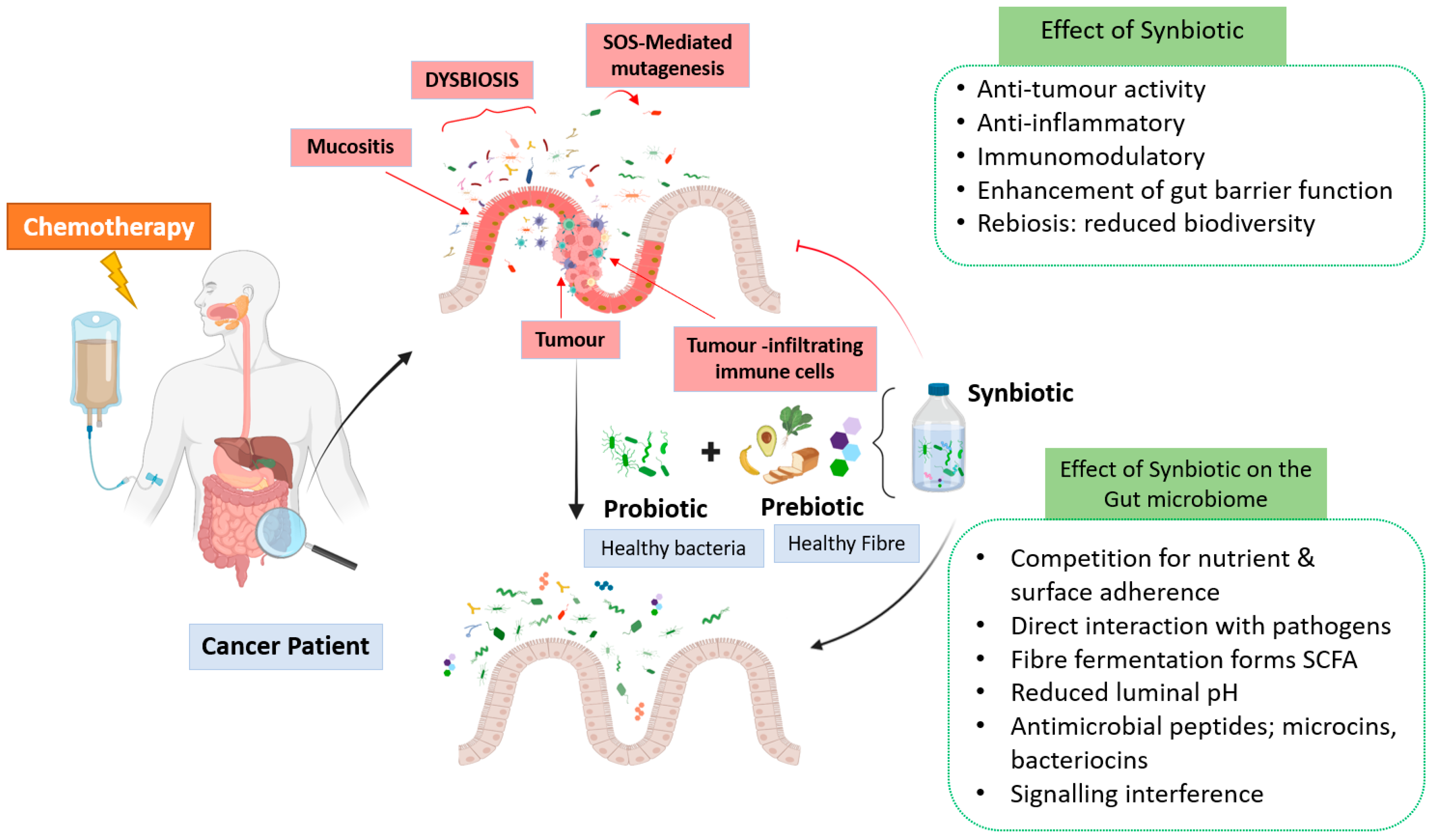
4.3. Synbiotics
5. Synbiotic Therapy to Alleviate Chemotherapy-Associated Symptoms
5.1. Effect of Prebiotics
5.2. Effect of Probiotics
| Beneficial Mechanism of Probiotics | Type of Probiotics | Relevance to Chemotherapy | References |
|---|---|---|---|
| The colonization and normalization of dysbiotic gut microbiota | Bifidobacterium, Lactobacillus reuteri, Lactobacillus rhamnosus GG, Butyricicoccus pullicaecorum, Faecalibacterium prausnitzii, Roseburia hominis, Eubacterium hallii, and Anaerostipes caccae | Chemotherapy may cause the dysbiosis of gut microbiota. Probiotics have been reported to be helpful in re-establishing the microbial communities in the gut. This has been found to be efficient in reducing the chemotherapy-associated gastrointestinal side effects, such as mucositis and diarrhoea. | [38] |
| Bacterial competition | Bifidobacterium and Lactobacillus | The depletion of gut microbiota due to chemotherapy results in the abundance of pathogenic bacteria in the gut. Probiotic consumption can outnumber the pathogenic bacteria by bacterial competition and thus reduced chemotherapy-associated side effects. | [38,123] |
| Cell adhesion | Lactobacillus rhamnosus, Lactobacillus plantarum, and Lactobacillus johnsonii | Chemotherapy damages the gut mucosa and results in the loss of gut microbiota. Probiotics possess the property of adherence and hence can adhere to mucosa in order to enhance the population of beneficial microbes in the gut. | [113] |
| Intestinal barrier integrity | Escherichia coli Nissle 1917, Lactobacillus reuteri, Lactobacillus rhamnosus GG, and Lactobacillus plantarum | Chemotherapy causes the impairment of the intestinal barrier. The maintenance of the intestinal barrier is the key to control dysbiosis and thus septic infections. Probiotics help to strengthen the integrity of the intestinal barrier. | [124,125] |
| The modulation of the immune system | Lactobacillus salivarius, Lactobacillus casei Shirota, Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus acidophilus, Streptococcus thermophilus, Bifidobacterium breve, and Bifidobacterium bifidum | Chemotherapy may weaken the immune system and compromise its ability to fight against infection. Probiotics regulate the immune response by modulating the functions of immune cells, such as macrophages, dendritic cells, as well as T and B lymphocytes. | [126,127] |
5.3. The Effect of Synbiotics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AIHW. Cancer in Australia. Available online: https://www.aihw.gov.au/getmedia/8c9fcf52-0055-41a0-96d9-f81b0feb98cf/aihw-can-123.pdf.aspx?inline=true (accessed on 27 December 2019).
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 24 May 2020).
- Cancer Research Unit. General Cancer Information. Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general (accessed on 4 January 2020).
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bastian, S.E.P.; Howarth, G.S. Newly Developed Synbiotics and the Chemotherapy-Damaged Gut. J. Evid.-Based Complement. Altern. Med. 2013, 18, 198–208. [Google Scholar] [CrossRef]
- Gibson, E.; Monje, M. Effect of cancer therapy on neural stem cells: Implications for cognitive function. Curr. Opin. Oncol. 2012, 24, 672–678. [Google Scholar] [CrossRef]
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643. [Google Scholar] [CrossRef]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Liu, B.; Ezeogu, L.; Zellmer, L.; Yu, B.; Xu, N.; Joshua Liao, D. Protecting the normal in order to better kill the cancer. Cancer Med. 2015, 4, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Neugut, A.I.; Prigerson, H.G. Curative, Life-Extending, and Palliative Chemotherapy: New Outcomes Need New Names. Oncologist 2017, 22, 883–885. [Google Scholar] [CrossRef]
- Lawrence, J.; Cameron, D.; Argyle, D. Species differences in tumour responses to cancer chemotherapy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140233. [Google Scholar] [CrossRef]
- Skipper, H.E.; Schabel, F.M., Jr.; Mellett, L.B.; Montgomery, J.A.; Wilkoff, L.J.; Lloyd, H.H.; Brockman, R.W. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother. Rep. 1970, 54, 431–450. [Google Scholar]
- Mihlon, F.t.; Ray, C.E., Jr.; Messersmith, W. Chemotherapy agents: A primer for the interventional radiologist. Semin. Interv. Radiol. 2010, 27, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Longo, D.L. Cancer Chemotherapy and Biotherapy: Principles and Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Fizazi, K.; Zelek, L. Is ‘one cycle every three or four weeks’ obsolete? A critical review of dose-dense chemotherapy in solid neoplasms. Ann. Oncol. 2000, 11, 133–149. [Google Scholar] [CrossRef]
- Padma, V.V. An overview of targeted cancer therapy. Biomedicine 2015, 5, 19. [Google Scholar] [CrossRef]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar] [PubMed]
- Secombe, K.R.; Van Sebille, Y.Z.A.; Mayo, B.J.; Coller, J.K.; Gibson, R.J.; Bowen, J.M. Diarrhea Induced by Small Molecule Tyrosine Kinase Inhibitors Compared With Chemotherapy: Potential Role of the Microbiome. Integr. Cancer Ther. 2020, 19, 1534735420928493. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Sonis, S.T.; Keefe, D.M. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother. Pharmacol. 2008, 62, 33–41. [Google Scholar] [CrossRef]
- Li, Z.; Ibrahim, N.K.; Wathen, J.K.; Wang, M.; Mante Menchu, R.P.; Valero, V.; Theriault, R.; Buzdar, A.U.; Hortobagyi, G.N. Colitis in patients with breast carcinoma treated with taxane-based chemotherapy. Cancer 2004, 101, 1508–1513. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef]
- Cassidy, J.; Misset, J.L. Oxaliplatin-related side effects: Characteristics and management. Semin. Oncol. 2002, 29, 11–20. [Google Scholar] [CrossRef]
- Markman, M.; Zanotti, K.; Webster, K.; Belinson, J.; Rose, P. Toxicity associated with carboplatin/paclitaxel/Irinotecan use in advanced ovarian cancer: Preliminary analysis. Oncolology 2003, 17, 34–35. [Google Scholar]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- McCollum, A.D.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Macdonald, J.S.; Benson, A.B., 3rd; Fuchs, C.S. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J. Natl. Cancer Inst. 2002, 94, 1160–1167. [Google Scholar] [CrossRef]
- Stentoft, J. The toxicity of cytarabine. Drug. Saf. 1990, 5, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Armand, J.P.; Ribrag, V.; Harrousseau, J.L.; Abrey, L. Reappraisal of the use of procarbazine in the treatment of lymphomas and brain tumors. Ther. Clin. Risk Manag. 2007, 3, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H.; Loehrer, P.J. Ifosfamide chemotherapy for pancreatic carcinoma. Cancer Chemother. Pharmacol. 1986, 18, S51–S54. [Google Scholar] [CrossRef]
- Panasci, L.; Shenouda, G.; Begin, L.; Pollak, M.; Reinke, A.; Margolese, R. Mitomycin C and mitoxantrone chemotherapy for advanced breast cancer: Efficacy with minimal gastrointestinal toxicity and alopecia. Cancer Chemother. Pharmacol. 1990, 26, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Amon, P.; Sanderson, I. What is the microbiome? Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 257. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A. Peptidyl hormones of endocrine cells origin in the gut--their discovery and physiological relevance. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 11–27. [Google Scholar]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Basile, M.S.; Nicolosi, D.; Genovese, C.; Libra, M.; Salmeri, M. Benefits of using probiotics as adjuvants in anticancer therapy. World Acad. Sci. J. 2019, 1, 125–135. [Google Scholar] [CrossRef]
- Engevik, M.A.; Versalovic, J. Biochemical Features of Beneficial Microbes: Foundations for Therapeutic Microbiology. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. N. Am. 2011, 91, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Marchix, J.; Goddard, G.; Helmrath, M.A. Host-Gut Microbiota Crosstalk in Intestinal Adaptation. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 149–162. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Zhu, W. Crosstalk Between The Immune Receptors and Gut Microbiota. Curr. Protein Pept. Sci. 2015, 16, 622–631. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The human microbiome: Our second genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 2017, 168, 928–943.e911. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Sen, P.; Orešič, M. Metabolic Modeling of Human Gut Microbiota on a Genome Scale: An Overview. Metabolites 2019, 9, 22. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 2015, 349, 1101–1106. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther. 2006, 5, 1265–1269. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.P.; Rao, K.; Young, V.B. Probiotics for prevention of Clostridium difficile infection. Curr. Opin. Gastroenterol. 2018, 34, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Fayol-Messaoudi, D.; Berger, C.N.; Coconnier-Polter, M.H.; Liévin-Le Moal, V.; Servin, A.L. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 6008–6013. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T.; et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012, 8, e1002714. [Google Scholar] [CrossRef]
- Konieczna, P.; Groeger, D.; Ziegler, M.; Frei, R.; Ferstl, R.; Shanahan, F.; Quigley, E.M.; Kiely, B.; Akdis, C.A.; O’Mahony, L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut 2012, 61, 354–366. [Google Scholar] [CrossRef]
- Miller, L.E.; Lehtoranta, L.; Lehtinen, M.J. The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis. Nutrients 2017, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 2007, 9, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Gibson, G.R.; Fuller, R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J. Nutr. 2000, 130, 391s–395s. [Google Scholar] [CrossRef] [PubMed]
- Azcarate-Peril, M.A.; Ritter, A.J.; Savaiano, D.; Monteagudo-Mera, A.; Anderson, C.; Magness, S.T.; Klaenhammer, T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA 2017, 114, E367–E375. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 2017, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Walker, W.A. The role of gut-associated lymphoid tissues and mucosal defence. Br. J. Nutr. 2005, 93, S41–S48. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef]
- Ito, H.; Takemura, N.; Sonoyama, K.; Kawagishi, H.; Topping, D.L.; Conlon, M.A.; Morita, T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J. Agric. Food Chem. 2011, 59, 5771–5778. [Google Scholar] [CrossRef]
- Grimoud, J.; Durand, H.; de Souza, S.; Monsan, P.; Ouarne, F.; Theodorou, V.; Roques, C. In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int. J. Food Microbiol. 2010, 144, 42–50. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Jairath, S. Prebiotics, probiotics and synbiotics: An overview. J. Pharm. Educ. Res. 2010, 1, 13–36. [Google Scholar]
- Su, P.; Henriksson, A.; Mitchell, H. Prebiotics enhance survival and prolong the retention period of specific probiotic inocula in an in vivo murine model. J. Appl. Microbiol. 2007, 103, 2392–2400. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Ndagijimana, M.; Cruciani, F.; Carnevali, P.; Candela, M.; Guerzoni, M.E.; Brigidi, P. Impact of a synbiotic food on the gut microbial ecology and metabolic profiles. BMC Microbiol. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lu, P.; Li, M.X.; Cai, X.L.; Xiong, W.Y.; Hou, H.J.; Ha, X.Q. A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine 2019, 98, e16618. [Google Scholar] [CrossRef]
- Wong, V.W.; Won, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Li, K.C.; Chan, H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Spano, G.; Fiocco, D. β-Glucans and Probiotics. Am. J. Immunol. 2017, 13, 34–44. [Google Scholar] [CrossRef]
- Russo, P.; López, P.; Capozzi, V.; De Palencia, P.F.; Dueñas, M.T.; Spano, G.; Fiocco, D. Beta-glucans improve growth, viability and colonization of probiotic microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef] [PubMed]
- Jaskari, J.; Kontula, P.; Siitonen, A.; Jousimies-Somer, H.; Mattila-Sandholm, T.; Poutanen, K. Oat beta-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 1998, 49, 175–181. [Google Scholar] [CrossRef]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H.; Bozkurt, O.; Ozaslan, E.; Baldane, S.; Berk, V.; Inanc, M.; Duran, A.O.; Dikilitas, M.; Er, O.; Ozkan, M. Positive effects of oral β-glucan on mucositis and leukopenia in colorectal cancer patients receiving adjuvant FOLFOX-4 combination chemotherapy. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 3641–3644. [Google Scholar] [CrossRef] [PubMed]
- Schell, K.R.; Fernandes, K.E.; Shanahan, E.; Wilson, I.; Blair, S.E.; Carter, D.A.; Cokcetin, N.N. The Potential of Honey as a Prebiotic Food to Re-engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 2022, 9, 957932. [Google Scholar] [CrossRef]
- Ezz El-Arab, A.M.; Girgis, S.M.; Hegazy, E.M.; Abd El-Khalek, A.B. Effect of dietary honey on intestinal microflora and toxicity of mycotoxins in mice. BMC Complement. Altern. Med. 2006, 6, 6. [Google Scholar] [CrossRef]
- Li, Y.; Long, S.; Liu, Q.; Ma, H.; Li, J.; Xiaoqing, W.; Yuan, J.; Li, M.; Hou, B. Gut microbiota is involved in the alleviation of loperamide-induced constipation by honey supplementation in mice. Food Sci. Nutr. 2020, 8, 4388–4398. [Google Scholar] [CrossRef] [PubMed]
- Shamala, T.R.; Shri Jyothi, Y.; Saibaba, P. Stimulatory effect of honey on multiplication of lactic acid bacteria under in vitro and in vivo conditions. Lett. Appl. Microbiol. 2000, 30, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst. Rev. 2011, 2011, Cd000978. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Jeong, Y.M.; Lee, H.S.; Lee, Y.J.; Hwang, S.H. Effects of honey on oral mucositis in patients with head and neck cancer: A meta-analysis. Laryngoscope 2015, 125, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Xia, R.; Sun, Z.H.; Sun, L.; Min, X.; Liu, C.; Zhang, H.; Zhu, Y.M. Effects of honey use on the management of radio/chemotherapy-induced mucositis: A meta-analysis of randomized controlled trials. Int. J. Oral. Maxillofac. Surg. 2016, 45, 1618–1625. [Google Scholar] [CrossRef]
- Co, J.L.; Mejia, M.B.; Que, J.C.; Dizon, J.M. Effectiveness of honey on radiation-induced oral mucositis, time to mucositis, weight loss, and treatment interruptions among patients with head and neck malignancies: A meta-analysis and systematic review of literature. Head. Neck 2016, 38, 1119–1128. [Google Scholar] [CrossRef]
- Maria-Aggeliki, K.S.; Nikolaos, K.L.; Kyrias, G.M.; Vassilis, K.E. The potential clinical impact of probiotic treatment for the prevention and/or anti-inflammatory therapeutic effect against radiation induced intestinal mucositis. A review. Recent. Pat. Inflamm. Allergy Drug. Discov. 2009, 3, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Holec, V.; Drgona, L.; Hainova, K.; Ciernikova, S.; Zajac, V. Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement. Ther. Med. 2013, 21, 712–723. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A.M. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017, 8, 521–543. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, v139–v151. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Morelli, L.; Galbiati, F.; Rocchetti, S.; Coppola, S.; Beneduce, A.; Gilardini, C.; Zonenschain, D.; Nespoli, A.; Braga, M. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J. Gastroenterol. 2010, 16, 167–175. [Google Scholar] [CrossRef]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef]
- Consoli, M.L.; da Silva, R.S.; Nicoli, J.R.; Bruña-Romero, O.; da Silva, R.G.; de Vasconcelos Generoso, S.; Correia, M.I. Randomized Clinical Trial: Impact of Oral Administration of Saccharomyces boulardii on Gene Expression of Intestinal Cytokines in Patients Undergoing Colon Resection. JPEN J. Parenter. Enter. Nutr. 2016, 40, 1114–1121. [Google Scholar] [CrossRef]
- Wardill, H.R.; Van Sebille, Y.Z.A.; Ciorba, M.A.; Bowen, J.M. Prophylactic probiotics for cancer therapy-induced diarrhoea: A meta-analysis. Curr. Opin. Support. Palliat. Care 2018, 12, 187–197. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public. Health 2021, 18, 4265. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Hueso, T.; Ekpe, K.; Mayeur, C.; Gatse, A.; Joncquel-Chevallier Curt, M.; Gricourt, G.; Rodriguez, C.; Burdet, C.; Ulmann, G.; Neut, C.; et al. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: The role of mucosal strengthening. Gut Microbes 2020, 12, 1800897. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotics: Progress toward novel therapies for intestinal diseases. Curr. Opin. Gastroenterol. 2010, 26, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Geier, M.S.; Yazbeck, R.; Torres, D.M.; Butler, R.N.; Howarth, G.S. Lactobacillus fermentum BR11 and fructo-oligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr. Cancer 2008, 60, 757–767. [Google Scholar] [CrossRef]
- Theodoropoulos, G.E.; Memos, N.A.; Peitsidou, K.; Karantanos, T.; Spyropoulos, B.G.; Zografos, G. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann. Gastroenterol. 2016, 29, 56–62. [Google Scholar]
- Flesch, A.T.; Tonial, S.T.; Contu, P.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef]
- Polakowski, C.B.; Kato, M.; Preti, V.B.; Schieferdecker, M.E.M.; Ligocki Campos, A.C. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019, 58, 40–46. [Google Scholar] [CrossRef]
- Krebs, B. Prebiotic and Synbiotic Treatment before Colorectal Surgery-Randomised Double Blind Trial. Coll. Antropol. 2016, 40, 35–40. [Google Scholar]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K.; et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef]
- Farshi Radvar, F.; Mohammad-Zadeh, M.; Mahdavi, R.; Andersen, V.; Nasirimotlagh, B.; Faramarzi, E.; Lotfi Yagin, N. Effect of synbiotic supplementation on matrix metalloproteinase enzymes, quality of life and dietary intake and weight changes in rectal cancer patients undergoing neoadjuvant chemoradiotherapy. Mediterr. J. Nutr. Metab. 2020, 13, 225–235. [Google Scholar] [CrossRef]
- Fukaya, M.; Yokoyama, Y.; Usui, H.; Fujieda, H.; Sakatoku, Y.; Takahashi, T.; Miyata, K.; Niikura, M.; Sugimoto, T.; Asahara, T.; et al. Impact of synbiotics treatment on bacteremia induced during neoadjuvant chemotherapy for esophageal cancer: A randomised controlled trial. Clin. Nutr. 2021, 40, 5781–5791. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Drug Names | Mechanism of Action | Common Side Effects | References |
|---|---|---|---|---|
| Tubulin modifying agents | Docetaxel and paclitaxel | Inhibit the mitotic process of cells by interfering with the tubulin polymerisation process in order to induce cell death. | Ischaemic colitis, nausea, fatigue, flushing, fever, diarrhoea, acute abdominal pain, neutropenia, septicaemia, hyperglycaemia, gastrointestinal haemorrhage, bowel perforation, neuropathy, dyspnoea, peritonitis, and tenderness. | [21,22] |
| Platinum-based drugs | Cisplatin and oxaliplatin | Cause DNA damage to induce cell death. | Nausea, vomiting, diarrhoea, constipation, stomatitis, gastro-oesophageal reflux, anorexia, cachexia, asthenia, melena, dry mouth, gum inflammation, haemoptysis, colitis, ileus, pancreatitis, hepatic sinusoidal dilatation, rectal haemorrhage, haemorrhoids, tenesmus renal and neural toxicity, cardiotoxicity, ototoxicity, alopecia, and bone marrow suppression. | [23,24,25] |
| DNA intercalator drugs | Anthracyclines, doxorubicin, daunorubicin, idarubicin, and epirubicin | Inhibit DNA isomerase II and DNA replication to cause cell death. | Cardiac toxicity, nausea, vomiting, stomatitis, oesophageal ulceration, colonic ulceration, anorexia, and rarely tongue hyperpigmentation. | [10] |
| Antimetabolites | 5-fluorouracil, capecitabine, 6-mercaptopurine, cytarabine, gemcitabine, and methotrexate | Induce cell death during the S-phase of the cell cycle or by inhibiting the enzymes responsible for nucleic acid production | Fever, nausea, vomiting, gingivitis, pharyngitis, gastrointestinal ulceration, abdominal pain, loss of appetite, haematemesis, melena, diarrhoea, constipation, stomatitis, bowel necrosis, pancreatitis, hyperbilirubinemia. hepatic failure, hyperbilirubinemia, dyspepsia, anorexia, bone marrow suppression, and leukopenia. | [10,26,27] |
| Alkylating agents | Mechlorethamine, melphalan, chlorambucil, cyclophosphamide, ifosfamide, carmustine (BCNU), lomustine (CCNU), mitomycin C, dacarbazine, and procarbazine | Cause reactions with different components of DNA to induce cell death | Nausea, vomiting, abdominal pain, diarrhoea, constipation, melena, stomatitis, anorexia, dry mouth, leukopenia, thrombocytopenia, encephalopathy, bone marrow suppression, and haematuria. | [28,29,30] |
| Targeted biological agents (cellular kinases and monoclonal antibodies) | Alemtuzumab, bevacizumab, cetuximab, gemtuzumab, ozogamicin, tiuxetan, 131I-tositumomab, panitumumab, rituximab, trastuzumab, bortezomib, dasatinib, erlotinib, gefitinib, imatinib, lapatinib, sorafenib, and sunitinib | Induce cell death by targeting a specific molecule in cancer cells. | Nausea, vomiting, diarrhoea, anorexia, stomatitis, abdominal pain, hepatotoxicity, cardiotoxicity, proteinuria, skin rashes, thrombosis, hypertension, myelosuppression, peripheral neuropathy, and interstitial lung disease. | [10,18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.K.; Beckett, J.M.; Kalpurath, K.; Ishaq, M.; Ahmad, T.; Eri, R.D. Synbiotics as Supplemental Therapy for the Alleviation of Chemotherapy-Associated Symptoms in Patients with Solid Tumours. Nutrients 2023, 15, 1759. https://doi.org/10.3390/nu15071759
Singh NK, Beckett JM, Kalpurath K, Ishaq M, Ahmad T, Eri RD. Synbiotics as Supplemental Therapy for the Alleviation of Chemotherapy-Associated Symptoms in Patients with Solid Tumours. Nutrients. 2023; 15(7):1759. https://doi.org/10.3390/nu15071759
Chicago/Turabian StyleSingh, Neeraj K., Jeffrey M. Beckett, Krishnakumar Kalpurath, Muhammad Ishaq, Tauseef Ahmad, and Rajaraman D. Eri. 2023. "Synbiotics as Supplemental Therapy for the Alleviation of Chemotherapy-Associated Symptoms in Patients with Solid Tumours" Nutrients 15, no. 7: 1759. https://doi.org/10.3390/nu15071759
APA StyleSingh, N. K., Beckett, J. M., Kalpurath, K., Ishaq, M., Ahmad, T., & Eri, R. D. (2023). Synbiotics as Supplemental Therapy for the Alleviation of Chemotherapy-Associated Symptoms in Patients with Solid Tumours. Nutrients, 15(7), 1759. https://doi.org/10.3390/nu15071759








