Nutritional Status, Refeeding Syndrome and Some Associated Factors of Patients at COVID-19 Hospital in Vietnam
Abstract
1. Introduction
2. Materials and Methods
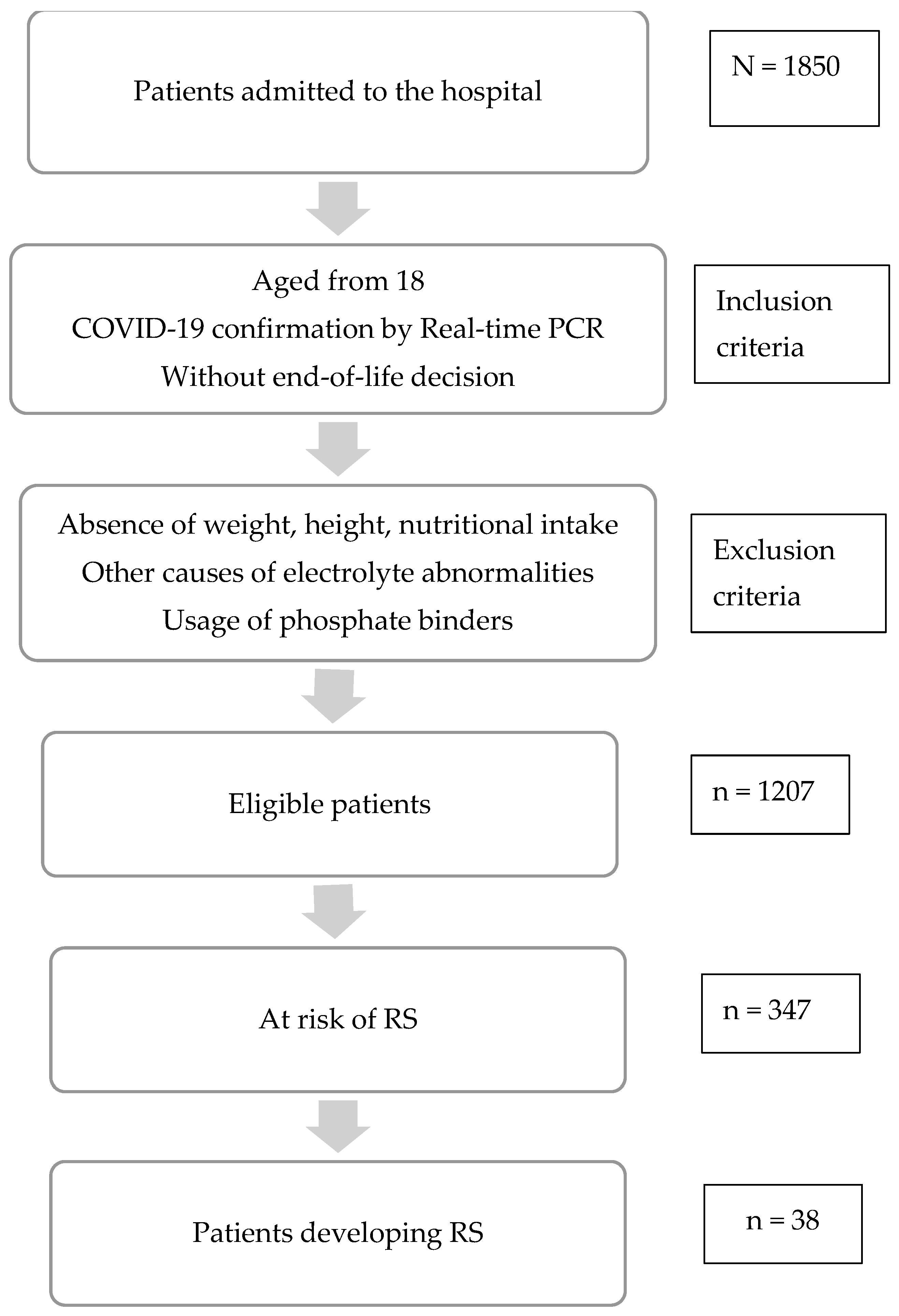
2.1. Study Design and Subjects
2.2. Socioeconomic Characteristics and Clinical Information
2.3. Classification of Severity of COVID-19
- -
- Mild: Patients had non-specific clinical symptoms such as fever, dry cough, sore throat, stuffy nose, fatigue, headache, muscle aches, loss of taste, smell, or diarrhea., breathing rate < 20 breaths/min and SpO2 > 96% when breathing air, normal or minimal damage chest X-ray. The patients are alert and can self-service.
- -
- Moderate: Patient are alert, had signs of pneumonia with shortness of breath, including rapid breathing at 20–25 times/minute, lungs crackles and no signs of severe respiratory distress, and SpO2 94–96% on room air. The patient might have difficulty breathing on exertion such as when walking around the house or going up the stairs. Patient had fast or slow pulse, dry skin, tachycardia, normal blood pressure.
- -
- Severe: Patients had signs of pneumonia accompanied by any of the following: breathing > 25 breaths/minute; severe shortness of breath, contraction of accessory respiratory muscles, or SpO2 < 94% when breathing room air. Patients had tachycardia or possibly bradycardia, and BP normal or elevated. Patient might be restless, lethargic, and tired. Patients had chest X-ray and chest CT that showed lesions with lesions more than 50%. Patients had PaO2/FiO2 200–300 mmHg, lung ultrasound showing multiple B-lines.
- -
- Critical: Patient had breathing at >30 breaths/minute or <10 breaths/minute, with signs of severe respiratory failure such as labored or irregular breathing. Patient had decreased consciousness or were in coma. Patients had tachycardia or possibly bradycardia, and low blood pressure. Patient had little urine or anuria. Patient had chest X-ray and chest CT showing lesions, with lesions more than 50%. Patient had PaO2/FiO2 < 200, respiratory acidosis, and blood lactate > 2 mmol/L. Patient had lung ultrasound showing multiple B-lines.
2.4. Assessment of Nutritional Status
2.5. Assessment of Risk of Refeeding Syndrome and Diagnosis of Refeeding Syndrome (RS)
| Significant risk for RS: 1 out of following criteria | Moderate risk for RS: 2 out of following criteria |
| - BMI: <16.0 kg/m2 | - BMI: 16–18.5 kg/m2 |
| - Weight loss about 10% in 6 months | - Weight loss about 5% in 1 month |
| - Severe muscle mass loss, subcutaneous fat loss | - Moderate muscle mass loss, subcutaneous fat loss |
| - Caloric intake before admission < 50% energy requirement in 1 week or longer | - Caloric intake before admission < 75% energy requirement in 1 week or longer |
| - Moderately or significantly depletion of serum potassium, phosphorus | - Minimally depletion of serum potassium, phosphorus |
- A decrease in any 1, 2, or 3 of serum phosphorus, potassium, and/or magnesium levels by 10–20% (mild RS), 20–30% (moderate RS), or >30% and/or organ dysfunction resulting from a decrease in any of these and/or due to thiamin deficiency (severe RS).
- And occurring within 5 days of reinitiating or substantially increasing energy provision
2.6. Data Collection and Analysis
3. Results
3.1. Characteristics of the Study Participants
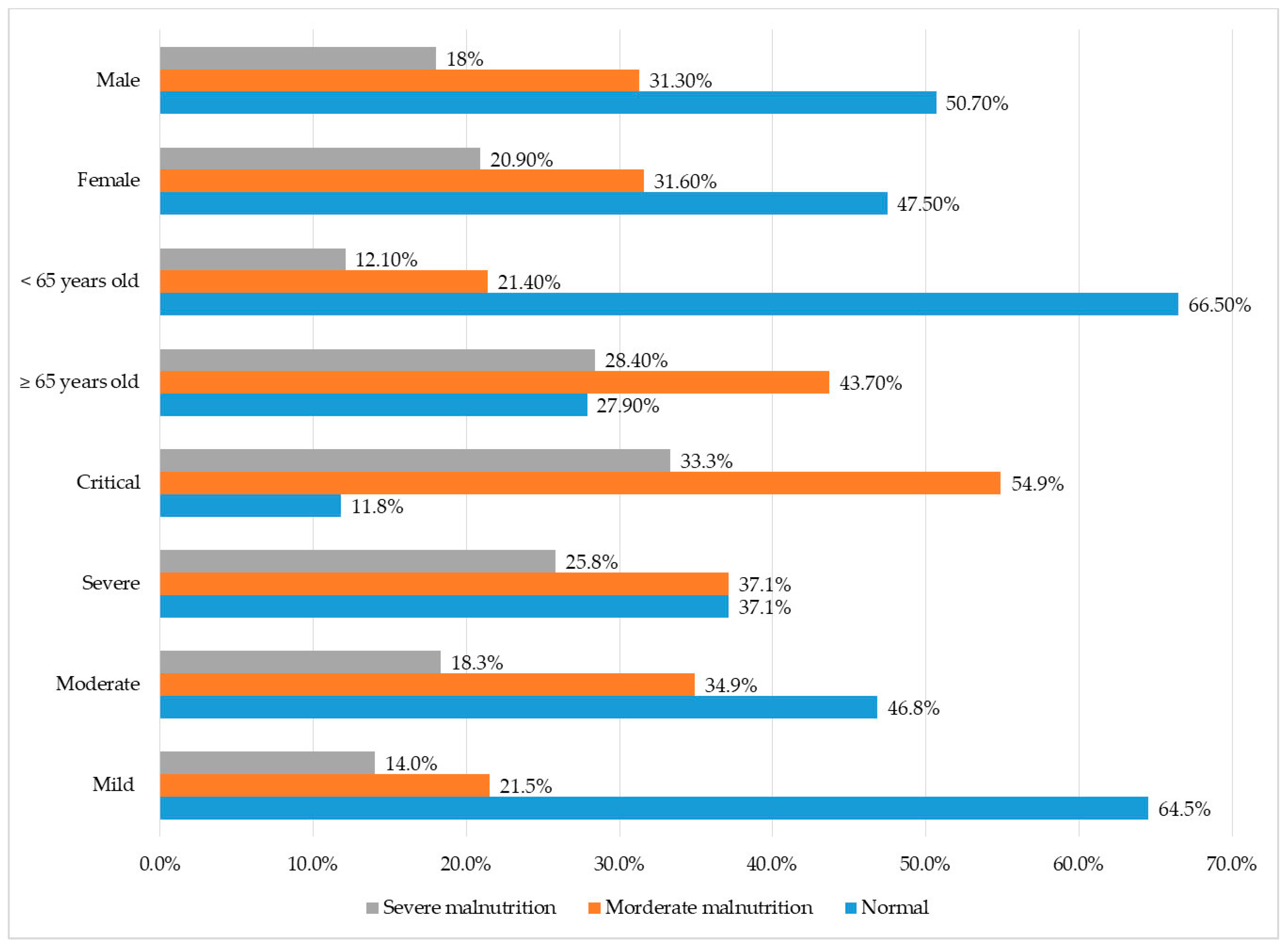
3.2. Nutritional Status among COVID-19 Patients
3.3. Refeeding Syndrome among COVID-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haraj, N.E.; El Aziz, S.; Chadli, A.; Dafir, A.; Mjabber, A.; Aissaoui, O.; Barrou, L.; El Hamidi, C.E.; Nsiri, A.; Harrar, R.A.; et al. Nutritional status assessment in patients with COVID-19 after discharge from the intensive care unit. Clin. Nutr. ESPEN 2021, 41, 423–428. [Google Scholar] [CrossRef] [PubMed]
- COVID Live—Coronavirus Statistics—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 13 June 2022).
- Carretero Gómez, J.; Mafé Nogueroles, M.C.; Garrachón Vallo, F.; Escudero Álvarez, E.; Maciá Botejara, E.; Miramontes González, J.P. Inflammation, malnutrition, and SARS-CoV-2 infection: A disastrous combination. Rev. Clin. Esp. 2020, 220, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- The Digestive System Is a Potential Route of 2019-nCov Infection: A Bioinformatics Analysis Based on Single-Cell Transcriptomes|bioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1 (accessed on 13 June 2022).
- da Silva, J.S.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Du, Z.; Zhu, F.; Cao, Z.; An, Y.; Gao, Y.; Jiang, B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020, 395, e52. [Google Scholar] [CrossRef] [PubMed]
- Recinella, G.; Marasco, G.; Serafini, G.; Maestri, L.; Bianchi, G.; Forti, P.; Zoli, M. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: A monocentric study. Aging Clin. Exp. Res. 2020, 32, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Sasdelli, A.S.; Ravaioli, F.; Baracco, B.; Battaiola, C.; Bocedi, G.; Brodosi, L.; Leoni, L.; Mari, G.A.; Musio, A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin. Nutr. 2021, 40, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- An Updated HACOR Score for Predicting the Failure of Noninvasive Ventilation: A Multicenter Prospective Observational Study|Critical Care|Full Text. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-022-04060-7 (accessed on 11 February 2023).
- Pourfridoni, M.; Abbasnia, S.M.; Shafaei, F.; Razaviyan, J.; Heidari-Soureshjani, R. Fluid and Electrolyte Disturbances in COVID-19 and Their Complications. BioMed Res. Int. 2021, 2021, e6667047. [Google Scholar] [CrossRef] [PubMed]
- Clinical Features and Potential Risk Factors for Discerning the Critical Cases and Predicting the Outcome of Patients with COVID-19—Wang—2020—Journal of Clinical Laboratory Analysis—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/jcla.23547 (accessed on 15 June 2022).
- The Role of the Mineralocorticoid Receptor in Inflammation: Focus on Kidney and Vasculature—FullText—American Journal of Nephrology 2017, Vol. 46, No. 4—Karger Publishers. Available online: https://www.karger.com/Article/Fulltext/480652 (accessed on 15 June 2022).
- Vahdat Shariatpanahi, Z.; Shariatpanahi, M.; Shahbazi, E.; Shahbazi, S. Refeeding Syndrome and Its Related Factors in Critically Ill Coronavirus Disease 2019 Patients: A Prospective Cohort Study. Front. Nutr. 2022, 9, 830457. [Google Scholar] [CrossRef] [PubMed]
| Criteria | Quantity (n) | Percentage (%) | |
|---|---|---|---|
| Gender | Male | 603 | 49.9 |
| Female | 604 | 50.1 | |
| Age | 58.9 ± 20.7 (18–103) | ||
| Weight (kg) | 57.8 ± 11.3 (30–130) | ||
| Height (cm) | 160 ± 8 | ||
| BMI (kg/m2) | 22.3 ± 3.3 | ||
| Comorbidities | None | 344 | 28.5 |
| One–Two background diseases | 586 | 48.6 | |
| More than three ones | 277 | 22.9 | |
| Level of COVID-19 condition | Mild | 636 | 52.7 |
| Moderate | 235 | 19.5 | |
| Severe | 132 | 10.9 | |
| Critical | 204 | 16.9 | |
| Oxygen therapy | None | 749 | 62.1 |
| Mask | 200 | 16.6 | |
| Nasal prong | 118 | 9.8 | |
| HFNC | 19 | 1.6 | |
| NIV (CIPAP or BIPAP) | 15 | 1,2 | |
| Mechanical ventilation/ ECMO | 106 | 8.8 | |
| Laboratory Tests | Mean Value According to Severity of COVID-19 | ||||
|---|---|---|---|---|---|
| Mild (n = 636) | Moderate (n = 235) | Severe (n = 132) | Critical (n = 204) | p Value | |
| Albumin (g/L) (n = 424) | 35.6 ± 7 [18.1–49.3] (n = 105) | 32.2 ± 5.1 [17.6–42.5] (n = 86) | 30.9 ± 5 [14.2–42.4] (n = 73) | 28.5 ± 4.5 [17.5–39.8] (n = 160) | p < 0.05 * |
| Pre-albumin (mg/dL) (n = 29) | 15 ± 5.7 [8–24] (n = 6) | 15 (n = 1) | 13.5 ± 4.9 [8–20] (n = 4) | 11.9 ± 5.2 [3–19] (n = 18) | p > 0.05 ** |
| Na (mmol/L) (n = 1033) | 135.6 ± 4.6 [107–155] (n = 504) | 132.9 ± 5 [115–145] (n = 212) | 134.4 ± 5.5 [110–157] (n = 125) | 134.7 ± 7.6 [106–163] (n = 192) | p < 0.05 ** |
| K (mmol/L) (n = 1033) | 3.8 ± 0.4 [2.2–5.5] (n = 504) | 3.9 ± 0.5 [2.8–5.4] (n = 212) | 4 ± 0.7 [2.2–6.2] (n = 125) | 4.1 ± 0.7 [2.3–6.7] (n = 192) | p < 0.05 ** |
| p (mmol/L) (n = 45) | 0 | 1 ± 0.2 [0.9–1.2] (n = 4) | 1.2 ± 0.5 [0.6–1.8] (n = 4) | 1.1 ± 0.4 [0.4–2.3] (n = 37) | p > 0.05 * |
| Triglyceride (mmol/L) (n = 22) | 1.6 ± 0.9 [0.3–2.9] (n = 6) | 2.2 ± 2.2 [0.6–6.1] (n = 5) | 1.8 ± 1.1 [0.8–3.4] (n = 4) | 3.9 ± 3 [1.1–11.7] (n = 10) | p > 0.05 ** |
| Malnutrition According to GLIM | Malnutrition According to BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal n (%) | Malnutrition n (%) | OR [95% CI] | p Value | Normal n (%) | Malnutrition n (%) | OR [95% CI] | p Value | |
| Age | ||||||||
| <65 (n = 663) | 441 (36.5) | 222 (18.4) | 5.1 (4–6.6) | p < 0.05 | 617 (51.1) | 46 (3.8) | 1.6 (1–2.4) | p < 0.05 |
| ≥65 (n = 544) | 152 (12.6) | 392 (32.5) | 487 (40.3) | 57 (4.7) | ||||
| Gender | ||||||||
| Male (n = 603) | 306 (25.4) | 297 (24.6) | 1.1 (0.9–1.4) | p > 0.05 | 558 (46.2) | 45 (3.7) | 1.3 (0.9–2) | p > 0.05 |
| Female (n = 604) | 287 (23.8) | 317 (26.3) | 546 (45.2) | 58 (4.8) | ||||
| Severity | ||||||||
| Mild and moderate (n = 871) | 520 (59.7) | 351 (40.3) | 5.3 (4–7.2) | p < 0.05 | 807 (67) | 64 (5.3) | 1.7 (1.1–2.6) | p < 0.05 |
| Severe and critical (n = 336) | 73 (21.7) | 263 (78.3) | 297 (24.6) | 39(3.2) | ||||
| Comorbidities | ||||||||
| No (n = 344) | 221 (66.1) | 123 (33.9) | 2.4 (1.8–3.1) | p < 0.05 | 316 (26.2) | 28 (2.3) | 1.1 (0.7–1.8) | p > 0.05 |
| Yes (n = 863) | 372 (43.1) | 491 (56.9) | 788 (65.3) | 75 (6.2) | ||||
| Feeding assistance | ||||||||
| No (n = 856) | 535 (44.3) | 321 (2.7) | 8.4 (6.1– 11.7) | p < 0.05 | 798 (66.1) | 58 (4.8) | 2 (1.3–3.1) | p < 0.05 |
| Yes (n = 351) | 58 (4.8) | 293 (24.3) | 306 (25.4) | 45 (3.7) | ||||
| Feeding route | ||||||||
| EN and/or PN (n = 1196) | 593 (49.1) | 603 (50) | p < 0.05 | 1095 (90.7) | 101 (8.4) | 2.4 (0.2–11.9) | p > 0.05 | |
| TPN (n = 11) | 0 | 11 (0.9) | 9 (0.7) | 2 (0.2) | ||||
| Malnutrition According to GLIM | Malnutrition According to BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal n (%) | Malnutrition n (%) | OR [95% CI] | p Value | Normal n (%) | Malnutrition n (%) | OR [95% CI] | p Value | |
| Albumin | ||||||||
| Normal (n = 109) | 49 (11.6) | 60 (14.1) | 2.8 (1.7–4.5) | p < 0.05 | 104 (24) | 7 (1.7) | 2.2 (1–6.1) | p > 0.05 |
| Hypoalbuminemia (n = 315) | 72 (17) | 243 (57.3) | 273 (64.4) | 42 (9.9) | ||||
| Pre-albumin | ||||||||
| Normal (n = 2) | 1 (3.4) | 1 (3.4) | 2.9 (0–235.2) | p > 0.05 | 2 (6.9) | 0 | p > 0.05 | |
| Low level (n = 27) | 7 (24.2) | 20 (69) | 24 (82.8) | 3 (10.3) | ||||
| Sodium | ||||||||
| Normal (n = 505) | 259 (25) | 246 (23.8) | 1.3 (1–1.7) | p < 0.05 | 462 (44.7) | 43 (4.2) | 1 (0.7–1.7) | p > 0.05 |
| Hyponatremia (n = 528) | 232 (22.5) | 296 (28.7) | 481 (46.6) | 47 (4.5) | ||||
| Potassium | ||||||||
| Normal (n = 933) | 444 (43) | 489 (47.3) | 1 (0.7–1.6) | p > 0.05 | 850 (82.3) | 83 (8) | 0.8 (0.3–1.7) | p > 0.05 |
| Hypokalemia (n = 100) | 47 (4.6) | 53 (5.1) | 93 (9) | 7 (0.7) | ||||
| Phosphorus | ||||||||
| Normal (n = 32) | 7 (15.6) | 25 (55.6) | 3.4 (0.4–163.8) | p > 0.05 | 29 (64.4) | 3 (6.8) | 1.8 (0.1–17.3) | p > 0.05 |
| Hypophosphatemia (n = 13) | 1 (2.2) | 12 (26.6) | 11 (24.4) | 2 (4.4) | ||||
| Severity of COVID-19 (n = 1207) | At Risk of RS (Baseline) | Incidence of RS | ||||
|---|---|---|---|---|---|---|
| n (%) | OR [95% CI] | p Value | n (%) | RR [95% CI] | p Value | |
| Mild and moderate | 163 (18.7) | 1 | <0.05 | 13 (1.5) | 1 | <0.05 |
| Severe and critical | 184 (54.8) | 5.3 [4–6.9] | 25 (7.4) | 2.47 [1.93–3.17] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.; Ta, T.V.; Bui, A.T.; Vo, S.N.; Nguyen, N.-L.T. Nutritional Status, Refeeding Syndrome and Some Associated Factors of Patients at COVID-19 Hospital in Vietnam. Nutrients 2023, 15, 1760. https://doi.org/10.3390/nu15071760
Nguyen LT, Ta TV, Bui AT, Vo SN, Nguyen N-LT. Nutritional Status, Refeeding Syndrome and Some Associated Factors of Patients at COVID-19 Hospital in Vietnam. Nutrients. 2023; 15(7):1760. https://doi.org/10.3390/nu15071760
Chicago/Turabian StyleNguyen, Linh Thuy, Thanh Van Ta, An Tuong Bui, Sy Nam Vo, and Ngoc-Lan Thi Nguyen. 2023. "Nutritional Status, Refeeding Syndrome and Some Associated Factors of Patients at COVID-19 Hospital in Vietnam" Nutrients 15, no. 7: 1760. https://doi.org/10.3390/nu15071760
APA StyleNguyen, L. T., Ta, T. V., Bui, A. T., Vo, S. N., & Nguyen, N.-L. T. (2023). Nutritional Status, Refeeding Syndrome and Some Associated Factors of Patients at COVID-19 Hospital in Vietnam. Nutrients, 15(7), 1760. https://doi.org/10.3390/nu15071760






