Low Zinc Alleviates the Progression of Thoracic Aortic Dissection by Inhibiting Inflammation
Abstract
1. Introduction
2. Methods and Material
2.1. Animals
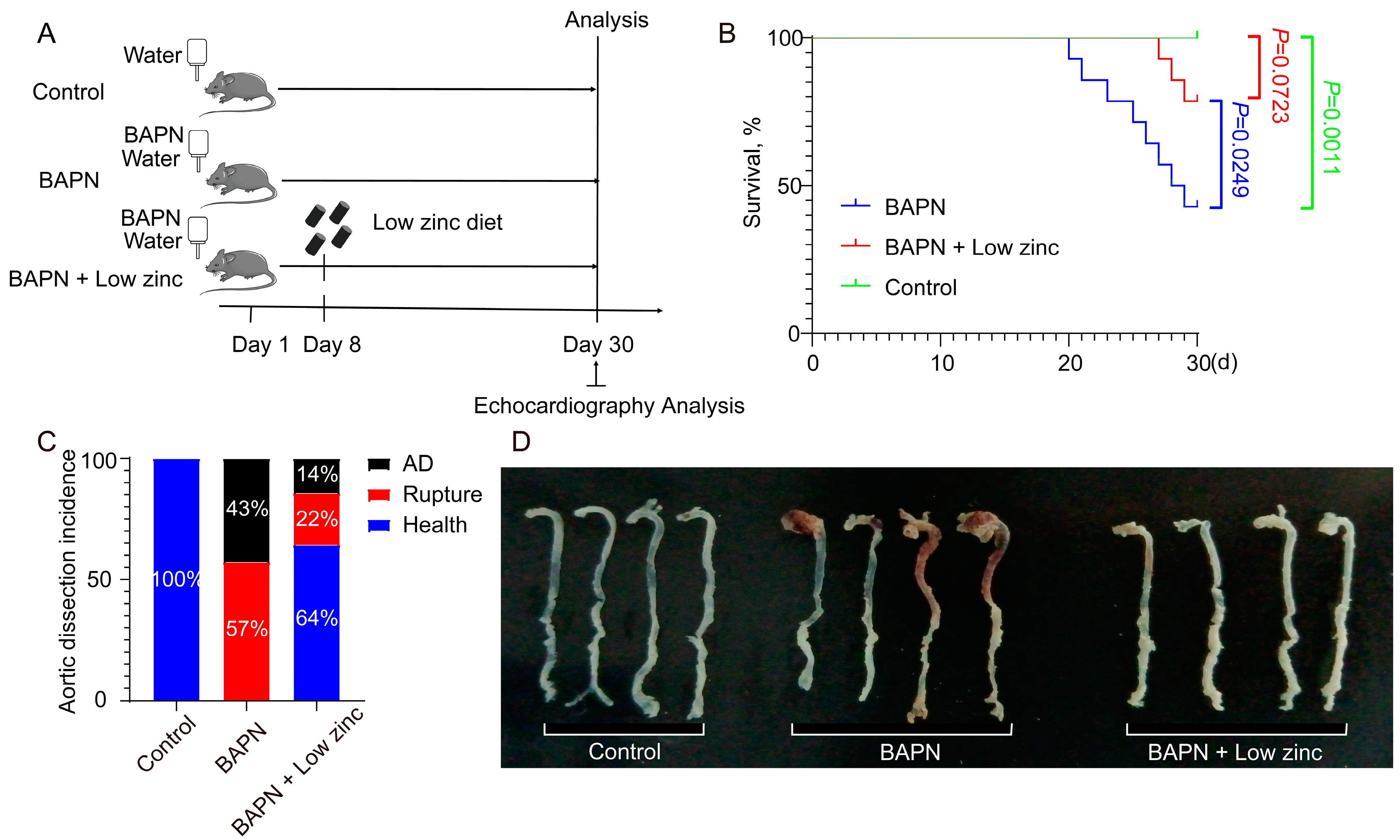
2.2. Experimental Design
2.3. Echocardiography Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Immunofluorescence Staining
2.6. Hematoxylin and Eosin (HE), Elastic Van Gieson (EVG), Masson’s Trichrome and Alcian Blue Staining
2.7. Real-Time Quantitative PCR (Q-PCR)
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Low Zinc Significantly Mitigates BAPN-Induced TAD Development in Mice
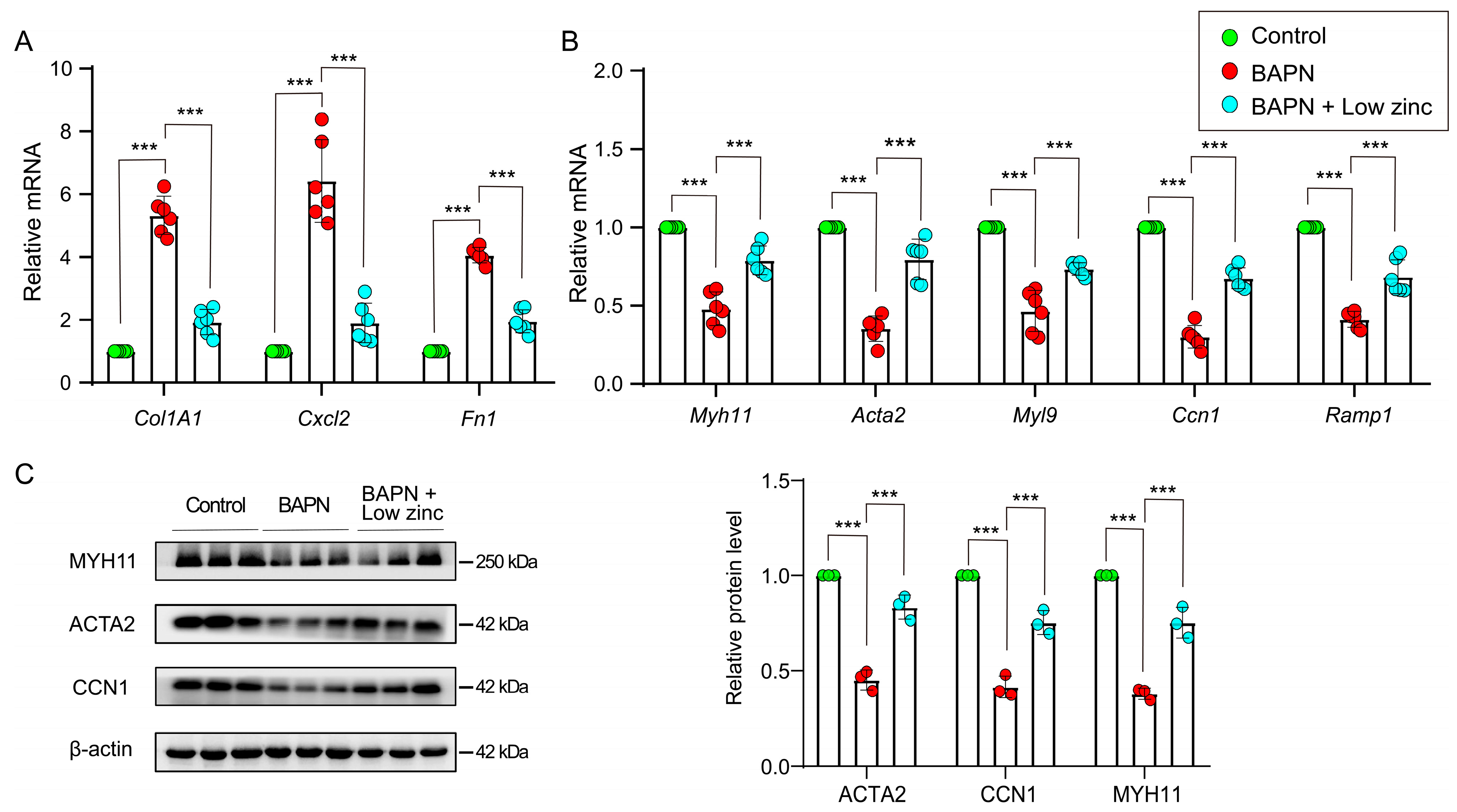
3.2. Low Zinc Inhibits the Transition of Contractile VSMCs to Synthetic VSMCs
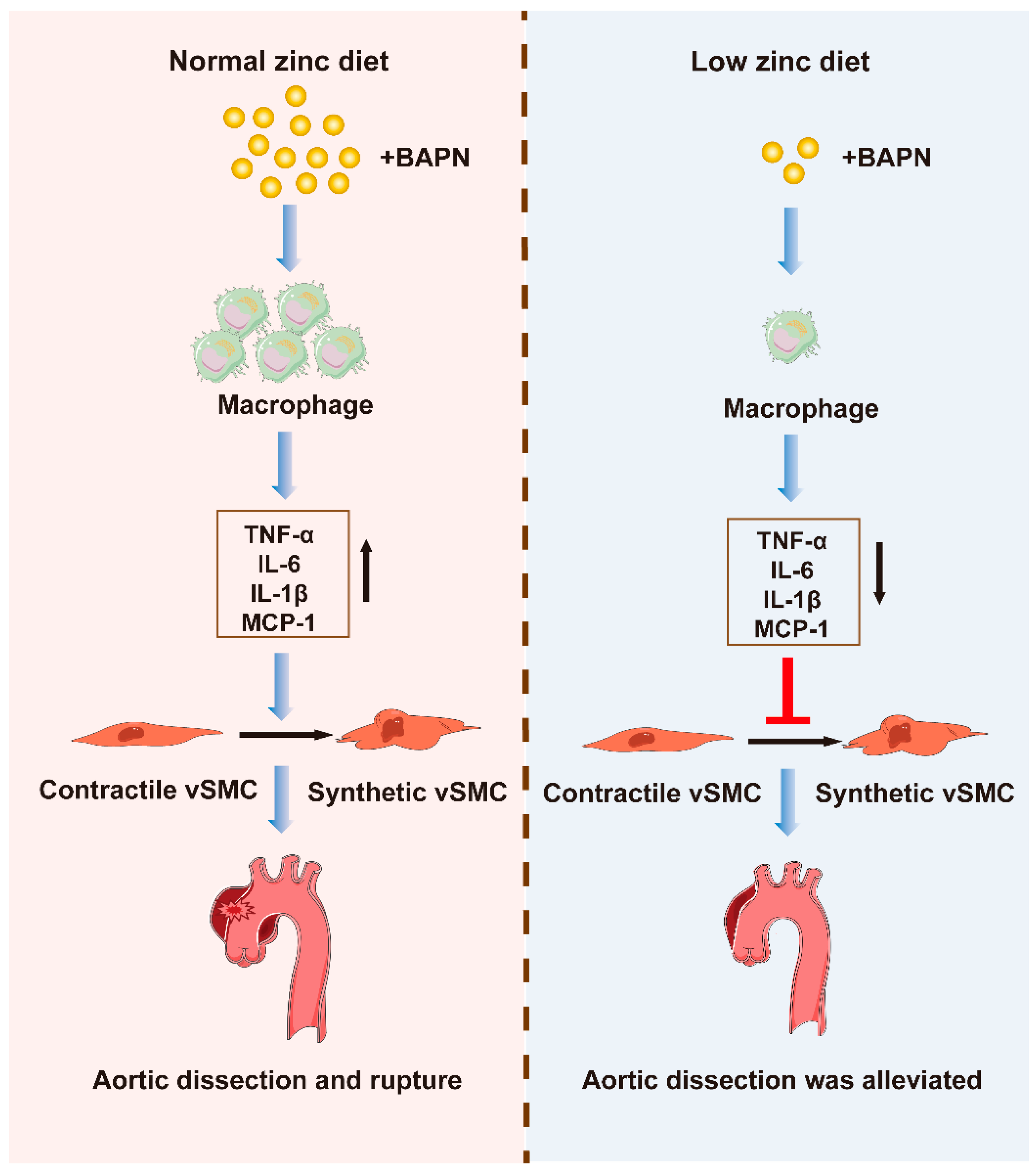
3.3. Low Zinc Alleviates TAD Development by Reducing Inflammation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.; et al. Aortic dissection. Nat. Rev. Dis. Prim. 2016, 2, 16053. [Google Scholar] [CrossRef] [PubMed]
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA 2000, 283, 897–903. [Google Scholar] [CrossRef]
- Clough, R.E.; Nienaber, C.A. Management of acute aortic syndrome. Nat. Rev. Cardiol. 2015, 12, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Dobnig, H.; Winklhofer-Roob, B.M.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; März, W. Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br. J. Nutr. 2009, 101, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Gupta, U.C.; Mittal, N.; Niaz, M.A.; Ghosh, S.; Rastogi, V. Epidemiologic study of trace elements and magnesium on risk of coronary artery disease in rural and urban Indian populations. J. Am. Coll. Nutr. 1997, 16, 62–67. [Google Scholar] [CrossRef]
- Giacconi, R.; Caruso, C.; Malavolta, M.; Lio, D.; Balistreri, C.R.; Scola, L.; Candore, G.; Muti, E.; Mocchegiani, E. Pro-inflammatory genetic background and zinc status in old atherosclerotic subjects. Ageing Res. Rev. 2008, 7, 306–318. [Google Scholar] [CrossRef]
- Socha, K.; Karwowska, A.; Kurianiuk, A.; Markiewicz-Żukowska, R.; Guzowski, A.; Gacko, M.; Hirnle, T.; Borawska, M.H. Estimation of Selected Minerals in Aortic Aneurysms-Impaired Ratio of Zinc to Lead May Predispose? Biol. Trace Elem. Res. 2021, 199, 2811–2818. [Google Scholar] [CrossRef]
- Pincemail, J.; Defraigne, J.O.; Cheramy-Bien, J.P.; Dardenne, N.; Donneau, A.F.; Albert, A.; Labropoulos, N.; Sakalihasan, N. On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm. Redox Rep. 2012, 17, 139–144. [Google Scholar] [CrossRef]
- Kurianiuk, A.; Socha, K.; Gacko, M.; Błachnio-Zabielska, A.; Karwowska, A. The Relationship between the Concentration of Cathepsin A, D, and E and the Concentration of Copper and Zinc, and the Size of the Aneurysmal Enlargement in the Wall of the Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2019, 55, 182–188. [Google Scholar] [CrossRef]
- Edvinsson, M.; Ilbäck, N.G.; Frisk, P.; Thelin, S.; Nyström-Rosander, C. Trace Element Changes in Thoracic Aortic Dissection. Biol. Trace Elem. Res. 2016, 169, 159–163. [Google Scholar] [CrossRef]
- Gać, P.; Czerwińska, K.; Macek, P.; Jaremków, A.; Mazur, G.; Pawlas, K.; Poręba, R. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021, 82, 103553. [Google Scholar] [CrossRef]
- Zhao, L.; Oliver, E.; Maratou, K.; Atanur, S.S.; Dubois, O.D.; Cotroneo, E.; Chen, C.N.; Wang, L.; Arce, C.; Chabosseau, P.L.; et al. The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature 2015, 524, 356–360. [Google Scholar] [CrossRef]
- Rink, L.; Haase, H. Zinc homeostasis and immunity. Trends Immunol. 2007, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Kirchner, H.; Rink, L. The immunobiology of zinc. Immunol. Today 1997, 18, 519–521. [Google Scholar] [CrossRef]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452s–1456s. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182 (Suppl. S1), S62–S68. [Google Scholar] [CrossRef]
- Beck, F.W.; Prasad, A.S.; Kaplan, J.; Fitzgerald, J.T.; Brewer, G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef]
- Yin, Z.Q.; Han, H.; Yan, X.; Zheng, Q.J. Research Progress on the Pathogenesis of Aortic Dissection. Curr. Probl. Cardiol. 2022, 101249. [Google Scholar] [CrossRef]
- He, R.; Guo, D.C.; Estrera, A.L.; Safi, H.J.; Huynh, T.T.; Yin, Z.; Cao, S.N.; Lin, J.; Kurian, T.; Buja, L.M.; et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006, 131, 671–678. [Google Scholar] [CrossRef]
- Schillinger, M.; Domanovits, H.; Bayegan, K.; Hölzenbein, T.; Grabenwöger, M.; Thoenissen, J.; Röggla, M.; Müllner, M. C-reactive protein and mortality in patients with acute aortic disease. Intensive Care Med. 2002, 28, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, H.; Eggebrecht, H.; Boes, T.; Antoch, G.; Rosenbaum, S.; Ladd, S.; Bockisch, A.; Barkhausen, J.; Erbel, R. Detection of inflammation in patients with acute aortic syndrome: Comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart 2008, 94, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- del Porto, F.; Proietta, M.; Tritapepe, L.; Miraldi, F.; Koverech, A.; Cardelli, P.; Tabacco, F.; de Santis, V.; Vecchione, A.; Mitterhofer, A.P.; et al. Inflammation and immune response in acute aortic dissection. Ann. Med. 2010, 42, 622–629. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Guo, D.C.; Sun, W.; Papke, C.L.; Duraisamy, S.; Estrera, A.L.; Safi, H.J.; Ahn, C.; Buja, L.M.; Arnett, F.C.; et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J. Thorac. Cardiovasc. Surg. 2008, 136, 922–929.e1. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Pan, L.; Bai, P.; Weng, X.; Liu, J.; Chen, Y.; Chen, S.; Ma, X.; Hu, K.; Sun, A.; Ge, J. Legumain Is an Endogenous Modulator of Integrin αvβ3 Triggering Vascular Degeneration, Dissection, and Rupture. Circulation 2022, 145, 659–674. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Li, L.Y.; Jiao, X.L.; Jia, L.X.; Zhang, X.P.; Wang, Y.L.; Yang, S.; Li, J.; Du, J.; Wei, Y.X.; et al. Intermittent Hypoxia Alleviates β-Aminopropionitrile Monofumarate Induced Thoracic Aortic Dissection in C57BL/6 Mice. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 1000–1010. [Google Scholar] [CrossRef]
- Yang, H.; Yang, F.; Luo, M.; Chen, Q.; Liu, X.; Zhang, Y.; Zhu, G.; Chen, W.; Li, T.; Shu, C.; et al. Metabolomic Profile Reveals That Ceramide Metabolic Disturbance Plays an Important Role in Thoracic Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 826861. [Google Scholar] [CrossRef]
- Sawada, H.; Beckner, Z.A.; Ito, S.; Daugherty, A.; Lu, H.S. β-Aminopropionitrile-induced aortic aneurysm and dissection in mice. JVS Vasc. Sci. 2022, 3, 64–72. [Google Scholar] [CrossRef]
- Rombouts, K.B.; van Merrienboer, T.A.R.; Ket, J.C.F.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur. J. Clin. Investig. 2022, 52, e13697. [Google Scholar] [CrossRef]
- Wu, D.; Shen, Y.H.; Russell, L.; Coselli, J.S.; LeMaire, S.A. Molecular mechanisms of thoracic aortic dissection. J. Surg. Res. 2013, 184, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018, 71–72, 396–420. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Moehle, C.W.; Pei, H.; Walton, S.P.; Yang, Z.; Kron, I.L.; Lau, C.L.; Owens, G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009, 138, 1392–1399. [Google Scholar] [CrossRef]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Nicoletti, A.; Li, Z.; Michel, J.B. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012, 95, 194–204. [Google Scholar] [CrossRef]
- Cai, Y.L.; Wang, Z.W. The expression and significance of IL-6, IFN-γ, SM22α, and MMP-2 in rat model of aortic dissection. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 560–568. [Google Scholar] [PubMed]
- Zhang, L.; Liao, M.F.; Tian, L.; Zou, S.L.; Lu, Q.S.; Bao, J.M.; Pei, Y.F.; Jing, Z.P. Overexpression of interleukin-1β and interferon-γ in type I thoracic aortic dissections and ascending thoracic aortic aneurysms: Possible correlation with matrix metalloproteinase-9 expression and apoptosis of aortic media cells. Eur. J. Cardiothorac. Surg. 2011, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Li, Y.; Zhang, H. The Correlation between the Inflammatory Effects of Activated Macrophages in Atherosclerosis and Aortic Dissection. Ann. Vasc. Surg. 2022, 85, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Kooreman, N.G.; Jagger, A.; Wagenhäuser, M.U.; Mehrkens, D.; Wang, Y.; Kayama, Y.; Toyama, K.; Raaz, U.; Schellinger, I.N.; et al. Systemic Upregulation of IL-10 (Interleukin-10) Using a Nonimmunogenic Vector Reduces Growth and Rate of Dissecting Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1796–1805. [Google Scholar] [CrossRef]
- Wu, X.; Ye, J.; Cai, W.; Yang, X.; Zou, Q.; Lin, J.; Zheng, H.; Wang, C.; Chen, L.; Li, Y. LDHA mediated degradation of extracellular matrix is a potential target for the treatment of aortic dissection. Pharmacol. Res. 2022, 176, 106051. [Google Scholar] [CrossRef]
- Worley, J.R.; Baugh, M.D.; Hughes, D.A.; Edwards, D.R.; Hogan, A.; Sampson, M.J.; Gavrilovic, J. Metalloproteinase expression in PMA-stimulated THP-1 cells. Effects of peroxisome proliferator-activated receptor-gamma (PPAR gamma) agonists and 9-cis-retinoic acid. J. Biol. Chem. 2003, 278, 51340–51346. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Asadi, S.; Nilashi, M.; Ali Abumalloh, R.; Ghabban, N.M.A.; Mohd Yusuf, S.Y.; Supriyanto, E.; Samad, S. A literature review on beneficial role of vitamins and trace elements: Evidence from published clinical studies. J. Trace Elem. Med. Biol. 2021, 67, 126789. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374s–1377s. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Lei, P.; Appukuttan, A.T.; Renoir, T.; Foliaki, S.; Chen, F.; Adlard, P.A.; Hannan, A.J.; Bush, A.I. Brain Zinc Deficiency Exacerbates Cognitive Decline in the R6/1 Model of Huntington’s Disease. Neurotherapeutics 2020, 17, 243–251. [Google Scholar] [CrossRef]
- Knoell, D.L.; Julian, M.W.; Bao, S.; Besecker, B.; Macre, J.E.; Leikauf, G.D.; DiSilvestro, R.A.; Crouser, E.D. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit. Care Med. 2009, 37, 1380–1388. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Hong, J.; Che, Y.; Chen, R.; Hu, Z.; Hu, X.; Wu, Q.; Hu, J.; Zhang, M. Iron deficiency promotes aortic medial degeneration via destructing cytoskeleton of vascular smooth muscle cells. Clin. Transl. Med. 2021, 11, e276. [Google Scholar] [CrossRef]
- Socha, K.; Borawska, M.H.; Gacko, M.; Guzowski, A. Diet and the content of selenium and lead in patients with abdominal aortic aneurysm. Vasa 2011, 40, 381–389. [Google Scholar] [CrossRef]
- Talebi, S.; Ghaedi, E.; Sadeghi, E.; Mohammadi, H.; Hadi, A.; Clark, C.C.T.; Askari, G. Trace Element Status and Hypothyroidism: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2020, 197, 1–14. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Schachner, T.; Golderer, G.; Sarg, B.; Lindner, H.H.; Bonaros, N.; Mikuz, G.; Laufer, G.; Werner, E.R. The amounts of alpha 1 antitrypsin protein are reduced in the vascular wall of the acutely dissected human ascending aorta. Eur. J. Cardiothorac. Surg. 2010, 37, 684–690. [Google Scholar] [CrossRef]
- Allaire, E.; Schneider, F.; Saucy, F.; Dai, J.; Cochennec, F.; Michineau, S.; Zidi, M.; Becquemin, J.P.; Kirsch, M.; Gervais, M. New insight in aetiopathogenesis of aortic diseases. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Liu, O.; Qin, Y.W.; Zhang, H.J.; Lv, Y. Association of the polymorphisms of MMP-9 and TIMP-3 genes with thoracic aortic dissection in Chinese Han population. Acta Pharmacol. Sin. 2014, 35, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, M.; Peters, A.S.; Erhart, P.; Körfer, D.; Böckler, D.; Dihlmann, S. Inflammasomes in the Pathophysiology of Aortic Disease. Cells 2021, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, M.; Caulk, A.W.; Cilfone, N.A.; Gujja, S.; Qin, L.; Chen, P.Y.; Chen, Z.; Yousef, S.; Jiao, Y.; et al. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J. Clin. Investig. 2020, 130, 1233–1251. [Google Scholar] [CrossRef]
- Bax, M.; Romanov, V.; Junday, K.; Giannoulatou, E.; Martinac, B.; Kovacic, J.C.; Liu, R.; Iismaa, S.E.; Graham, R.M. Arterial dissections: Common features and new perspectives. Front. Cardiovasc. Med. 2022, 9, 1055862. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, Q.; Su, Y.; Sun, W.; Huang, Q.; Wei, W. Zinc and its regulators in pancreas. Inflammopharmacology 2019, 27, 453–464. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; An, P.; Zhao, W.; Xia, Y.; Qi, J.; Luo, J.; Luo, Y. Low Zinc Alleviates the Progression of Thoracic Aortic Dissection by Inhibiting Inflammation. Nutrients 2023, 15, 1640. https://doi.org/10.3390/nu15071640
Zhu L, An P, Zhao W, Xia Y, Qi J, Luo J, Luo Y. Low Zinc Alleviates the Progression of Thoracic Aortic Dissection by Inhibiting Inflammation. Nutrients. 2023; 15(7):1640. https://doi.org/10.3390/nu15071640
Chicago/Turabian StyleZhu, Lin, Peng An, Wenting Zhao, Yi Xia, Jingyi Qi, Junjie Luo, and Yongting Luo. 2023. "Low Zinc Alleviates the Progression of Thoracic Aortic Dissection by Inhibiting Inflammation" Nutrients 15, no. 7: 1640. https://doi.org/10.3390/nu15071640
APA StyleZhu, L., An, P., Zhao, W., Xia, Y., Qi, J., Luo, J., & Luo, Y. (2023). Low Zinc Alleviates the Progression of Thoracic Aortic Dissection by Inhibiting Inflammation. Nutrients, 15(7), 1640. https://doi.org/10.3390/nu15071640








