Effects of Caprylic Acid and Eicosapentaenoic Acid on Lipids, Inflammatory Levels, and the JAK2/STAT3 Pathway in ABCA1-Deficient Mice and ABCA1 Knock-Down RAW264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feed Configuration
2.3. Experimental Animals
2.4. Preparation of Fatty Acids
2.5. ABCA1-KD in RAW 264.7 Cells
2.6. Serum Lipid Profiles Measurement
2.7. Inflammatory Level Measurement
2.8. Real-Time PCR Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
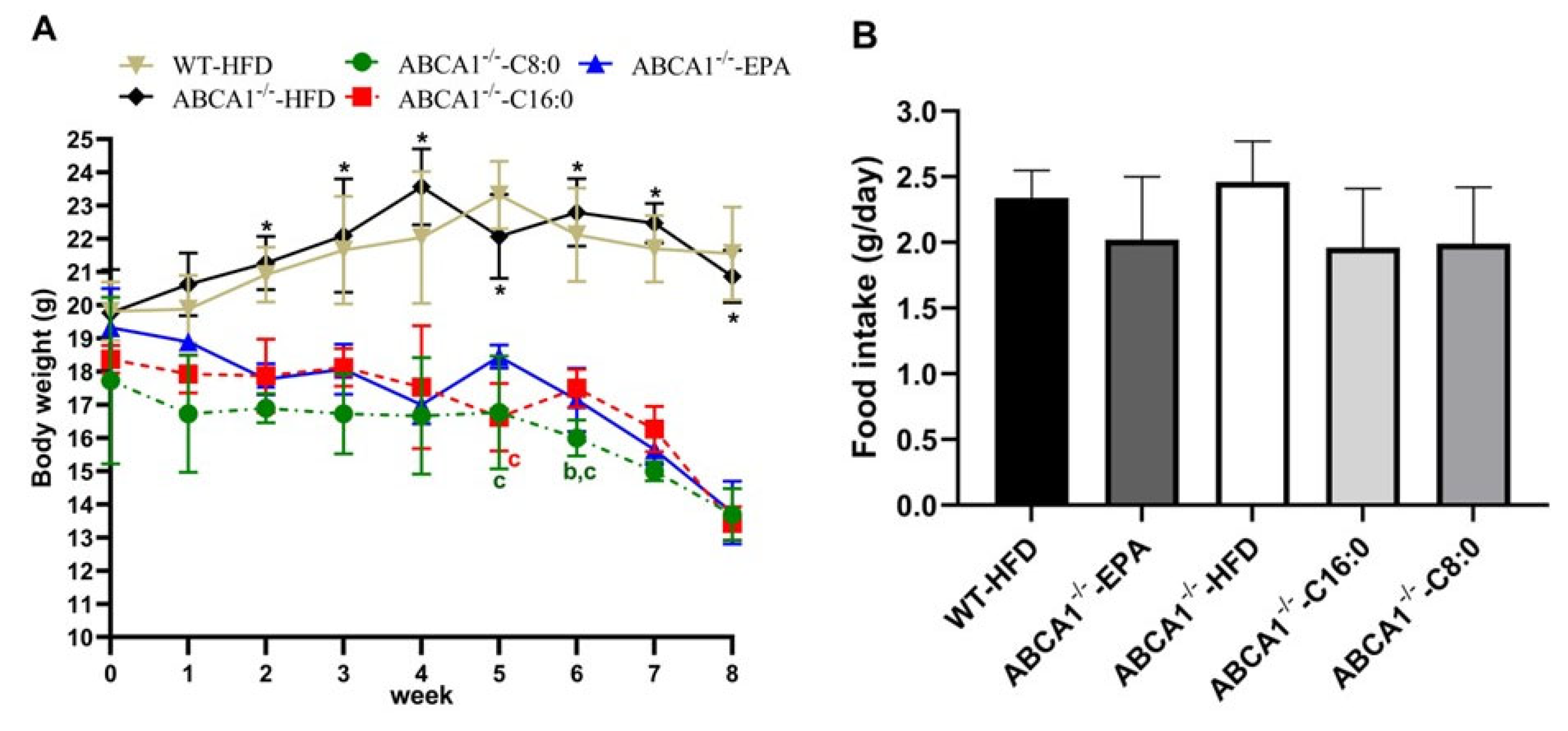
3.1. Body Weight of ABCA1−/− Mice
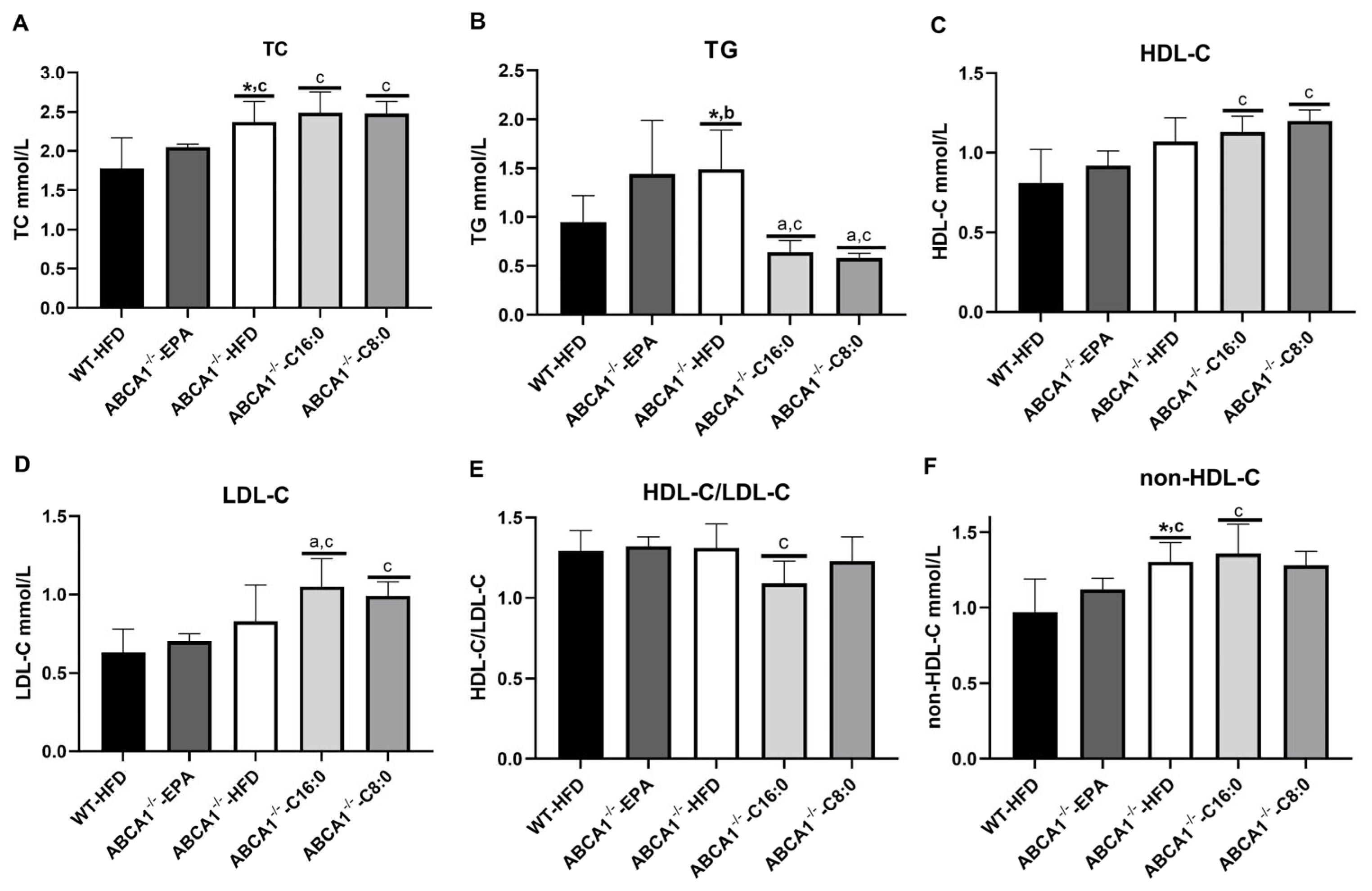
3.2. Serum Lipid Profiles in ABCA1−/− Mice
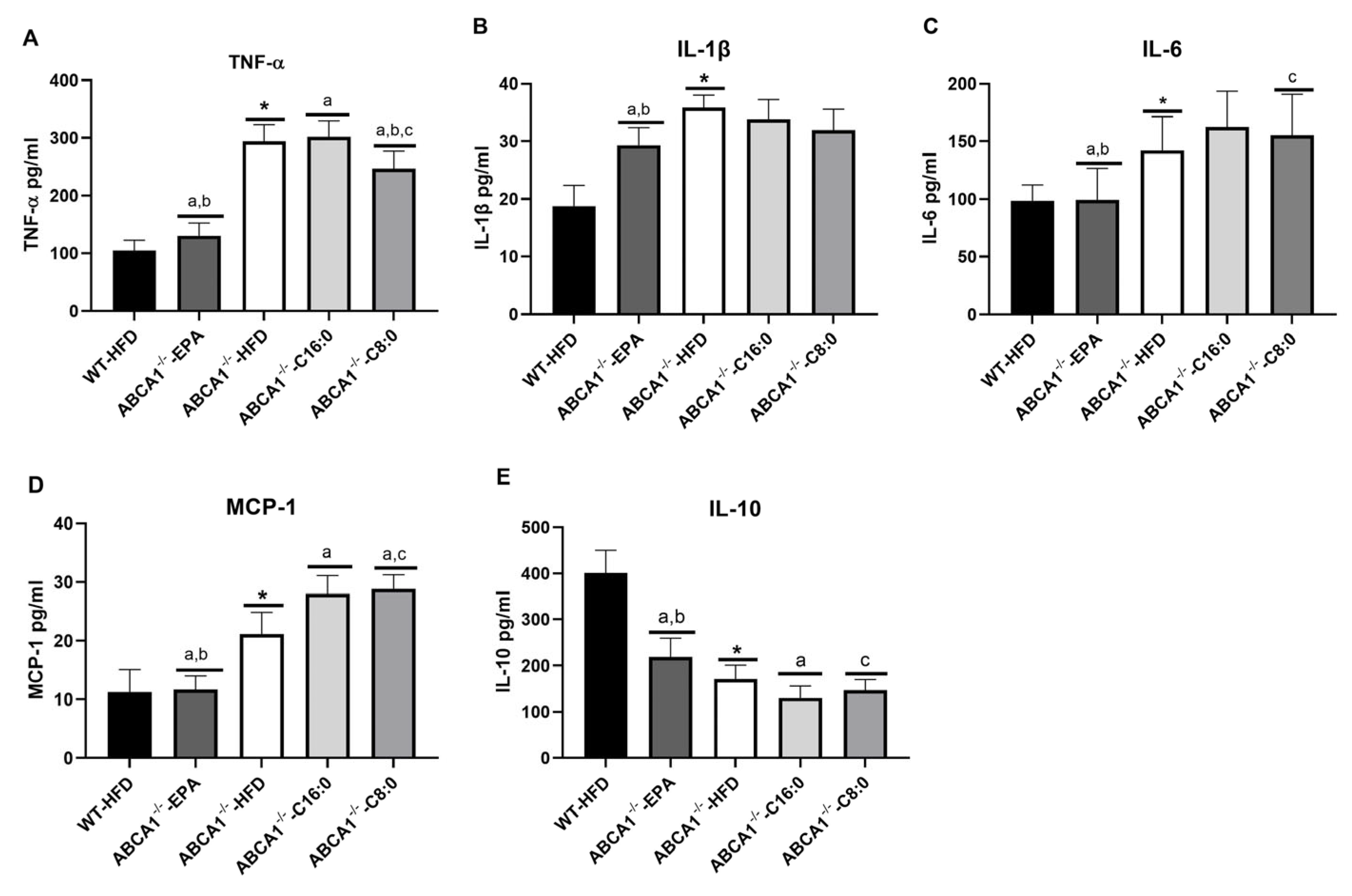
3.3. Serum Inflammatory Factors in ABCA1−/− Mice
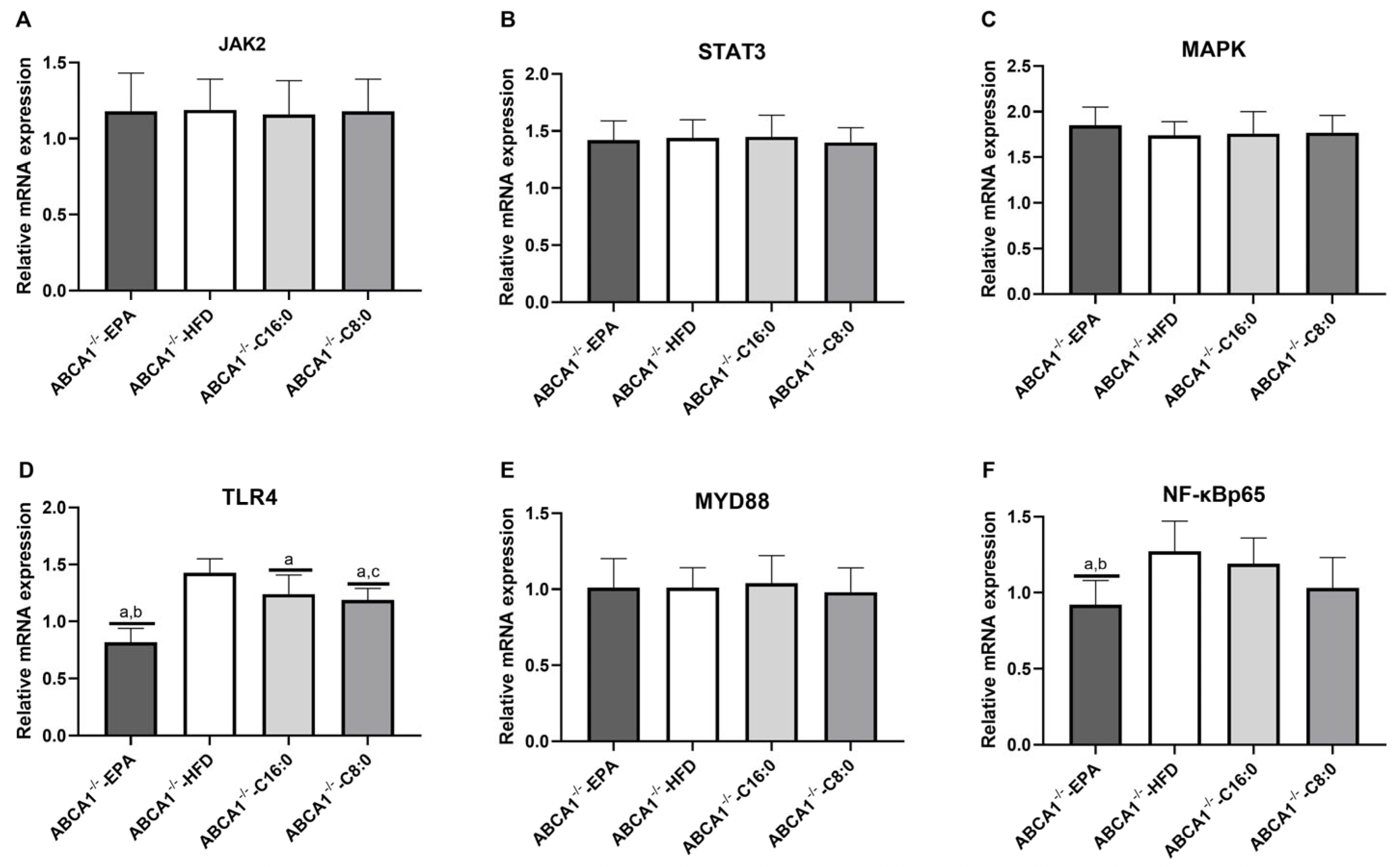
3.4. The mRNA Expression Levels of TLR4 and JAK2/STAT3 in the ABCA1−/− Mouse Aorta
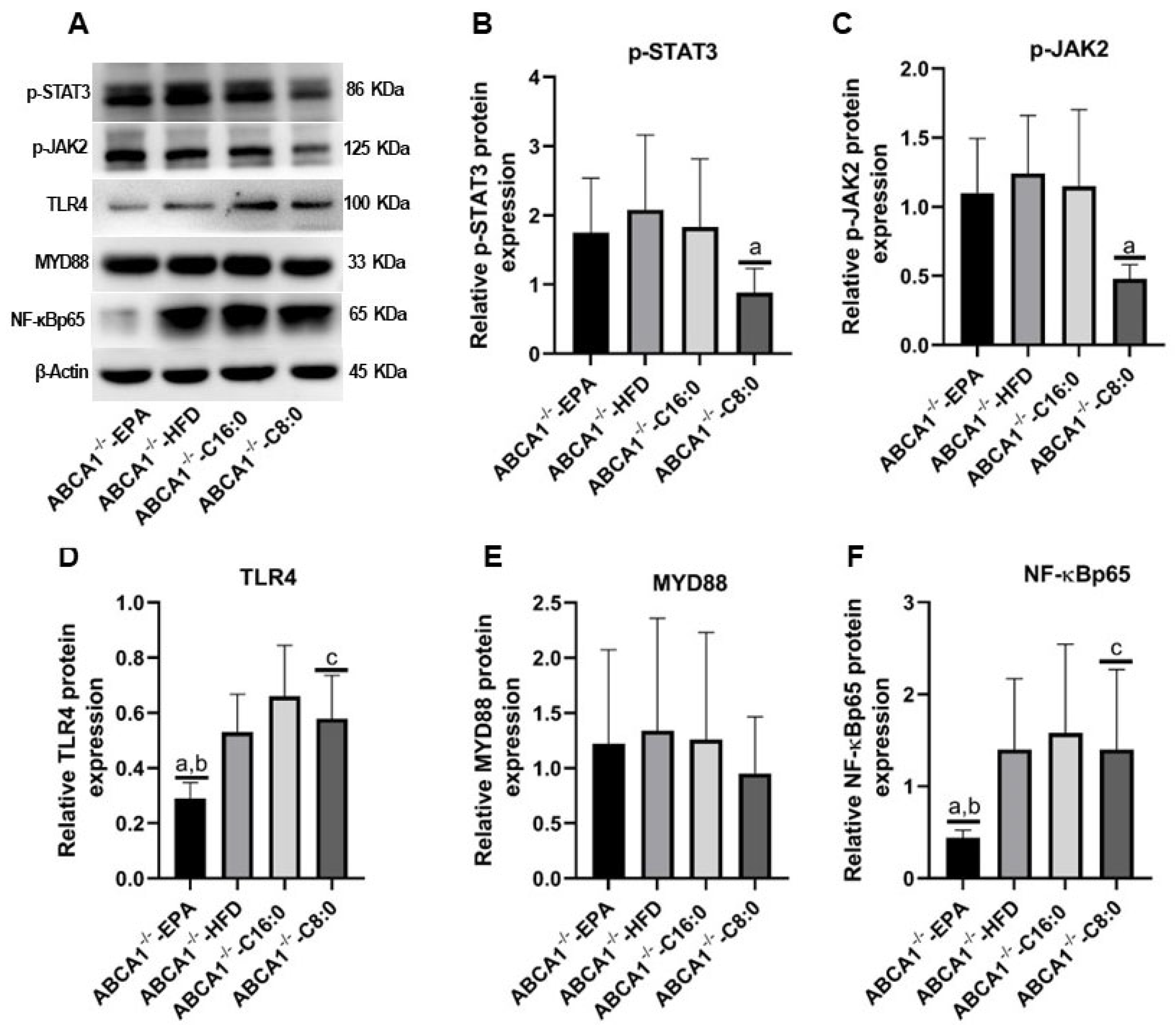
3.5. The Relative Protein Expression Levels of TLR4 and JAK2/STAT3 in the ABCA1−/− Mouse Aorta
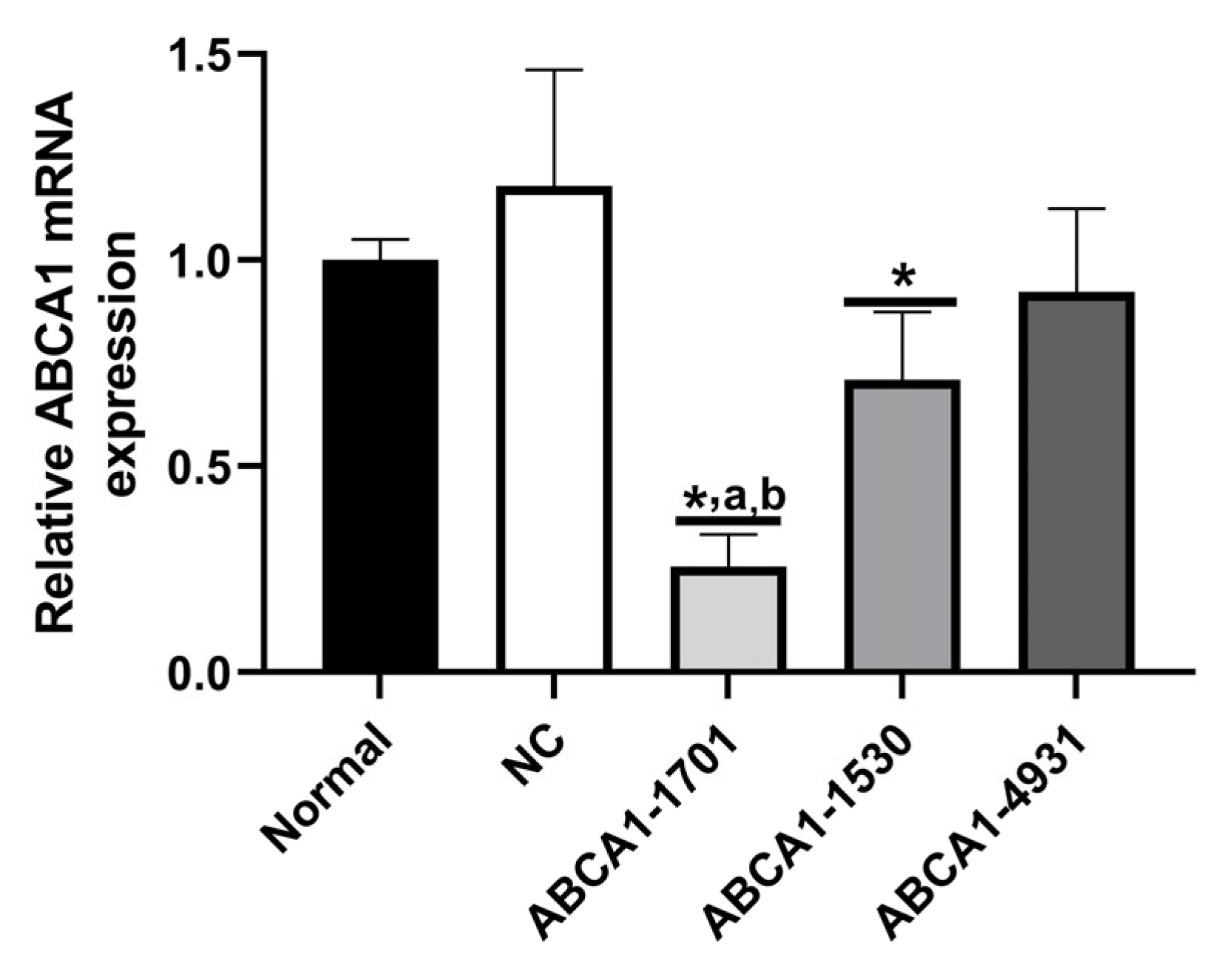
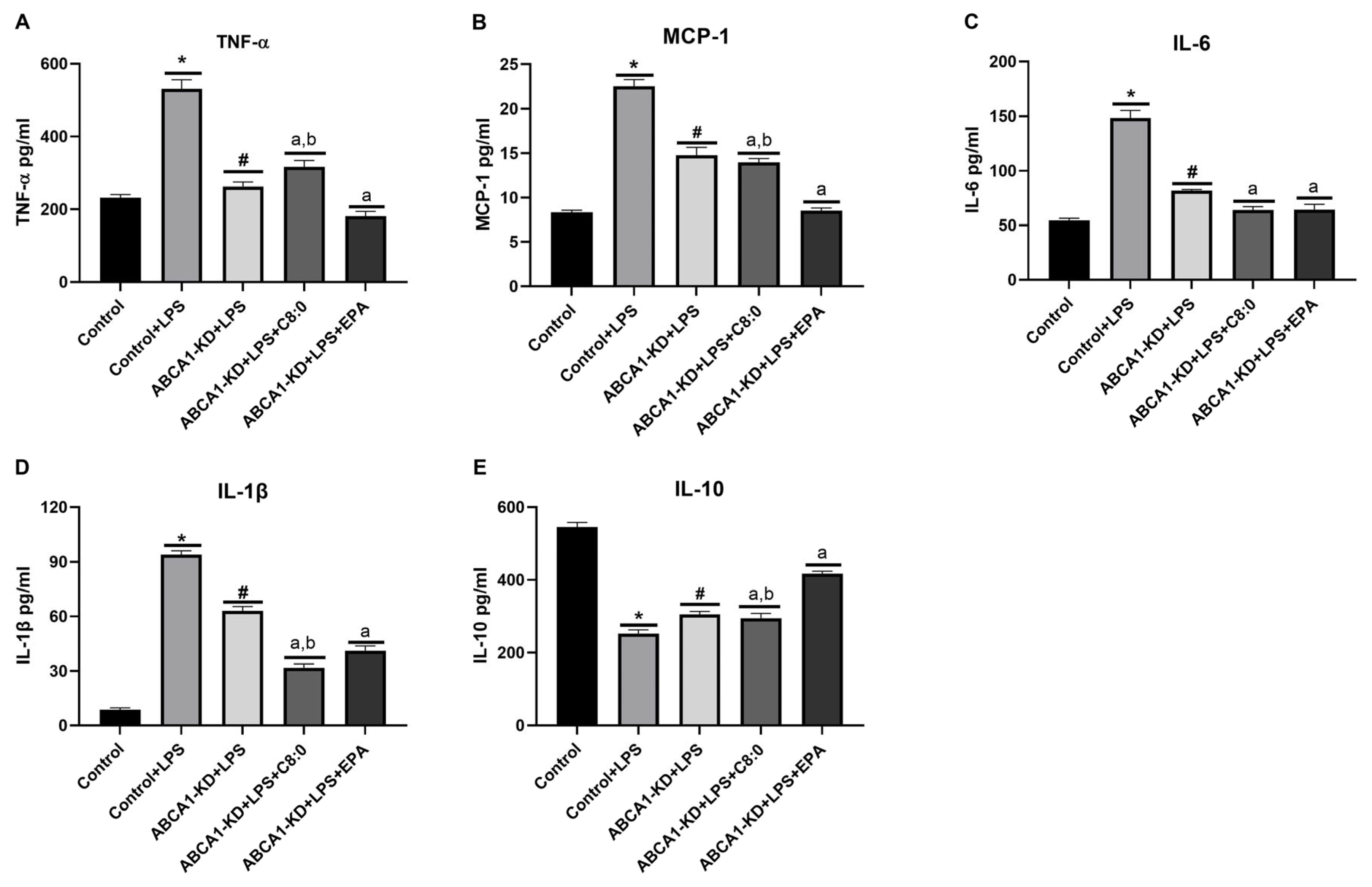
3.6. The Inflammatory Factors of ABCA1-KD RAW 264.7 Cells
3.7. The Protein Expression of JAK2/STAT3 in ABCA1-KD RAW 264.7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yusuf, S.; Rangarajan, S.; Teo, K.; Islam, S.; Li, W.; Liu, L.; Bo, J.; Lou, Q.; Lu, F.; Liu, T.; et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med. 2014, 371, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Islam, S.; Chow, C.K.; Rangarajan, S.; Dagenais, G.; Diaz, R.; Gupta, R.; Kelishadi, R.; Iqbal, R.; Avezum, A.; et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): A prospective epidemiological survey. Lancet 2011, 378, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Murphy, A.J.; Wang, M.; Pagler, T.A.; Vengrenyuk, Y.; Kappus, M.S.; Gorman, D.J.; Nagareddy, P.R.; Zhu, X.; Abramowicz, S.; et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ. Res. 2013, 112, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Liao, D.F.; Tang, C.K. ATP-binding membrane cassette transporter A1 (ABCA1): A possible link between inflammation and reverse cholesterol transport. Mol. Med. 2010, 16, 438–449. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Mujawar, Z.; Tamehiro, N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis 2010, 211, 361–370. [Google Scholar] [CrossRef]
- Yamagishi, K.; Hori, M.; Iso, H. Fish and omega-3 polyunsaturated fatty acids in relation to risk of cardiovascular disease. Nihon Rinsho. 2013, 71, 1552–1557. [Google Scholar]
- Sacks, F.M.; Campos, H. Polyunsaturated fatty acids, inflammation, and cardiovascular disease: Time to widen our view of the mechanisms. J. Clin. Endocrinol. Metab. 2006, 91, 398–400. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Zhu, H.; Hong, Y.; Wu, Z.; Hou, Y.; Li, Q.; Ding, B.; Yi, D.; Chen, H. Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun. 2013, 19, 504–515. [Google Scholar] [CrossRef]
- Decuypere, J.A.; Dierick, N.A. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: Concept, possibilities and limitations. An overview. Nutr. Res. Rev. 2003, 16, 193–210. [Google Scholar] [CrossRef]
- Nosaka, N.; Maki, H.; Suzuki, Y.; Haruna, H.; Ohara, A.; Kasai, M.; Tsuji, H.; Aoyama, T.; Okazaki, M.; Igarashi, O.; et al. Effects of margarine containing medium-chain triacylglycerols on body fat reduction in humans. J. Atheroscler. Thromb. 2003, 10, 290–298. [Google Scholar] [CrossRef]
- Rego Costa, A.C.; Rosado, E.L.; Soares-Mota, M. Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: A systematic review. Nutr. Hosp. 2012, 27, 103–108. [Google Scholar] [CrossRef]
- Bourque, C.; St-Onge, M.P.; Papamandjaris, A.A.; Cohn, J.S.; Jones, P.J. Consumption of an oil composed of medium chain triacyglycerols, phytosterols, and N-3 fatty acids improves cardiovascular risk profile in overweight women. Metabolism 2003, 52, 771–777. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Lamarche, B.; Mauger, J.F.; Jones, P.J. Consumption of a functional oil rich in phytosterols and medium-chain triglyceride oil improves plasma lipid profiles in men. J. Nutr. 2003, 133, 1815–1820. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, Y.; Wang, J.; Xu, Q.; Yu, X.; Yang, X.; Liu, Z.; Xue, C. Medium-chain triglycerides promote macrophage reverse cholesterol transport and improve atherosclerosis in ApoE-deficient mice fed a high-fat diet. Nutr. Res. 2016, 36, 964–973. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhang, P.; Liu, Y.H.; Xu, Q.; Zhang, Y.; Li, H.Z.; Liu, L.; Liu, Y.M.; Yang, X.Y.; Xue, C.Y. Caprylic Acid Improves Lipid Metabolism, Suppresses the Inflammatory Response and Activates the ABCA1/p-JAK2/p-STAT3 Signaling Pathway in C57BL/6J Mice and RAW264.7 Cells. Biomed. Environ. Sci. 2022, 35, 95–106. [Google Scholar] [CrossRef]
- Nofer, J.R. Signal transduction by HDL: Agonists, receptors, and signaling cascades. Handb. Exp. Pharmacol. 2015, 224, 229–256. [Google Scholar] [CrossRef]
- Tang, C.; Houston, B.A.; Storey, C.; LeBoeuf, R.C. Both STAT3 activation and cholesterol efflux contribute to the anti-inflammatory effect of apoA-I/ABCA1 interaction in macrophages. J. Lipid Res. 2016, 57, 848–857. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, C.; Xu, Q.; Zhang, Y.; Li, H.; Li, F.; Liu, Y.; Guo, C. Caprylic acid suppresses inflammation via TLR4/NF-kappaB signaling and improves atherosclerosis in ApoE-deficient mice. Nutr. Metab. 2019, 16, 40. [Google Scholar] [CrossRef]
- Aiello, R.J.; Brees, D.; Francone, O.L. ABCA1-deficient mice: Insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 972–980. [Google Scholar] [CrossRef]
- Orso, E.; Broccardo, C.; Kaminski, W.E.; Bottcher, A.; Liebisch, G.; Drobnik, W.; Gotz, A.; Chambenoit, O.; Diederich, W.; Langmann, T.; et al. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 2000, 24, 192–196. [Google Scholar] [CrossRef]
- Christiansen-Weber, T.A.; Voland, J.R.; Wu, Y.; Ngo, K.; Roland, B.L.; Nguyen, S.; Peterson, P.A.; Fung-Leung, W.P. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 2000, 157, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Babashamsi, M.M.; Koukhaloo, S.Z.; Halalkhor, S.; Salimi, A.; Babashamsi, M. ABCA1 and metabolic syndrome; a review of the ABCA1 role in HDL-VLDL production, insulin-glucose homeostasis, inflammation and obesity. Diabetes Metab. Syndr. 2019, 13, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Langmann, T.; Klucken, J.; Reil, M.; Liebisch, G.; Luciani, M.F.; Chimini, G.; Kaminski, W.E.; Schmitz, G. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): Evidence for sterol-dependent regulation in macrophages. Biochem. Biophys. Res. Commun. 1999, 257, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mauerer, R.; Ebert, S.; Langmann, T. High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages. Exp. Mol. Med. 2009, 41, 126–132. [Google Scholar] [CrossRef]
- Shen, J.; Hafeez, A.; Stevenson, J.; Yang, J.; Yin, C.; Li, F.; Wang, S.; Du, H.; Ji, X.; Rafols, J.A.; et al. Omega-3 fatty acid supplement prevents development of intracranial atherosclerosis. Neuroscience 2016, 334, 226–235. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, R.; Zhang, Y.; Xu, Q.; Zhang, J.; Zhang, Y.; Zheng, Z.; Yu, X.; Jing, H.; et al. A good response to oil with medium- and long-chain fatty acids in body fat and blood lipid profiles of male hypertriglyceridemic subjects. Asia Pac. J. Clin. Nutr. 2009, 18, 351–358. [Google Scholar]
- Xue, C.; Liu, Y.; Wang, J.; Zhang, R.; Zhang, Y.; Zhang, J.; Zhang, Y.; Zheng, Z.; Yu, X.; Jing, H.; et al. Consumption of medium- and long-chain triacylglycerols decreases body fat and blood triglyceride in Chinese hypertriglyceridemic subjects. Eur. J. Clin. Nutr. 2009, 63, 879–886. [Google Scholar] [CrossRef]
- Parks, J.S.; Chung, S.; Shelness, G.S. Hepatic ABC transporters and triglyceride metabolism. Curr. Opin. Lipidol. 2012, 23, 196–200. [Google Scholar] [CrossRef]
- Drobnik, W.; Lindenthal, B.; Lieser, B.; Ritter, M.; Christiansen Weber, T.; Liebisch, G.; Giesa, U.; Igel, M.; Borsukova, H.; Büchler, C.; et al. ATP-binding cassette transporter A1 (ABCA1) affects total body sterol metabolism. Gastroenterology 2001, 120, 1203–1211. [Google Scholar] [CrossRef]
- Haghpassand, M.; Bourassa, P.A.; Francone, O.L.; Aiello, R.J. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J. Clin. Investig. 2001, 108, 1315–1320. [Google Scholar] [CrossRef]
- Westerterp, M.; Tsuchiya, K.; Tattersall, I.W.; Fotakis, P.; Bochem, A.E.; Molusky, M.M.; Ntonga, V.; Abramowicz, S.; Parks, J.S.; Welch, C.L.; et al. Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Endothelial Cells Accelerates Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1328–1337. [Google Scholar] [CrossRef]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef]
- Westerterp, M.; Bochem, A.E.; Yvan-Charvet, L.; Murphy, A.J.; Wang, N.; Tall, A.R. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ. Res. 2014, 114, 157–170. [Google Scholar] [CrossRef]
- Chai, A.B.; Ammit, A.J.; Gelissen, I.C. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir. Res. 2017, 18, 41. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef]
- Haversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Ouhtit, A.; Halade, G.V.; Rahman, M.M. Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content. Nutrients 2019, 11, 2701. [Google Scholar] [CrossRef]
- Daci, A.; Ozen, G.; Uyar, I.; Civelek, E.; Yildirim, F.I.A.; Durman, D.K.; Teskin, O.; Norel, X.; Uydes-Dogan, B.S.; Topal, G. Omega-3 polyunsaturated fatty acids reduce vascular tone and inflammation in human saphenous vein. Prostaglandins Other Lipid Mediat. 2017, 133, 29–34. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, G.J.; Yin, K.; Xia, X.D.; Gong, D.; Zhao, Z.W.; Chen, L.Y.; Zheng, X.L.; Tang, X.E.; Tang, C.K. Apolipoprotein A-1 Binding Protein Inhibits Inflammatory Signaling Pathways by Binding to Apolipoprotein A-1 in THP-1 Macrophages. Circ. J. 2018, 82, 1396–1404. [Google Scholar] [CrossRef]
- Soro-Paavonen, A.; Westerbacka, J.; Ehnholm, C.; Taskinen, M.R. Metabolic syndrome aggravates the increased endothelial activation and low-grade inflammation in subjects with familial low HDL. Ann. Med. 2006, 38, 229–238. [Google Scholar] [CrossRef]
- He, P.; Gelissen, I.C.; Ammit, A.J. Regulation of ATP binding cassette transporter A1 (ABCA1) expression: Cholesterol-dependent and–independent signaling pathways with relevance to inflammatory lung disease. Respir. Res. 2020, 21, 250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Olefsky, J.M.; Osborn, O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 2011, 32, 543–550. [Google Scholar] [CrossRef]
- Aiello, R.J.; Brees, D.; Bourassa, P.A.; Royer, L.; Lindsey, S.; Coskran, T.; Haghpassand, M.; Francone, O.L. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 630–637. [Google Scholar] [CrossRef]

| Indicators | Primer | Sequence | Primer Bank ID |
|---|---|---|---|
| β-actin | Forward | 5′-GGCTGTATTCCCCTCCATCG -3′ | 6671509a1 |
| Reverse | 5′-CCAGTTGGTAACAATGCCATGT -3′ | ||
| JAK2 | Forward | 5′-TTGTGGTATTACGCCTGTGTATC-3′ | 6680508a1 |
| Reverse | 5′-ATGCCTGGTTGACTCGTCTAT-3′ | ||
| STAT3 | Forward | 5′-CAATACCATTGACCTGCCGAT-3′ | 13277852a1 |
| Reverse | 5′-GAGCGACTCAAACTGCCCT-3′ | ||
| MAPK | Forward | 5′-GGCTCGGCACACTGATGAT-3′ | 6754632a1 |
| Reverse | 5′-TGGGGTTCCAACGAGTCTTAAA-3′ | ||
| TLR4 | Forward | 5′-ATGGCATGGCTTACACCACC-3′ | 10946594a1 |
| Reverse | 5′-GAGGCCAATTTTGTCTCCACA-3′ | ||
| MYD88 | Forward | 5′-TCATGTTCTCCATACCCTTGGT-3′ | 26354939a1 |
| Reverse | 5′-AAACTGCGAGTGGGGTCAG-3′ | ||
| NF-κBp65 | Forward | 5′-CACCGGATTGAAGAGAAGCG-3′ | 30047197a1 |
| Reverse | 5′-AAGTTGATGGTGCTGAGGGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, P.; Liu, Y.; Liu, Z.; Xu, Q.; Zhang, Y.; Liu, L.; Yang, X.; Li, L.; Xue, C. Effects of Caprylic Acid and Eicosapentaenoic Acid on Lipids, Inflammatory Levels, and the JAK2/STAT3 Pathway in ABCA1-Deficient Mice and ABCA1 Knock-Down RAW264.7 Cells. Nutrients 2023, 15, 1296. https://doi.org/10.3390/nu15051296
Zhang X, Zhang P, Liu Y, Liu Z, Xu Q, Zhang Y, Liu L, Yang X, Li L, Xue C. Effects of Caprylic Acid and Eicosapentaenoic Acid on Lipids, Inflammatory Levels, and the JAK2/STAT3 Pathway in ABCA1-Deficient Mice and ABCA1 Knock-Down RAW264.7 Cells. Nutrients. 2023; 15(5):1296. https://doi.org/10.3390/nu15051296
Chicago/Turabian StyleZhang, Xinsheng, Peng Zhang, Yinghua Liu, Zhao Liu, Qing Xu, Yong Zhang, Lu Liu, Xueyan Yang, Liya Li, and Changyong Xue. 2023. "Effects of Caprylic Acid and Eicosapentaenoic Acid on Lipids, Inflammatory Levels, and the JAK2/STAT3 Pathway in ABCA1-Deficient Mice and ABCA1 Knock-Down RAW264.7 Cells" Nutrients 15, no. 5: 1296. https://doi.org/10.3390/nu15051296
APA StyleZhang, X., Zhang, P., Liu, Y., Liu, Z., Xu, Q., Zhang, Y., Liu, L., Yang, X., Li, L., & Xue, C. (2023). Effects of Caprylic Acid and Eicosapentaenoic Acid on Lipids, Inflammatory Levels, and the JAK2/STAT3 Pathway in ABCA1-Deficient Mice and ABCA1 Knock-Down RAW264.7 Cells. Nutrients, 15(5), 1296. https://doi.org/10.3390/nu15051296






