Increased Fruit and Vegetable Consumption Mitigates Oxidative Damage and Associated Inflammatory Response in Obese Subjects Independent of Body Weight Change
Abstract
1. Introduction
2. Material and Methods
2.1. Measurements
2.2. Statistics and Analysis
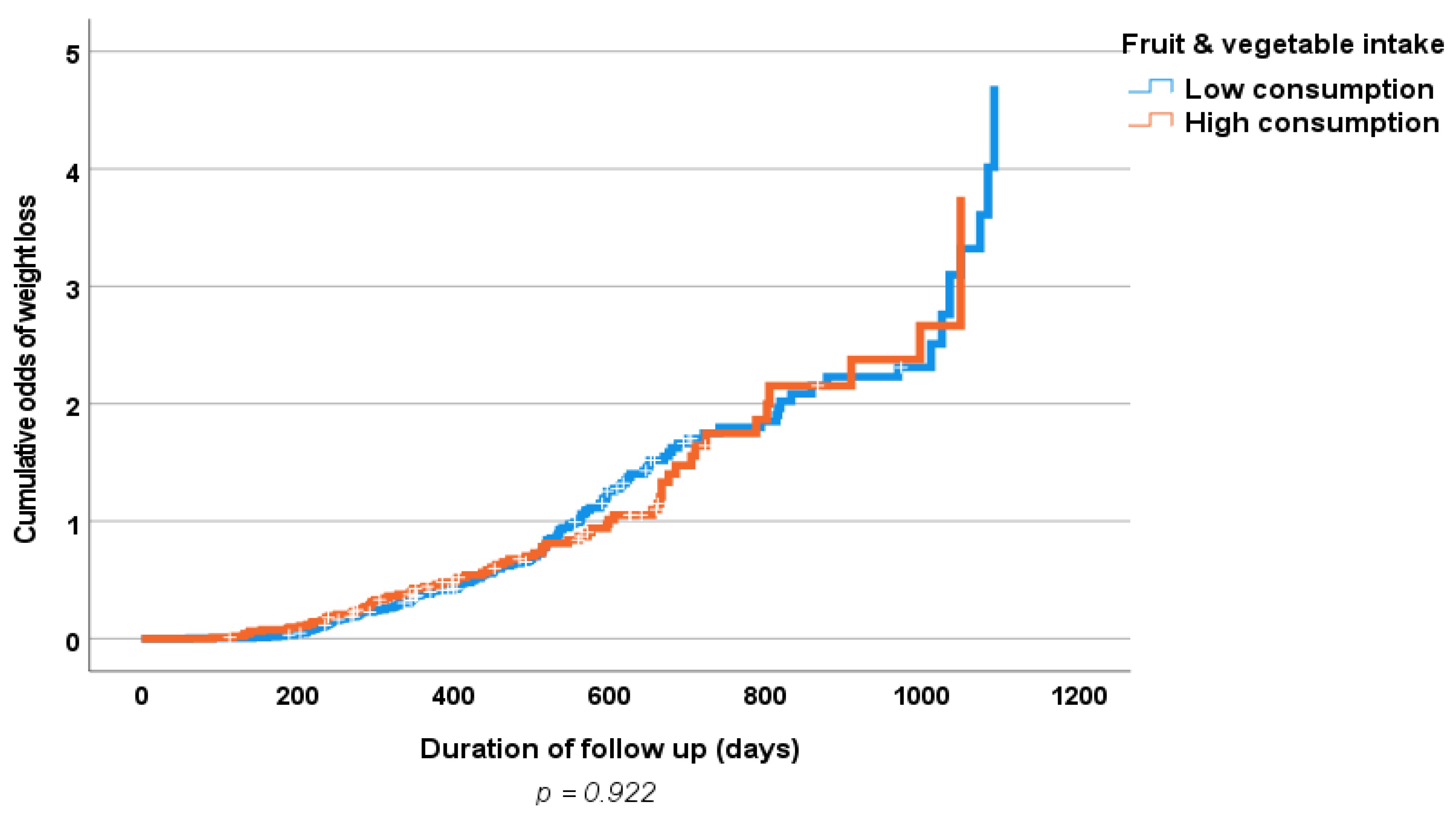
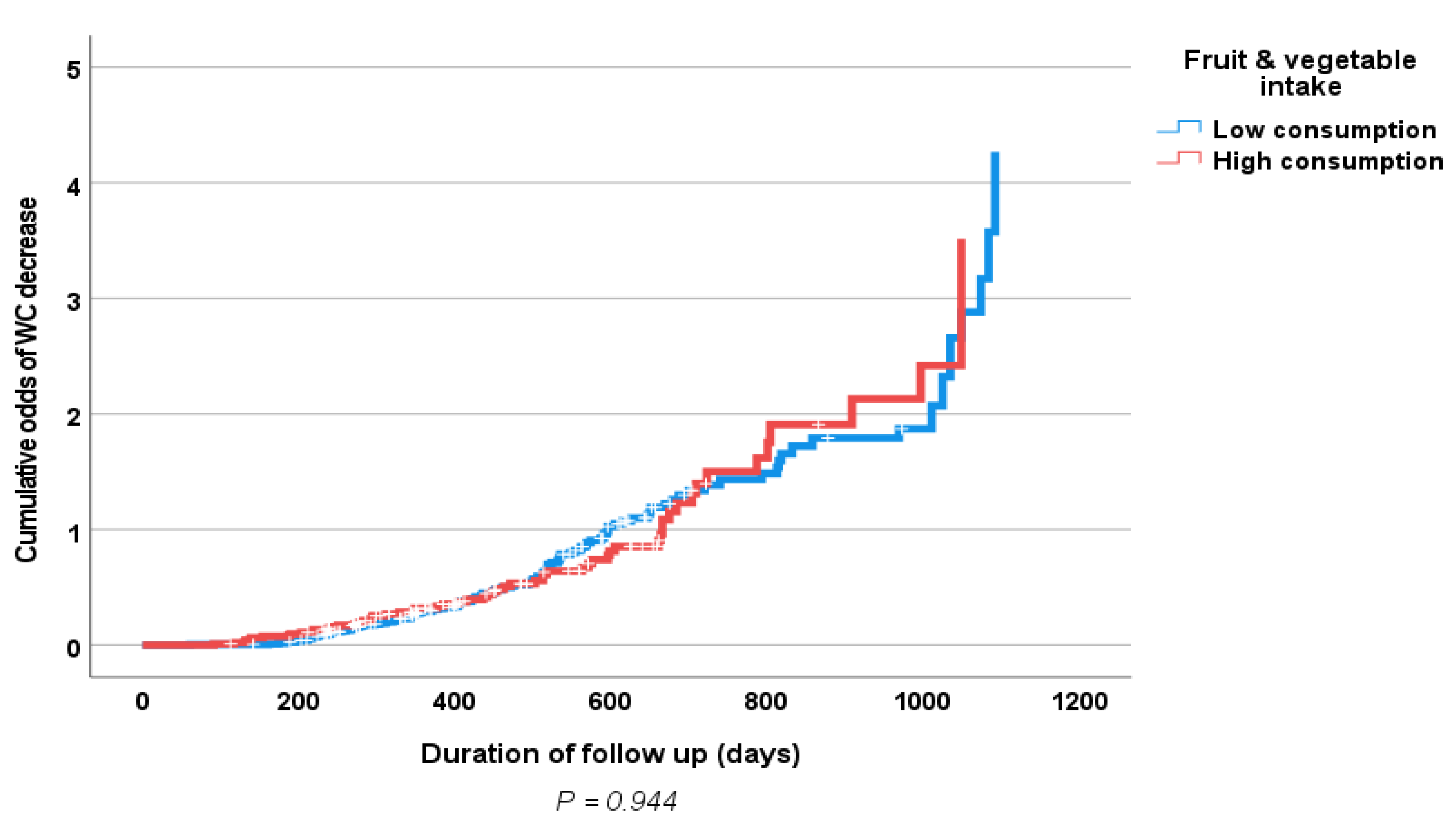
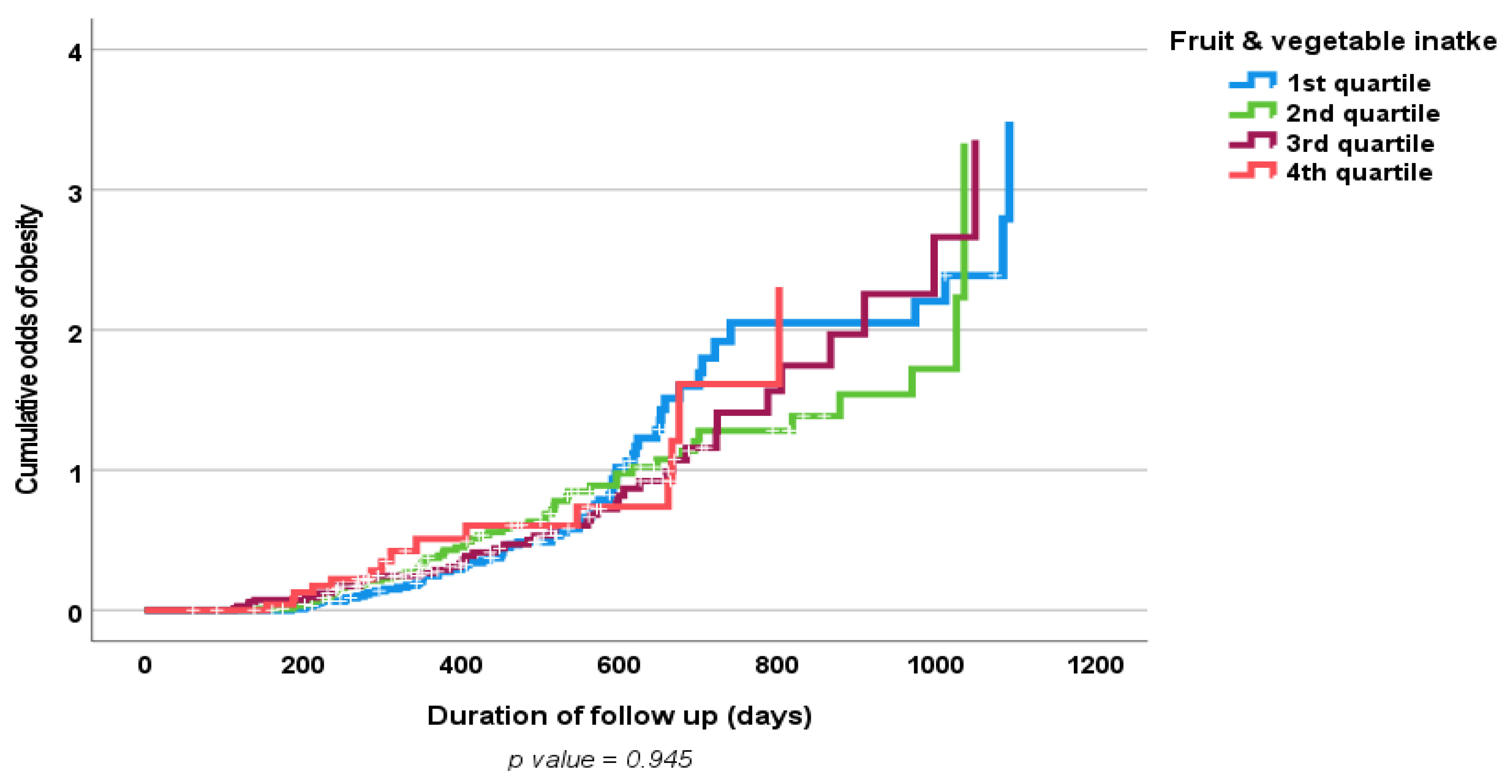
3. Results
4. Discussion
4.1. Fruit and Vegetable-Mechanisms of Action
4.2. Antioxidants, Oxidative Damage, and Inflammation
4.3. Effects of Fruit and Vegetable Consumption on Weight and WC Loss
4.4. Limitations and Strength of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKinsey Global Institute. Overcoming Obesity: An Initial Economic Analysis. Available online: http://www.mckinsey.com/insights/economic_studies/how_the_world_could_better_fight_obesity (accessed on 20 March 2023).
- McLellan, F. Obesity rising to alarming levels around the world. Lancet 2002, 359, 1412. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bakir, A.; Abi Saab, B.; Roglic, G.; King, H. Glucose intolerance and associated factors in the multi-ethnic population of the United Arab Emirates: Results of a national survey. Diabetes Res. Clin. Pract. 2005, 69, 188–195. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and risk of myocardial infarction in 27,000 participants from 52 countrries: A case-control study. Lancet 2006, 366, 1640–1649. [Google Scholar]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- The Report of a British Nutrition Foundation Task Force. Cardiovascular Disease, Diet, Nutrition and Emerging Risk Factors; Blackwell Publishing Ltd.: Oxford, UK, 2005; ISBN 1-4051-01660. [Google Scholar]
- John, J.H.; Ziebland, S.; Yudkin, P.; Roe, L.S.; Neil, H.A.W.; Oxford Fruit and Vegetable Study Group. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet 2002, 359, 1969–1974. [Google Scholar]
- Gariballa, S.; Kosanovic, M.; Yasin, J.; El Essa, A. Oxidative damage and inflammation in obese diabetic Emirati subjects. Nutrients 2014, 6, 4872–4880. [Google Scholar] [CrossRef]
- Tohill, B.C.; Seymour, J.; Serdula, M.; Kettel-Khan, L.; Rolls, B.J. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr. Rev. 2004, 62, 365–374. [Google Scholar] [CrossRef]
- Pomerleau, J.; Lock, K.; Knai, C.; McKee, M. Interventions designed to increase adult fruit and vegetable intake can be effective: A systematic review of the literature. J. Nutr. 2005, 135, 2486–2495. [Google Scholar] [CrossRef]
- Ledoux, T.A.; Hingle, M.D.; Baranowski, T. Relationship of fruit and vegetable intake with adiposity: A systematic review. Obes. Rev. 2011, 12, e143–e150. [Google Scholar] [CrossRef]
- Yarnell, J.W.; Fehily, A.M.; Milbank, J.E.; Sweetnam, P.M.; Walker, C.L. A short dietary questionnaire for use in epidemiological survey: Comparison with weighed dietary records. Hum. Nutr. Appl. Nutr. 1983, 37, 103–112. [Google Scholar] [PubMed]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Oxidative damage and associated inflammatory risk factors in obese Emirati women. Saudi Med. J. 2017, 38, 960–964. [Google Scholar] [PubMed]
- Sarwer, D.B.; Green, A.V.S.; Vetter, M.L.; Wadden, T.A. Behavior therapy for obesity: Where are we now? Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef]
- The Lancet. Global Burden of Diseases, Injuries, and Risk Factors Study. 2013. Available online: http://www.thelancet.com/themed/global-burden-of-disease (accessed on 20 March 2023).
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Capewell, S. United Nations’ dietary policies to prevent cardiovascular disease. BMJ 2011, 343, d5747. [Google Scholar]
- Malhotra, A.; Maruthappu, M.; Stephenson, T. Healthy eating: An NHS priority A sure way to improve health outcomes for NHS staff and the public. Postgrad. Med. J. 2014, 90, 671–672. [Google Scholar]
- Available online: http://wholesomewave.org/wholesome-wave-fruit-vegetable-prescription-program-launches-in-new-york-city-public-hospitals-targets-those-at-risk-of-obesity (accessed on 20 March 2023).
- Pereira, M.A.; Ludwig, D.S. Dietary fiber and body-weight regulation: Observations and mechanisms. Pediatr. Clin. North Am. 2001, 48, 969–980. [Google Scholar]
- Du, H.; van der A, D.L.; Boshuizen, H.C.; Forouhi, N.G.; Wareham, N.J.; Halkjær, J.; Tjønneland, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am. J. Clin. Nutr. 2010, 91, 329–336. [Google Scholar] [CrossRef]
- Hill, M.F. Emerging role for antioxidant therapy in protection against diabetic cardiac complications: Experimental and clinical evidence for utilization of classic and new antioxidants. Curr. Cardiol. Rev. 2008, 4, 259–268. [Google Scholar]
- Evans, J.L. Antoxidants: Do they have a role in the treatment of insulin resistance? Indian J. Med. Res. 2007, 125, 355–372. [Google Scholar] [PubMed]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef] [PubMed]
- Puchau, B.; Zulet, M.A.; de Echávarri, A.G.; Hermsdorff, H.H.M.; Martínez, J.A. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young men. Nutrition 2010, 26, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Moslehi, N.; Bastan, S.; Azizi, F. Colors of fruits and vegetables and 3-year changes of cardiometabolic risk factors in adults: Tehran lipid and glucose study. Eur. J. Clin. Nutr. 2015, 69, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Katcher, H.I.; Legro, R.S.; Kunselman, A.R.; Gillies, P.J.; Demers, L.M.; Bagshaw, D.M.; Kris-Etherton, P.M. The effects of a whole grain–enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 79–90. [Google Scholar] [CrossRef]
- Koh-Banerjee, P.; Rimm, E.B. Whole grain consumption and weight gain: A review of the epidemiological evidence, potential mechanisms and opportunities for future research. Proc. Nutr. Soc. 2003, 62, 25–29. [Google Scholar]
- Holmes, B.; Dick, K.; Nelson, M. A comparison of four dietary assessment methods in materially deprived households in England. Public Health Nutr. 2008, 11, 444–456. [Google Scholar] [CrossRef]

| BMI | ||||
|---|---|---|---|---|
| Normal Risk (BMI ≤ 25) (n = 69) | High Health Risk (BMI 25.1–29.9) (n = 284) | Increased Health Risk (BMI ≥ 30) (n = 584) | p Value | |
| Age (years) | 34 (12) | 39 (12) | 40 (12) | 0.001 |
| Females, n (%) | 53 (77) | 167 (59) | 491 (84) | 0.001 |
| Previous diabetes, n(%) | 6 (9) | 39 (14) | 86 (15) | 0.390 |
| Previous hypertension, n(%) | 4 (6) | 31 (11) | 104 (18) | 0.001 |
| Waist circumference (cm) | 81 (11) | 90 (8) | 103 (13) | 0.001 |
| Systolic BP (mmHg) | 117 (9) | 121 (15) | 123 (14) | 0.001 |
| Diastolic BP (mmHg) | 74 (6) | 74 (8) | 76 (10) | 0.051 |
| Total cholesterol (mmol/L) | 4.7 (1) | 5.0 (0.9) | 4.9 (0.9) | 0.073 |
| Triglycerides (mmol/L) | 1.1 (0.6) | 1.38 (1) | 1.42 (1) | 0.482 |
| HDL (mmol/L) | 1.39 (0.4) | 1.21 (0.4) | 1.10 (0.3) | 0.048 |
| HbA1c, = (%) | 5.4 (0.6) | 5.9 (1) | 6.0 (1) | 0.001 |
| Glucose (mmol/L) | 5.8 (2) | 8.8 (10) | 8.9 (11) | 0.060 |
| 1st Quartile (0.5 to 3.0 Servings/Day) (n = 159) | 2nd Quartile (3.5 to 4.5 Servings/Day) (n = 177) | 3rd Quartile (4.6–5.6 Servings/Day) (n = 189) | 4th Quartile (5.7 to 9.8 Servings/Day) (n = 166) | p Value * | |
|---|---|---|---|---|---|
| Body weight (kg) | 85.6 (17) | 86.5 (16) | 86.5 (16) | 86.2 (15) | 0.947 |
| Body mass index | 34.3 (5) | 33.3 (6) | 33.0 (6) | 33.3 (6) | 0.165 |
| Waist circumference (cm) | 101 (14) | 99.5 (13) | 99.9 (14) | 98.2 (12) | 0.359 |
| Systolic BP (mmHg) | 121 (14) | 119 (14) | 123 (14) | 122 (13) | 0.080 |
| Diastolic BP (mmHg) | 74 (9) | 72 (9) | 76 (11) | 77 (10) | 0.001 |
| HbA1c (%) | 6.0 (1) | 5.8 (0.7) | 6.1 (1.4) | 6.0 (1.5) | 0.715 |
| Glucose (mmol/L) | 11.6 (16) | 9.3 (9) | 11.2 (14) | 6.8 (4) | 0.059 |
| hs-CRP (mg/L) | 8.1 (8) | 5.7 (7) | 4.2 (5) | 4.1 (4) | 0.001 |
| TNFα (pg/mL) | 13.7 (4.2) | 11.9 (5.7) | 7.7 (5) | 6.4 (4.7) | 0.001 |
| Glutathione (GSH) (nM/mL) | 6.3 (4.1) | 6.0 (3.5) | 7.3 (5.6) | 7.5 (3) | 0.418 |
| Superoxide dismutase (U/mL) | 4.4 (3) | 3.5 (1.5) | 2.9 (1.3) | 2.5 (0.9) | 0.001 |
| Catalase (nmol/min/mL) | 33 (20) | 46 (32) | 62 (33) | 71 (33) | 0.001 |
| Glutathione peroxidase (ng/mL) | 64 (102) | 100 (89) | 158 (81) | 206 (71) | 0.001 |
| TBARS (nmol/mL) | 30 (13) | 27 (15) | 18 (12) | 18 (13) | 0.001 |
| Protein carbonyl (nmol/mL) | 95 (51) | 93 (64) | 121 (63) | 149 (73) | 0.001 |
| 1st Quartile (0.5 to 2.8 Servings/Day) (n = 116) | 2nd Quartile (2.9 to 3.8 Servings/Day) (n = 99) | 3rd Quartile (3.9–4.9 Servings/Day) (n = 113) | 4th Quartile (5.0 to 9.8 Servings/Day) (n = 101) | p Value * | |
|---|---|---|---|---|---|
| Body weight (kg) | 82 (15) | 83.5 (17) | 77.5 (12) | 82 (19) | 0.108 |
| Body mass index | 32.4 (5) | 33.3 (7) | 31.5 (4) | 32.3 (5) | 0.250 |
| Waist circumference (cm) | 97 (13) | 97 (16) | 94 (13) | 94 (13) | 0.521 |
| Systolic BP (mmHg) | 118 (11) | 119 (10) | 119 (11) | 118 (9) | 0.819 |
| Diastolic BP (mmHg) | 73 (9) | 72 (7) | 72 (8) | 71 (8) | 0.578 |
| HbA1c (%) | 6.1 (1.4) | 5.9 (1.1) | 6.0 (1.4) | 5.7 (0.9) | 0.318 |
| Glucose (mmol/L) | 6.8 (6) | 6.2 (5) | 5.8 (4) | 6.5 (4) | 0.488 |
| hs-CRP (mg/L) | 6.0 (6) | 6.5 (8) | 4.1 (5.5) | 3.5 (3.4) | 0.011 |
| TNFα (pg/mL) | 7.8 (3.7) | 7.7 (4.1) | 6.4 (4.4) | 5.1 (2.9) | 0.001 |
| Glutathione (GSH) (nM/mL) | 5.6 (3.4) | 6.7 (4) | 6.8 (3.6) | 5.8 (3.3) | 0.512 |
| Superoxide dismutase (U/mL) | 4.8 (3.5) | 4.8 (3.4) | 3.7 (3.3) | 3.1 (1.4) | 0.003 |
| Catalase (nmol/min/mL) | 49 (19) | 51 (25) | 68 (32) | 72 (29) | 0.001 |
| Glutathione peroxidase (ng/mL) | 52 (21) | 76 (43) | 116 (100) | 126 (49) | 0.001 |
| TBARS (nmol/mL) | 31 (11) | 27 (11) | 21 (10) | 20 (12) | 0.001 |
| Protein carbonyl (nmol/mL) | 73 (55) | 88 (68) | 111 (86) | 137 (72) | 0.001 |
| Mean (SE) | Low Consumption (≤4.4 Servings/Day) | High Consumption (>4.4 Servings/Day) | Two-Sided p Values * |
|---|---|---|---|
| (n = 374) | (n = 295) | ||
| Calorie intake (Kal/day) | 1085 (631) | 1274 (716) | 0.191 |
| Body weight (kg) | 86 (17) | 88.6 (17) | 0.670 |
| Body mass index | 33.8 (6) | 33.0 (5) | 0.111 |
| Waist circumference (cm) | 100 (13) | 99 (13) | 0.179 |
| Systolic BP (mmHg) | 120 (13) | 122 (14) | 0.064 |
| Diastolic BP (mmHg) | 73 (14) | 77 (11) | 0.001 |
| HbA1c (%) | 5.9 (0.8) | 6.0 (1.5) | 0.346 |
| Glucose (mmol/L) | 10.6 (14) | 9.0 (9) | 0.220 |
| hs-CRP (mg/L) | 6.7 (7) | 4.1 (4) | 0.001 |
| TNFα (pg/mL) | 12.5 (5) | 7.0 (5) | 0.001 |
| Glutathione (GSH) (nM/mL) | 6.2 (4) | 7.8 (5) | 0.036 |
| Superoxide dismutase (U/mL) | 3.8 (2.3) | 2.7 (1.2) | 0.001 |
| Catalase (nmol/min/mL) | 41 (28) | 67 (33) | 0.001 |
| Glutathione peroxidase (ng/mL) | 87 (95) | 181 (80) | 0.001 |
| TBARS (nmol/mL) | 28 (14) | 18 (12) | 0.001 |
| Protein carbonyl (nmol/mL) | 98 (61) | 133 (70) | 0.001 |
| Mean (SE) | Low Consumption (≤3.7 Servings/Day) | High Consumption (>3.7 Servings/Day) | Two-Sided p Values * |
|---|---|---|---|
| (n = 200) | (n = 229) | ||
| Calorie intake (Kal/day) | 1155 (735) | 1149 (634) | 0.966 |
| Body weight (kg) | 82.9 (16) | 79 (15) | 0.047 |
| Body mass index | 32.9 (6) | 31.7 (4) | 0.055 |
| Waist circumference (cm) | 96.9 (14) | 93.6 (13) | 0.054 |
| Systolic BP (mmHg) | 118 (10) | 119 (10) | 0.196 |
| Diastolic BP (mmHg) | 73 (8) | 72 (8) | 0.693 |
| HbA1c (%) | 6.1 (1.3) | 5.8 (1.1) | 0.093 |
| Glucose (mmol/L) | 6.8 (5) | 6.0 (4) | 0.165 |
| hs-CRP (mg/L) | 6.7 (7) | 3.8 (4) | 0.001 |
| TNFα (pg/mL) | 8.0 (4) | 5.7 (3) | 0.001 |
| Glutathione (GSH) (nM/mL) | 5.9 (4) | 6.6 (3.6) | 0.350 |
| Superoxide dismutase (U/mL) | 4.66 (3.4) | 3.56 (2.4) | 0.014 |
| Catalase (nmol/min/mL) | 49 (19) | 68 (31) | 0.001 |
| Glutathione peroxidase (ng/mL) | 60 (33) | 119 (75) | 0.001 |
| TBARS (nmol/mL) | 30 (11) | 21 (11) | 0.001 |
| Protein carbonyl (nmol/mL) | 78 (59) | 124 (80) | 0.001 |
| Variable | Regression Coefficient | Standard Error | Odd Ratio for Unit Change (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | −0.020 | 0.018 | 0.980 (0.947–1.014) | 0.249 |
| Sex (male/female) | −0.248 | 0.574 | 0.780 (0.253–2.405) | 0.666 |
| Marital status (married, unmarried, divorced) | 0.311 | 0.207 | 1.364 (0.909–2.047) | 0.133 |
| Level of education (Primary, secondary, graduate) | −0.226 | 0.136 | 0.797 (611–1.040) | 0.095 |
| Baseline physical activity (Not active, moderately active, very active) | 0.867 | 0.403 | 2.380 (1.08–5.245) | 0.032 |
| Difference between baseline and follow up physical activity | −0.564 | 0.376 | 0.569 (0.272–1.189) | 0.134 |
| Baseline fruit and vegetable consumption (servings/day) | −0.011 | 0.023 | 0.989 (0.946–1.034) | 0.638 |
| Difference between baseline and follow-up fruit and vegetable consumption | 0.007 | 0.020 | 1.007 (0.969–1.047) | 0.727 |
| Variable | Regression Coefficient | Standard Error | Odd Ratio for Unit Change (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 0.007 | 0.015 | 1.007 (0.978–1.036) | 0.636 |
| Sex (male/female) | 0.282 | 0.531 | 1.326 (0.468–3.757) | 0.596 |
| Marital status (married, unmarried, divorced) | 0.017 | 0.165 | 1.017 (0.736–1.407) | 0.917 |
| Level of education (primary, secondary, graduate) | −0.056 | 0.112 | 0.946 (0.760–1.177) | 0.616 |
| Baseline physical activity (not active, moderately active, very active) | 0.365 | 0.304 | 1.440 (0.793–2.616) | 0.231 |
| Difference between baseline and follow-up physical activity | −0.372 | 0.286 | 0.689 (0.393–1.207) | 0.193 |
| Baseline fruit and vegetable consumption (servings/day) | −0.013 | 0.019 | 0.987 (0.952–1.024) | 0.495 |
| Difference between baseline and follow-up fruit and vegetable consumption | 0.010 | 0.016 | 1.010 (0.979–1.042) | 0.540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gariballa, S.; Al-Bluwi, G.S.M.; Yasin, J. Increased Fruit and Vegetable Consumption Mitigates Oxidative Damage and Associated Inflammatory Response in Obese Subjects Independent of Body Weight Change. Nutrients 2023, 15, 1638. https://doi.org/10.3390/nu15071638
Gariballa S, Al-Bluwi GSM, Yasin J. Increased Fruit and Vegetable Consumption Mitigates Oxidative Damage and Associated Inflammatory Response in Obese Subjects Independent of Body Weight Change. Nutrients. 2023; 15(7):1638. https://doi.org/10.3390/nu15071638
Chicago/Turabian StyleGariballa, Salah, Ghada S. M. Al-Bluwi, and Javed Yasin. 2023. "Increased Fruit and Vegetable Consumption Mitigates Oxidative Damage and Associated Inflammatory Response in Obese Subjects Independent of Body Weight Change" Nutrients 15, no. 7: 1638. https://doi.org/10.3390/nu15071638
APA StyleGariballa, S., Al-Bluwi, G. S. M., & Yasin, J. (2023). Increased Fruit and Vegetable Consumption Mitigates Oxidative Damage and Associated Inflammatory Response in Obese Subjects Independent of Body Weight Change. Nutrients, 15(7), 1638. https://doi.org/10.3390/nu15071638







