Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria, Information Sources, and Search Strategy
2.2. Data Extraction
2.3. Outcomes
2.4. Data Synthesis and Assessment of the Risk of Bias
3. Results
3.1. Study Selection
3.2. Study Characteristics
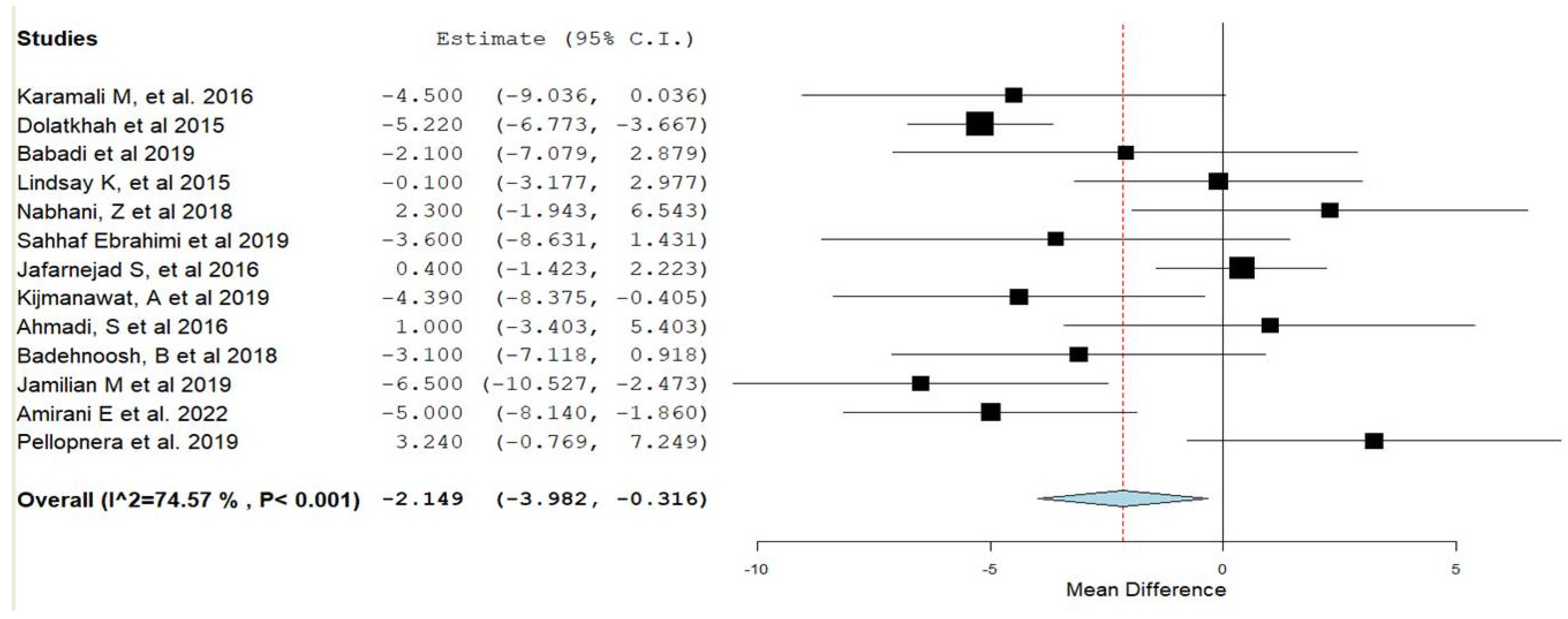
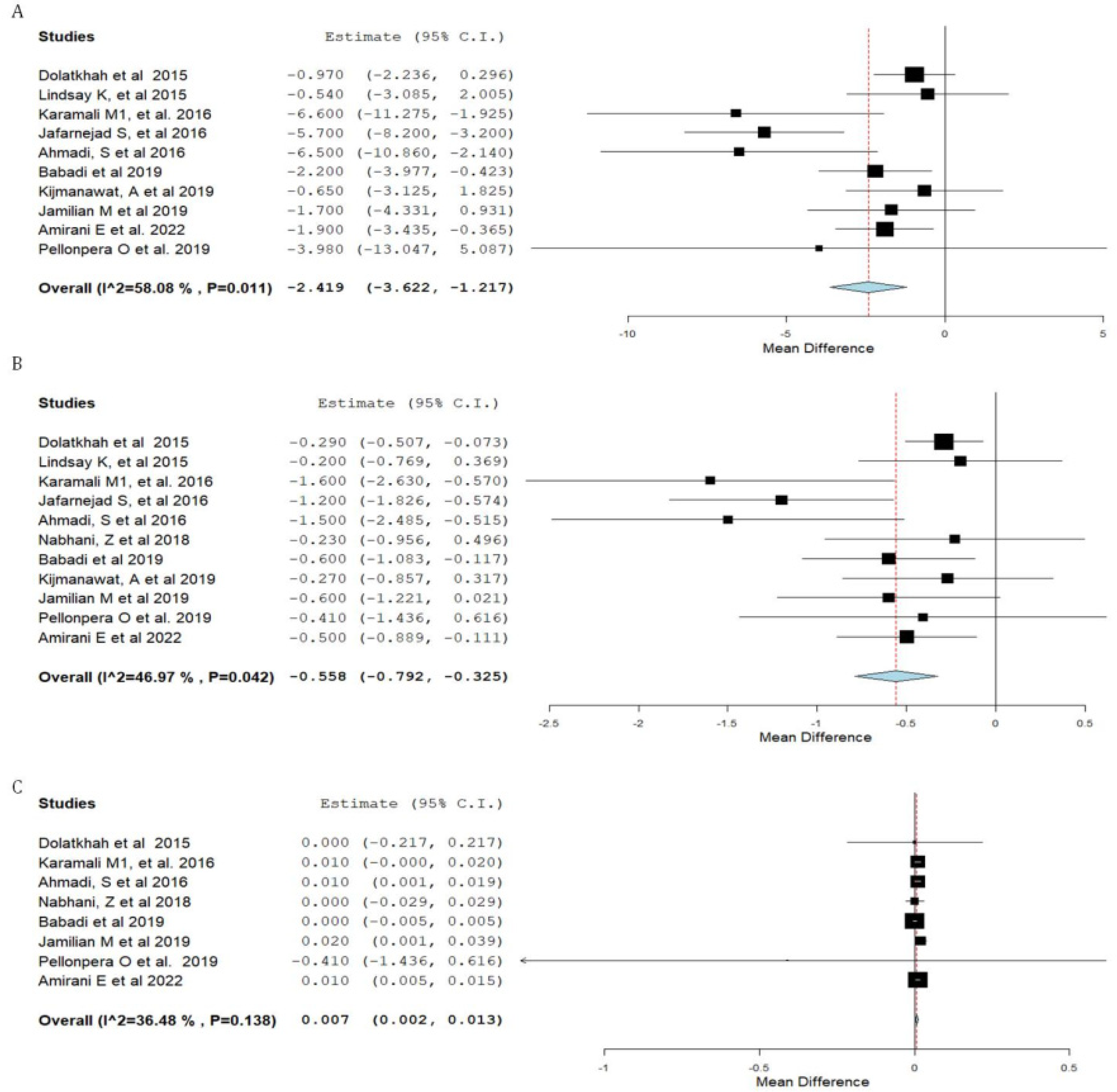
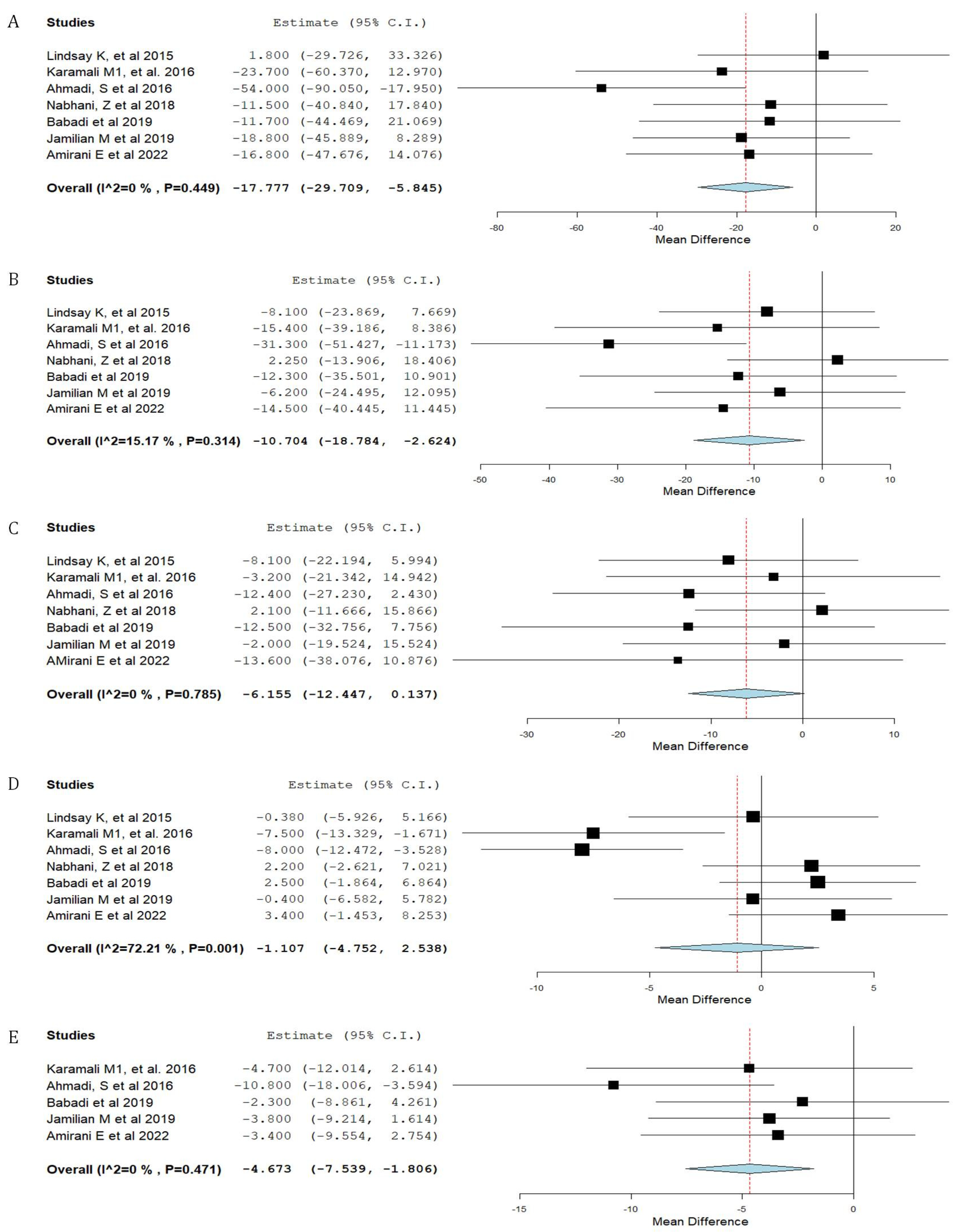
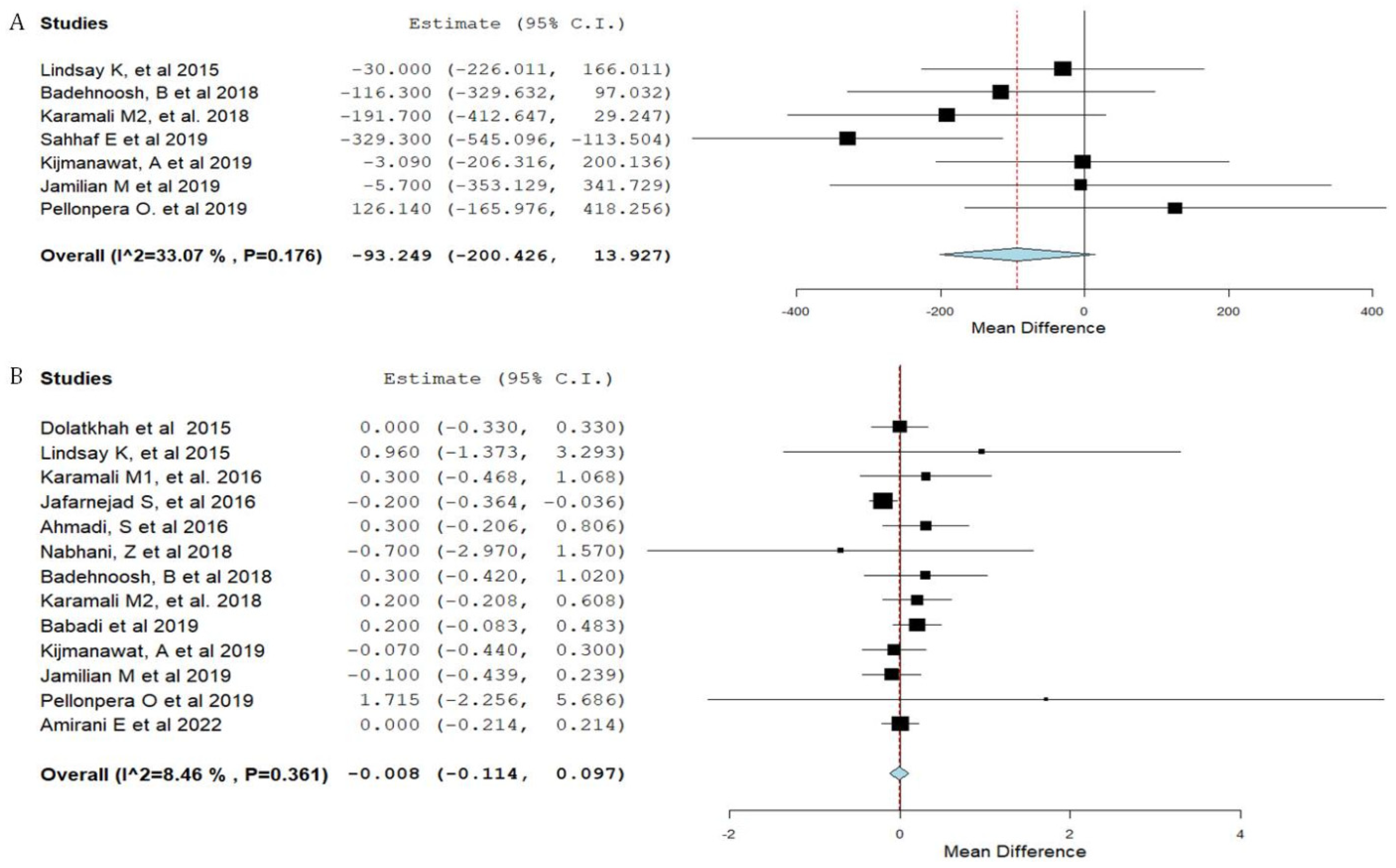
3.3. Synthesis of Results
4. Discussion
4.1. Main Findings
4.2. Comparison with Existing Literature
4.3. Strengths, Limitations and Suggestions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, S.; Bock, C.; Wetzel, M.; Maul, H.; Loerbroks, A. The prevalence of gestational diabetes in advanced economies. J. Perinat. Med. 2012, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Dallanora, S.; Medeiros, d.S.; Deon, R.G.; Tracey, C.A.; Freitas-Vilela, A.A.; Wurdig Roesch, L.F.; Mendes, R.H. Do probiotics effectively ameliorate glycemic control during gestational diabetes? A systematic review. Arch. Gynecol. Obstet. 2018, 298, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Poomalar, G.K. Changing trends in management of gestational diabetes mellitus. World J. Diabetes 2015, 6, 284–295. [Google Scholar] [CrossRef]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A. Screening, diagnosis, and management of gestational diabetes mellitus. Am. Fam. Physician 2015, 91, 460–467. [Google Scholar]
- Yefet, E.; Schwartz, N.; Sliman, B.; Ishay, A.; Nachum, Z. Good glycemic control of gestational diabetes mellitus is associated with the attenuation of future maternal cardiovascular risk: A retrospective cohort study. Cardiovasc. Diabetol. 2019, 18, 75. [Google Scholar] [CrossRef]
- Schwartz, N.; Green, M.S.; Yefet, E.; Nachum, Z. Modifiable risk factors for gestational diabetes recurrence. Endocrine 2016, 54, 714–722. [Google Scholar] [CrossRef]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics improve glucose and lipid metabolism in pregnant women: A meta-analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [PubMed]
- Yefet, E.; Colodner, R.; Strauss, M.; Letova, Y.G.Z.; Nachum, Z. A Randomized Controlled Open Label Crossover Trial to Study Vaginal Colonization of Orally Administered Lactobacillus Reuteri RC-14 and Rhamnosus GR-1 in Pregnant Women at High Risk for Preterm Labor. Nutrients 2020, 12, 1141. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef]
- Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics: A potential role in the prevention of gestational diabetes? Acta Diabetol. 2012, 49, S1–S13. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.G.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed]
- Sahhaf, E.F.; Homayouni, R.A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: A randomized placebo-controlled trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e1–496.e11. [Google Scholar] [CrossRef]
- Ahmadi, S.; Jamilian, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1394–1401. [Google Scholar] [CrossRef]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern. Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2018, 11, 1227–1235. [Google Scholar] [CrossRef]
- Karamali, M.; Nasiri, N.; Taghavi, S.N.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Pregnancy Outcomes in Gestational Diabetes. Probiotics Antimicrob. Proteins 2018, 10, 496–503. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Pellonpera, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef]
- Amirani, E.; Asemi, Z.; Taghizadeh, M. The effects of selenium plus probiotics supplementation on glycemic status and serum lipoproteins in patients with gestational diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2022, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]
- de Veciana, M.; Major, C.A.; Morgan, M.A.; Asrat, T.; Toohey, J.S.; Lien, J.M.; Evans, A.T. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N. Engl. J. Med. 1995, 333, 1237–1241. [Google Scholar] [CrossRef]
- Schwartz, N.; Green, M.S.; Yefet, E.; Nachum, Z. Postprandial glycemic control during gestational diabetes pregnancy predicts the risk of recurrence. Sci. Rep. 2018, 8, 6350. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Naghibi, R.M.; Rahimi, F.A.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71–74. [Google Scholar]
- Lindsay, K.L.; Kennelly, M.; Culliton, M.; Smith, T.; Maguire, O.C.; Shanahan, F.; Brennan, L.; McAuliffe, F.M. Probiotics in obese pregnancy do not reduce maternal fasting glucose: A double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am. J. Clin. Nutr. 2014, 99, 1432–1439. [Google Scholar] [CrossRef]
- Neri, C.; Serafino, E.; Morlando, M.; Familiari, A. Microbiome and Gestational Diabetes: Interactions with Pregnancy Outcome and Long-Term Infant Health. J. Diabetes Res. 2021, 2021, 9994734. [Google Scholar] [CrossRef]
- Gohir, W.; Whelan, F.J.; Surette, M.G.; Moore, C.; Schertzer, J.D.; Sloboda, D.M. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 2015, 6, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Belitsos, P.; Sifakis, S.; Dafopoulos, K.; Siristatidis, C.; Pappa, K.I.; Iliodromiti, Z. Role of Adipokines and Other Inflammatory Mediators in Gestational Diabetes Mellitus and Previous Gestational Diabetes Mellitus. Int. J. Endocrinol. 2012, 2012, 549748. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.; Gruppuso, P.A.; Petzold, K.; Brambilla, D.; Hiilesmaa, V.; Teramo, K.A. Hyperinsulinemia and Macrosomia in the Fetus of the Diabetic Mother. Diabetes Care 1994, 17, 640–648. [Google Scholar] [CrossRef]
- Catalano, P.M.; Hauguel-De Mouzon, S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am. J. Obstet. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Kamińska, K.; Stenclik, D.; Błażejewska, W.; Bogdański, P.; Moszak, M. Probiotics in the Prevention and Treatment of Gestational Diabetes Mellitus (GDM): A Review. Nutrients 2022, 14, 4303. [Google Scholar] [CrossRef]

| First Author, Year | Country | Number of Subjects | Probiotic Intervention | Probiotic Dose/Day | Intervention Period | Primary Outcome | Results | Quality Score * |
|---|---|---|---|---|---|---|---|---|
| Dolatkhah et al., 2015 [22] | Turkey | Probiotics n = 29 placebo n = 27 | Lactobacillus acidophilus LA-5, Bifidobacterium BB-12, Streptococcus thermophilus STY-31 and Lactobacillus delbrueckii bulgaricus LBY-27 | 4 biocap >4 × 109 CFU | 8 weeks | Weight gain and glucose metabolism | Decrease in FPG | 24 |
| Lindsay et al., 2015 [24] | Ireland | Probiotics n = 48 placebo n = 52 | Lactobacillus salivarius UCC118 | 100 mg of Lactobacillus salivarius UCC118 at a target dose of 109 CFU | 8 weeks | Fasting glucose | No impact on glycemic control among GDM patients | 25 |
| Karamali et al., 2016 [12] | Iran | Probiotics n = 30 Placebo n = 30 | L. acidophilus, L. casei and B. bifidum strains | 2 × 109 CFU/g each | 6 weeks | Glucose homoeostasis parameters | Decrease in FPG and serum insulin levels | 25 |
| Jafarnejad et al., 2016 [14] | Iran | Probiotics n = 37 Placebo n = 35 | VSL#3 (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. bulgaricus) | 112.5 × 109 CFU | 8 weeks | Glycemic control and inflammatory status | FPG, HbA1c, HOMA-IR, and insulin levels remained unchanged | 22 |
| Ahmadi et al., 2016 [25] | Iran | Probiotics n = 35 Placebo n = 35 | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum plus 0.8 g inulin | 2 × 109 colony-forming units/g each | 6 weeks | Insulin metabolism | Decrease in serum insulin levels | 25 |
| Nabhani et al., 2018 [26] | Iran | Probiotics n = 45 Placebo n = 45 | Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus gasseri | 500 mg of Lactobacillus probiotic strains consisting of L. acidophilus (5 × 1010 CFU/g), L. plantarum (1.5 × 1010 CFU/g), L. fermentum (7 × 109 CFU/g), L. gasseri (2 × 1010 CFU/g) | 6 weeks | Glucose homoeostasis parameters | No effect on FPG and insulin resistance/sensitivity indices | 25 |
| Badehnoosh et al., 2018 [27] | Iran | Probiotics n = 30 Placebo n = 30 | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | 2 × 109 CFU/g each | 6 weeks | Inflammatory markers | Decrease in FPG, no effect on pregnancy outcomes | 24 |
| Karamali et al., 2018 [29] | Iran | Probiotics n = 30 Placebo n = 30 | Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g) and Bifidobacterium bifidum (2 × 109 CFU/g) strains plus 800 mg inulin | 2 × 109 CFU/g each | 6 weeks | Inflammatory markers | No effect on birth weight | 23 |
| Babadi et al., 2019 [28] | Iran | Probiotics n = 24 Placebo n = 24 | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum | 2 × 109 CFU/g each | 6 weeks | Gene expression of PPAR-γ | Decrease in FPG, serum insulin levels and insulin resistance; increased insulin sensitivity | 23 |
| Sahhaf Ebrahimi et al., 2019 [23] | Iran | Probiotics n = 42 Placebo n = 42 | Probiotic yoghurt containing Lactobacillus acidophilus and Bifidobacterium lactis | 300 g/day of probiotic yoghurt (contained 106 Lactobacillus acidophilus and Bifidobacterium lactis | 8 weeks | Glycemic parameters | Decrease in FPG and HbA1c, lower birth weight and fewer macrosome neonates in the probiotic group | 24 |
| Kijmanawat et al., 2019 [30] | Thailand | Probiotics n = 28 Placebo n = 29 | Lactobacillus acidophilus and Bifidobacterium bifidum | 1 × 109 CFU/g each | 4 weeks | Glycemic control | Decrease in FPG and serum insulin levels, and increased insulin sensitivity | 25 |
| Jamilian et al., 2019 [31] | Iran | Probiotics n = 29 Placebo n = 28 | Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum | 8 × 109 CFU/day | 6 weeks | Insulin metabolism | Decrease in FPG and serum insulin levels | 25 |
| Pellonpera et al., 2019 [34] | Finland | Probiotics n = 27 Placebo n = 22 | Lactobacillus rhamnosus and Bifidobacterium animalis ssp. lactis | 1 × 1010 CFU each | From the first study visit, throughout the pregnancy, and until 6 months postpartum | The incidence of GDM | No difference in FPG, insulin resistance, maternal weight gain and neonatal birth weight | 24 |
| Amirani et al., 2022 [35] | Iran | Probiotics n = 26 Placebo n = 25 | Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis Bifidobacterium longum Additionally, selenium | 2 × 109 CFU/day each | 6 weeks | Insulin metabolism | Reduced fasting glucose, insulin concentrations, insulin resistance, triglycerides, total cholesterol, and low-density lipoprotein (LDL) cholesterol | 24 |
| Outcome | p Value |
|---|---|
| FPG | 0.03 |
| Fasting plasma Insulin | 0.048 |
| Neonatal birth weight | 0.95 |
| HOMA-IR | 0.005 |
| QUICKI | 0.57 |
| Triglycerides | 0.24 |
| VLDL cholesterol | 0.052 |
| Total cholesterol | 0.37 |
| LDL cholesterol | 0.43 |
| HDL cholesterol | 0.96 |
| Maternal weight gain | 0.93 |
| FPG (mg/dL) | Fasting Insulin (µIU/mL) | Neonatal Birth Weight (g) | HOMA-IR | QUICKI | TG (mg/dL) | VLDL (mg/dL) | Total Cholesterol (mg/dL) | |
|---|---|---|---|---|---|---|---|---|
| Lactobacillus acidophilus | −2.8 [(−4.7)–(−0.9)] | −2.7 [(−4.0)–(−1.3)] | −141 [(−262)–(−19)] | −0.6 [(−0.9)–(−0.4)] | 0.008 [0.002–0.01] | −21 [(−34)–(−8)] | −4.7 [(−7.5)–(−1.8)] | −12 [(−22)–(−1.7)] |
| Bifidobacterium bifidum | −3.7 [(−5.5)–(−2.0)] | −2.4 [(−3.8)–(−1.1)] | −88 [(−204)–(27)] | −0.7 [(−1.0)–(−0.4)] | 0.008 [0.002–0.015] | −23 [(−38)–(−9)] | −4.7 [(−7.5)–(−1.8)] | −16 [(−26)–(−6)] |
| Lactobacillus casei | −2.2 [(−4.5)–(0.1)] | −4.5 [(−7.9)–(−1.2)] | −153 [(−306)–(1)] | −1.1 [(−1.6)–(−0.6)] | 0.006 [(−0.002)–0.013] | −29 [(−54)–(−4)] | −5.8 [(−10.8)–(−0.8)] | −21 [(−34)–(−8)] |
| Maternal Weight Gain (Kg) | HDL (mg/dL) | LDL (mg/dL) | ||||||
| Lactobacillus acidophilus | −0.006 [(−0.1)–(0.1)] | −1.2 [(−5.5)–(3.0)] | −5.7 [(−12.7)–(1.4)] | |||||
| Bifidobacterium bifidum | 0.072 [(−0.05)–(0.2)] | −1.9 [(−6.9)–(3.0)] | −8.4 [(−16.6)–(−0.2)] | |||||
| Lactobacillus casei | 0.16 [(−0.01)–(0.34)] | −4.2 [(−11.3)–(2.9)] | −9.6 [(−19.6)–(0.4)] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yefet, E.; Bar, L.; Izhaki, I.; Iskander, R.; Massalha, M.; Younis, J.S.; Nachum, Z. Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1633. https://doi.org/10.3390/nu15071633
Yefet E, Bar L, Izhaki I, Iskander R, Massalha M, Younis JS, Nachum Z. Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients. 2023; 15(7):1633. https://doi.org/10.3390/nu15071633
Chicago/Turabian StyleYefet, Enav, Liron Bar, Ido Izhaki, Rula Iskander, Manal Massalha, Johnny S. Younis, and Zohar Nachum. 2023. "Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis" Nutrients 15, no. 7: 1633. https://doi.org/10.3390/nu15071633
APA StyleYefet, E., Bar, L., Izhaki, I., Iskander, R., Massalha, M., Younis, J. S., & Nachum, Z. (2023). Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients, 15(7), 1633. https://doi.org/10.3390/nu15071633







