The Influence of Maternal Folate Status on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection and Quality Assessment
2.4. Statistical Analysis
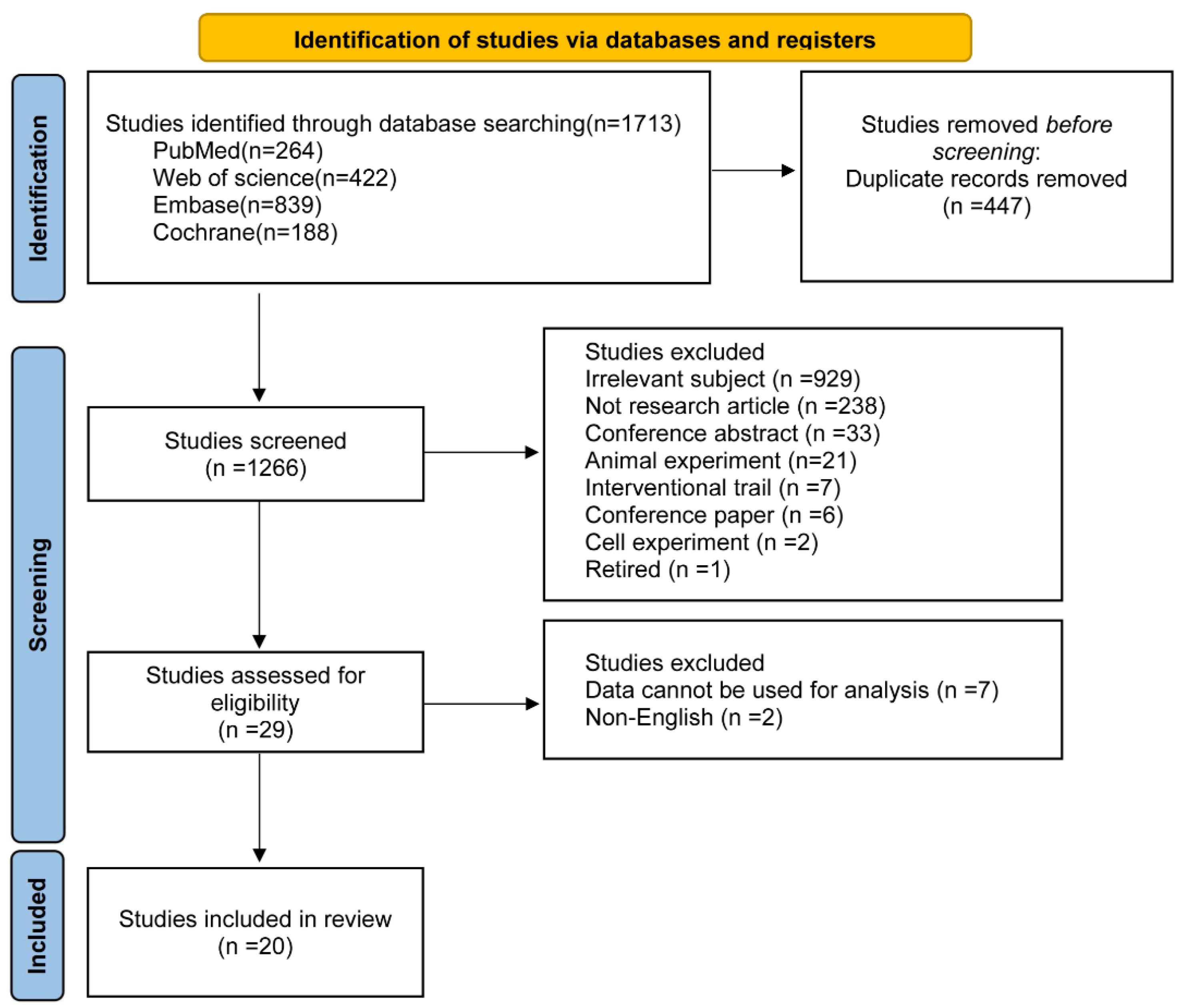
3. Results
3.1. Study Characteristics
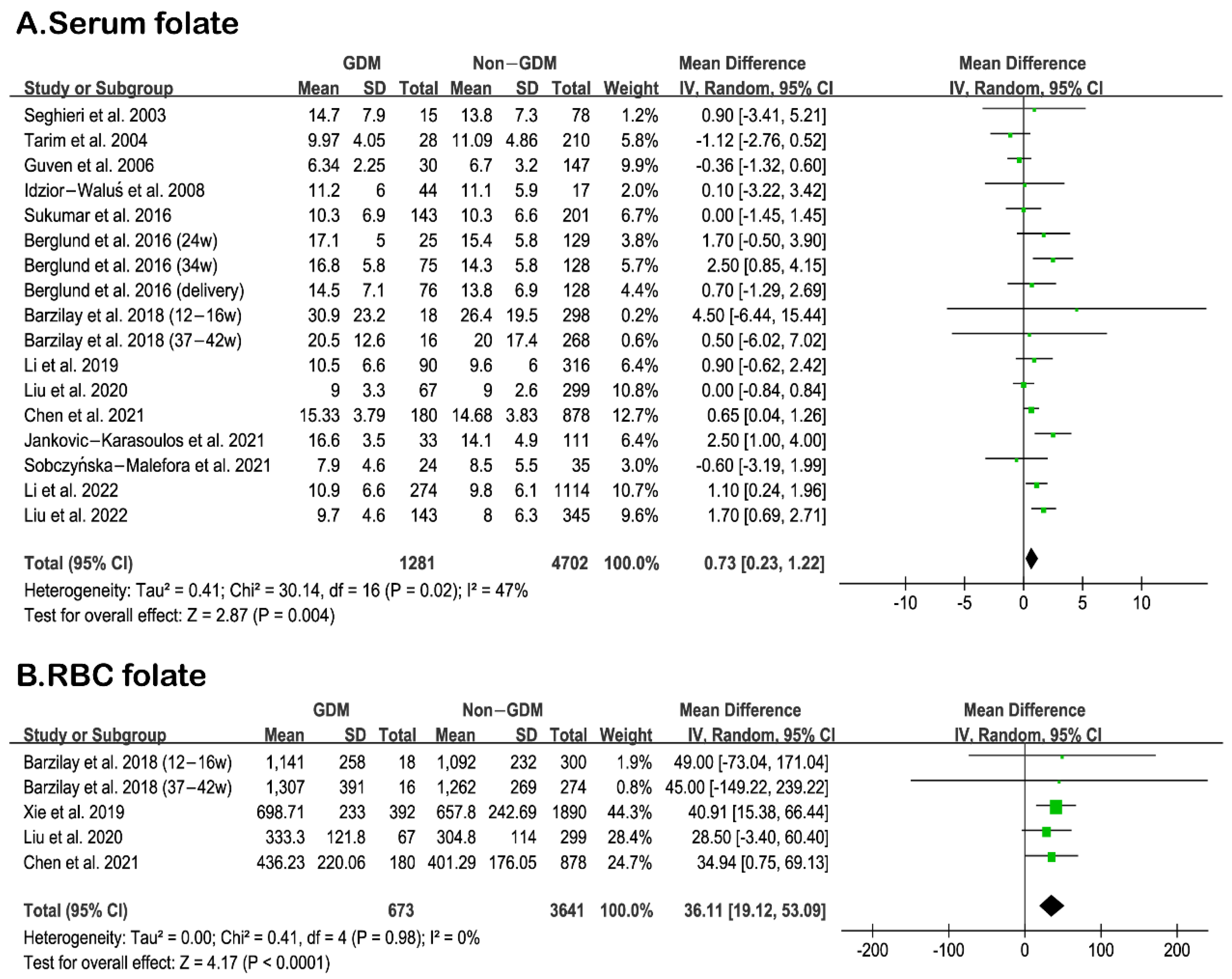
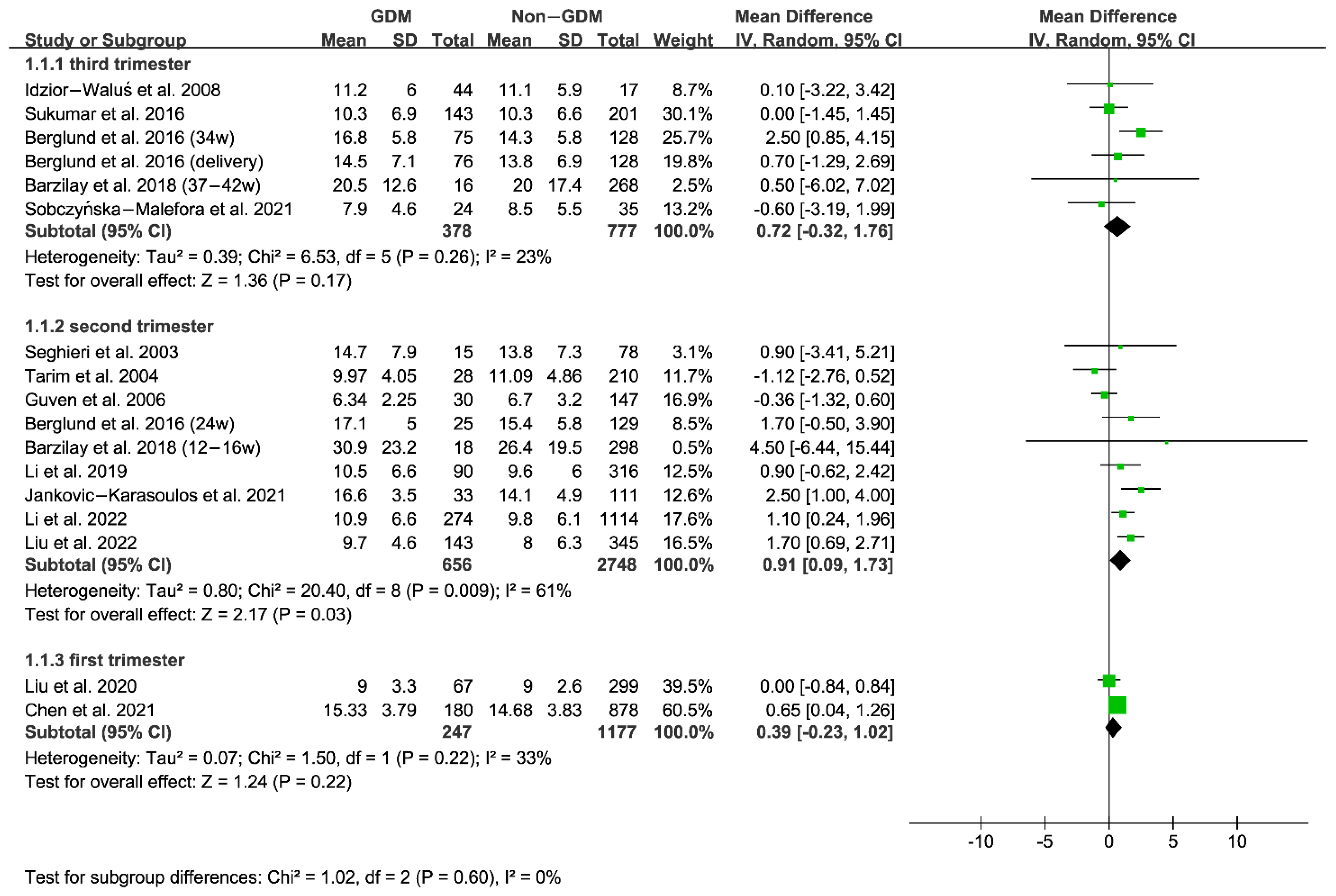
3.2. Comparison of Serum and RBC Folate Levels between GDM and Non-GDM Women
3.3. Relationship between Serum/RBC Folate and GDM Risk
3.4. Descriptive Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Erratum. 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S19–S40. Diabetes Care 2023, 46, 1106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. TEM 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef]
- Retnakaran, R.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B. Treatment of Gestational Diabetes Mellitus and Maternal Risk of Diabetes After Pregnancy. Diabetes Care 2023, 46, 587–592. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- van Weelden, W.; Seed, P.T.; Antoun, E.; Godfrey, K.M.; Kitaba, N.T.; Lillycrop, K.A.; Dalrymple, K.V.; Sobczyńska-Malefora, A.; Painter, R.C.; Poston, L.; et al. Folate and vitamin B12 status: Associations with maternal glucose and neonatal DNA methylation sites related to dysglycaemia, in pregnant women with obesity. J. Dev. Orig. Health Dis. 2022, 13, 168–176. [Google Scholar] [CrossRef]
- Czeizel, A.E. Is folic acid a risk factor for oral clefts? Eur. J. Epidemiol. 2013, 28, 841–843. [Google Scholar] [CrossRef]
- Burdge, G.C.; Lillycrop, K.A. Folic acid supplementation in pregnancy: Are there devils in the detail? Br. J. Nutr. 2012, 108, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Atta, C.A.M.; Fiest, K.M.; Frolkis, A.D.; Jette, N.; Pringsheim, T.; St Germaine-Smith, C.; Rajapakse, T.; Kaplan, G.G.; Metcalfe, A. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am. J. Public Health 2016, 106, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef]
- Chiba, H.; Fukui, A.; Fuchinoue, K.; Funamizu, A.; Tanaka, K.; Mizunuma, H. Expression of Natural Cytotoxicity Receptors on and Intracellular Cytokine Production by NK Cells in Women with Gestational Diabetes Mellitus. Am. J. Reprod. Immunol. 2016, 75, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Wang, D.P.; Zhang, L.L.; Zhang, F.; Wang, D.M.; Zhang, W.Y. Genomic expression profiles of blood and placenta reveal significant immune-related pathways and categories in Chinese women with gestational diabetes mellitus. Diabetes Med. J. Br. Diabet. Assoc. 2011, 28, 237–246. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Hill, J.C.; Veena, S.R.; Bhat, D.S.; Wills, A.K.; Karat, C.L.S.; Yajnik, C.S.; Fall, C.H.D. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009, 52, 2350–2358. [Google Scholar] [CrossRef]
- Zheng, L.D.; Linarelli, L.E.; Liu, L.; Wall, S.S.; Greenawald, M.H.; Seidel, R.W.; Estabrooks, P.A.; Almeida, F.A.; Cheng, Z. Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clin. Epigenet. 2015, 7, 60. [Google Scholar] [CrossRef]
- Bonamichi, B.D.S.F.; Lee, J. Unusual Suspects in the Development of Obesity-Induced Inflammation and Insulin Resistance: NK cells, iNKT cells, and ILCs. Diabetes Metab. J. 2017, 41, 229–250. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, S.; Hung, T.C.; Zheng, W.; Su, X. Association of pre- and early-pregnancy factors with the risk for gestational diabetes mellitus in a large Chinese population. Sci. Rep. 2021, 11, 7335. [Google Scholar] [CrossRef]
- Cheng, G.; Sha, T.; Gao, X.; He, Q.; Wu, X.; Tian, Q.; Yang, F.; Tang, C.; Wu, X.; Xie, Q.; et al. The Associations between the Duration of Folic Acid Supplementation, Gestational Diabetes Mellitus, and Adverse Birth Outcomes based on a Birth Cohort. Int. J. Environ. Res. Public Health 2019, 16, 4511. [Google Scholar] [CrossRef]
- Milman, N. Intestinal absorption of folic acid—New physiologic & molecular aspects. Indian J. Med. Res. 2012, 136, 725–728. [Google Scholar] [PubMed]
- Wang, L.; Hou, Y.; Meng, D.; Yang, L.; Meng, X.; Liu, F. Vitamin B12 and Folate Levels During Pregnancy and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 670289. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Z.; Zhang, J. Association between maternal folate status and gestational diabetes mellitus. Food Sci. Nutr. 2021, 9, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, J.; Guo, L. Effects of maternal folate and vitamin B12 on gestational diabetes mellitus: A dose-response meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022, 76, 1502–1512. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, S.; Lei, J.; Luo, L.; Wang, F. Periconceptional folate and gestational diabetes mellitus: A systematic review and meta-analysis of cohort studies. J. Matern.-Fetal Neonatal Med. 2022, 35, 6884–6893. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Chou, R.; Baker, W.L.; Bañez, L.L.; Iyer, S.; Myers, E.R.; Newberry, S.; Pincock, L.; Robinson, K.A.; Sardenga, L.; Sathe, N.; et al. Agency for Healthcare Research and Quality Evidence-based Practice Center methods provide guidance on prioritization and selection of harms in systematic reviews. J. Clin. Epidemiol. 2018, 98, 98–104. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Liu, X.-H.; Cao, Z.-J.; Chen, L.-W.; Zhang, D.-L.; Qu, X.-X.; Li, Y.-H.; Tang, Y.-P.; Bao, Y.-R.; Ying, H. The association between serum folate and gestational diabetes mellitus: A large retrospective cohort study in Chinese population. Public Health Nutr. 2022, 26, 1014–1021. [Google Scholar] [CrossRef]
- Yuan, X.; Han, X.; Zhou, W.; Long, W.; Wang, H.; Yu, B.; Zhang, B. Association of folate and vitamin B12 imbalance with adverse pregnancy outcomes among 11,549 pregnant women: An observational cohort study. Front. Nutr. 2022, 9, 947118. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassie, Y.; et al. Association of maternal vitamin B12 and folate levels in early pregnancy with gestational diabetes: A prospective UK cohort study (PRiDE study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Sobczyńska-Malefora, A.; Yajnik, C.S.; Harrington, D.J.; Hitman, G.A.; Finer, S. Vitamin B12 and Folate Markers Are Associated with Insulin Resistance During the Third Trimester of Pregnancy in South Asian Women, Living in the United Kingdom, with Gestational Diabetes and Normal Glucose Tolerance. J. Nutr. 2022, 152, 163–170. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chen, H.; Jiang, Y.; Wang, Y.; Wang, D.; Li, M.; Dou, Y.; Sun, X.; Huang, G.; et al. Association of Maternal Folate and Vitamin B12 in Early Pregnancy With Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2021, 44, 217–223. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Y.; Ma, L.; Yao, A.M.; Chen, X.Y.; Hou, Y.X.; Wu, L.P.; Xia, L.Y. Associations Between Gestational Diabetes Mellitus Risk and Folate Status in Early Pregnancy and MTHFR C677T Polymorphisms in Chinese Women. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xu, P.; Fu, Z.; Gu, X.; Li, H.; Cui, X.; You, L.; Zhu, L.; Ji, C.; Guo, X. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci. Nutr. 2019, 7, 3759–3765. [Google Scholar] [CrossRef]
- Tarim, E.; Bagis, T.; Kilicdag, E.; Erkanli, S.; Aslan, E.; Sezgin, N.; Kuscu, E. Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet. Et Gynecol. Scand. 2004, 83, 543–547. [Google Scholar] [CrossRef]
- Guven, M.A.; Kilinc, M.; Batukan, C.; Ekerbicer, H.C.; Aksu, T. Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch. Gynecol. Obstet. 2006, 274, 333–337. [Google Scholar] [CrossRef]
- Barzilay, E.; Moon, A.; Plumptre, L.; Masih, S.P.; Sohn, K.-J.; Visentin, C.E.; Ly, A.; Malysheva, O.; Croxford, R.; Caudill, M.A.; et al. Fetal one-carbon nutrient concentrations may be affected by gestational diabetes. Nutr. Res. 2018, 55, 57–64. [Google Scholar] [CrossRef]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.K.Y.; Shek, L.P.C.; Yap, F.K.P.; Tan, K.H.; Godfrey, K.M.; van Dam, R.M.; et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin. Nutr. 2018, 37, 940–947. [Google Scholar] [CrossRef]
- Berglund, S.K.; García-Valdés, L.; Torres-Espinola, F.J.; Segura, M.T.; Martínez-Zaldívar, C.; Aguilar, M.J.; Agil, A.; Lorente, J.A.; Florido, J.; Padilla, C.; et al. Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: An observational cohort study (PREOBE). BMC Public Health 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern. Child Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef]
- Seghieri, G.; Breschi, M.C.; Anichini, R.; De Bellis, A.; Alviggi, L.; Maida, I.; Franconi, F. Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metab. Clin. Exp. 2003, 52, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Idzior-Waluś, B.; Cyganek, K.; Sztefko, K.; Seghieri, G.; Breschi, M.C.; Waluś-Miarka, M.; Kawalec, E.; Seretny, M.; Sieradzki, J. Total plasma homocysteine correlates in women with gestational diabetes. Arch. Gynecol. Obstet. 2008, 278, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Lu, L.-P.; Yi, M.-H.; Shen, C.-Y.; Lu, G.-Q.; Jia, J.; Wu, H. Study on the correlation between homocysteine-related dietary patterns and gestational diabetes mellitus:a reduced-rank regression analysis study. BMC Pregnancy Childbirth 2022, 22, 306. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Yan, X.; Wang, Y.; Shi, C.; Wu, X.; Liu, H.; Zhang, L.; Zhang, X.; Liu, J.; et al. Joint effects of folate and vitamin B12 imbalance with maternal characteristics on gestational diabetes mellitus. J. Diabetes 2019, 11, 744–751. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Wang, Y.; Zhang, X.; Zhang, L.; Li, C.; Li, J.; Wang, C.; Liu, H.; Liu, J.; et al. Associations of Maternal rs1801131 Genotype in MTHFR and Serum Folate and Vitamin B12 with Gestational Diabetes Mellitus in Chinese Pregnant Women. Nutrients 2022, 14, 1169. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Xia, S.; Li, Z.; Liu, J. Vitamin B12 and gestational diabetes mellitus: A systematic review and meta-analysis. Br. J. Nutr. 2022, 129, 381–394. [Google Scholar] [CrossRef]
- He, J.; Jiang, D.; Cui, X.; Ji, C. Vitamin B12 status and folic acid/vitamin B12 related to the risk of gestational diabetes mellitus in pregnancy: A systematic review and meta-analysis of observational studies. BMC Pregnancy Childbirth 2022, 22, 587. [Google Scholar] [CrossRef]
- Kouroglou, E.; Anagnostis, P.; Daponte, A.; Bargiota, A. Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: A systematic review and meta-analysis. Endocrine 2019, 66, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.M.; Joubert, B.R.; Wu, M.C.; Olshan, A.F.; Håberg, S.E.; Ueland, P.M.; Nystad, W.; Nilsen, R.M.; Vollset, S.E.; Peddada, S.D.; et al. Neonatal genome-wide methylation patterns in relation to birth weight in the Norwegian Mother and Child Cohort. Am. J. Epidemiol. 2014, 179, 834–842. [Google Scholar] [CrossRef]
- Maher, A.; Sobczyńska-Malefora, A. The Relationship Between Folate, Vitamin B12 and Gestational Diabetes Mellitus With Proposed Mechanisms and Foetal Implications. J. Fam. Reprod. Health 2021, 15, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Morris, M.S.; Jacques, P.F. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc. Natl. Acad. Sci. USA 2007, 104, 19995–20000. [Google Scholar] [CrossRef]
- Cho, N.H.; Lim, S.; Jang, H.C.; Park, H.K.; Metzger, B.E. Elevated homocysteine as a risk factor for the development of diabetes in women with a previous history of gestational diabetes mellitus: A 4-year prospective study. Diabetes Care 2005, 28, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Rai, V. Folate pathway gene MTHFR C677T polymorphism and risk of lung cancer in Asian populations. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 9259–9264. [Google Scholar] [CrossRef]
- Wald, N.J. Folic acid and the prevention of neural-tube defects. N. Engl. J. Med. 2004, 350, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Green, N.S. Folic acid supplementation and prevention of birth defects. J. Nutr. 2002, 132, 2356S–2360S. [Google Scholar] [CrossRef]
- Barkai, G.; Arbuzova, S.; Berkenstadt, M.; Heifetz, S.; Cuckle, H. Frequency of Down’s syndrome and neural-tube defects in the same family. Lancet 2003, 361, 1331–1335. [Google Scholar] [CrossRef]


| ID | First Author | Country | Study Design | Sample Size | GDM(n) | Age | Test for GDM | GDM Criteria | Period for GDM Assessment | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Seghieri et al., 2003 [43] | Italy | Cross-sectional | 93 | 15 | GDM: 34.6 ± 3.1, Control: 32.3 ± 3.7 | 100 g OGTT | the American Diabetes Association | 24–28 weeks | 6 |
| 2 | Tarim et al., 2004 [37] | Turkey | Prospective cohort | 238 | 28 | GDM: 32 ± 4.03, Control: 26.83 ± 4.44 | 50 g OGTT | Carpenter and Coustan | 24–28 weeks | 6 |
| 3 | Guven et al., 2006 [38] | Turkey | Cross-sectional | 177 | 30 | GDM: 30.0 ± 4.3, Control: 28.6 ± 3.4 | 100 g OGTT | Carpenter and Coustan | 24–28 weeks | 7 |
| 4 | Idzior-Waluś et al., 2008 [44] | Poland | Prospective cohort | 61 | 44 | GDM: 30.5 ± 6.6, Control: 26.2 ± 4.0 | 75 g OGTT | WHO1999 | 26–32 weeks | 8 |
| 5 | Krishnaveni et al., 2009 [16] | India | Prospective cohort | 785 | 49 | 23 ± 4.5 | 100 g OGTT | Carpenter-Coustan criteria | 32 ± 2 weeks | 6 |
| 6 | Sukumar et al., 2016 [45] | UK | Case-control | 344 | 143 | GDM: 31.4 ± 5.8, Control: 29.6 ± 5.9 | 75 g OGTT | WHO1999 | 24–36 weeks | 7 |
| 7 | Berglund et al., 2016 [41] | Spain | Prospective cohort | 331 | 76 | GDM: 33.7 ± 4.6 | NA | NDDG | 24 weeks, 34 weeks, delivery | 7 |
| 8 | Barzilay et al., 2018 [39] | Canada | Prospective cohort | 368 | 16 | GDM: 34.4 ± 5.3, Control: 32.1 ± 4.8 | 50 g OGTT | Canadian Diabetes Association 2008 practice guidelines | 24–28 weeks | 6 |
| 9 | Lai et al., 2018 [40] | Singapore | Cross-sectional | 913 | 164 | <35, n = 705, ≥35, n = 208 | 75 g OGTT | 1999 World Health Organization standard criteria | 26–28 weeks | 8 |
| 10 | Xie et al., 2019 [36] | China | Prospective cohort | 2282 | 392 | GDM: 29.01 ± 3.15, Control: 27.89 ± 3.18 | 75 g OGTT | FPG ≥ 5.5 mmol/L, 2-h plasma glucose ≥ 8 mmol/L | 24–28 weeks | 9 |
| 11 | Li et al., 2019 [47] | China | Cross-sectional | 406 | 90 | 29.4 ± 4.5 | 75 g OGTT | IADPSG | 24–28 weeks | 8 |
| 12 | Liu et al., 2020 [35] | China | Prospective cohort | 366 | 67 | GDM: 30.5 ± 4.0, Control: 28.9 ± 3.5 | 75 g OGTT | IADPSG | 24–28 weeks | 8 |
| 13 | Jankovic-Karasoulos et al., 2021 [42] | Australia | Prospective cohort | 144 | 33 | GDM: 28.9 ± 5.2, Control: 27.9 ± 5.9 | NA | WHO 2016 | Around 26 weeks | 7 |
| 14 | Saravanan et al., 2021 [32] | UK | Prospective cohort | 4746 | NICE-GDM: 538, IADPSG-GDM: 633 | 30.51 ± 5.29 | 75 g OGTT | NICE, IADPSG | 26–28 weeks | 9 |
| 15 | Sobczyńska-Malefora et al., 2021 [33] | UK | Cross-sectional | 59 | 24 | GDM: 30.8 ± 5.2, Control: 27.7 ± 4.8 | 75 g OGTT | Local diagnostic | 28 weeks | 7 |
| 16 | Chen et al., 2021 [34] | China | Prospective cohort | 1058 | 180 | 30.24 ± 3.97 | 75 g OGTT | IADPSG | 24–28 weeks | 8 |
| 17 | Liu et al., 2022 [30] | China | Retrospective cohort | 42,478 | 5122 | NA | 75 g OGTT | IADPSG | 24–28 weeks | 7 |
| 18 | Yuan et al., 2022 [31] | China | Retrospective cohort | 11,549 | 965 | NA | NA | NA | NA | 8 |
| 19 | Liu et al., 2022 [46] | China | Case-control | 488 | 143 | GDM: 30.63 ± 4.64, Control: 28.51 ± 4.44 | 75 g OGTT | IADPSG | 24–28 weeks | 8 |
| 20 | Li et al., 2022 [48] | China | Case-control | 1388 | 274 | <30, n = 692, 30–35, n = 489, ≥35, n = 207 | 75 g OGTT | IADPSG | 24–28 weeks | 7 |
| ID | First Author | Folate Level (ng/mL) | Folate Status | Time for Folate Measurement | |
|---|---|---|---|---|---|
| GDM | Non-GDM | ||||
| 1 | Seghieri et al., 2003 [43] | 14.7 ± 7.9 | 13.8 ± 7.3 | serum folate | 24–28 weeks gestation (second trimester) |
| 2 | Tarim et al., 2004 [37] | 9.97 ± 4.05 | 11.09 ± 4.86 | serum folate | 24–28 weeks gestation (second trimester) |
| 3 | Guven et al., 2006 [38] | 6.34 ± 2.25 | 6.7 ± 3.2 | serum folate | 24–28 weeks gestation (second trimester) |
| 4 | Idzior-Waluś et al., 2008 [44] | 11.2 ± 6 | 11.1 ± 5.9 | serum folate | 26–32 weeks gestation (third trimester) |
| 5 | Berglund et al., 2016 [41] | 17.1 ± 5.0 | 15.4 ± 5.8 | serum folate | 24 weeks gestation (second trimester) |
| 16.8 ± 5.8 | 14.3 ± 5.8 | serum folate | 34 weeks gestation (third trimester) | ||
| 14.5 ± 7.1 | 13.8 ± 6.9 | serum folate | delivery (third trimester) | ||
| 6 | Sukumar et al., 2016 [45] | 10.3 ± 6.9 | 10.3 ± 6.6 | serum folate | 24–36 weeks gestation (third trimester) |
| 7 | Barzilay et al., 2018 [39] | 30.9 ± 23.2 | 26.4 ± 19.5 | serum folate | 12–16 weeks gestation (second trimester) |
| 20.5 ± 12.6 | 20 ± 17.4 | serum folate | 37–42 weeks gestation (third trimester) | ||
| 1141 ± 258 | 1092 ± 232 | RBC folate | 12–16 weeks gestation (second trimester) | ||
| 1307 ± 391 | 1262 ± 269 | RBC folate | 37–42 weeks gestation (third trimester) | ||
| 8 | Xie et al., 2019 [36] | 698.71 ± 233 | 657.80 ± 242.69 | RBC folate | 19–24 weeks gestation (second trimester) |
| 9 | Li et al., 2019 [47] | 10.5 ± 6.6 | 9.6 ± 6.0 | serum folate | 24–28 weeks gestation (second trimester) |
| 10 | Liu et al., 2020 [35] | 9.0 ± 3.3 | 9.0 ± 2.6 | serum folate | before 12 weeks gestation (first trimester) |
| 333.3 ± 121.8 | 304.8 ± 114 | RBC folate | before 12 weeks gestation (first trimester) | ||
| 11 | Sobczyńska-Malefora et al., 2021 [33] | 7.9 ± 4.6 | 8.5 ± 5.5 | serum folate | 28 weeks gestation (third trimester) |
| 12 | Chen et al., 2021 [34] | 436.23 ± 220.06 | 401.29 ± 176.05 | RBC folate | 9–13 weeks gestation (first trimester) |
| 15.33 ± 3.79 | 14.68 ± 3.83 | serum folate | 9–13 weeks gestation (first trimester) | ||
| 13 | Jankovic-Karasoulos et al., 2021 [42] | 16.6 ± 3.5 | 14.1 ± 4.9 | serum folate | 15 ± 1 weeks gestation (second trimester) |
| 14 | Liu et al., 2022 [46] | 9.7 ± 4.6 | 8.0 ± 6.3 | serum folate | 24–28 weeks gestation (second trimester) |
| 15 | Li et al., 2022 [48] | 10.9 ± 6.6 | 9.8 ± 6.1 | serum folate | 24–28 weeks gestation (second trimester) |
| ID | First Author | Outcome | Adjusted OR (95% CI) | Adjusted Factors | Time for Measurement |
|---|---|---|---|---|---|
| 1 | Krishnaveni et al., 2009 [16] | Serum folate As continuous variable | 1.0 (0.99, 1.0) | age, religion, socioeconomic status, parity and family history of diabetes | 30 ± 2 weeks gestation |
| 2 | Sukumar et al., 2016 [45] | Serum folate: ng/mL 3.1–18.7 <3.1 | Reference 0.89 (0.07, 11.38) | age, parity, ethnic origin, smoking, the gestation of bloods, serum B12, gestational BMI | 24–36 weeks gestation |
| 3 | Lai et al., 2018 [40] | Serum folate As continuous variable | 1.29 (1.01, 1.60) | maternal age, ethnicity, education, income, smoking, alcohol intake, physical activity, pre-pregnancy BMI, parity, family history of diabetes, and previous occurrence of GDM, plasma B6 and B12 | at 26 weeks gestation |
| 4 | Xie et al., 2019 [36] | RBC folate: ng/mL Q1: <398.6 Q2: 398.6–570.3 Q3: ≥570.3 As continuous variable | Reference 2.17 (1.20, 3.95) 2.76 (1.56, 4.89) 1.16 (1.03, 1.30) | maternal age, parity, and BMI | second trimester |
| 5 | Li et al., 2019 [47] | Serum folate: ng/mL Q1: <6.9 Q2: 6.9–12.2 Q3: ≥12.2 | Reference 1.12 (0.59, 2.13) 1.98 (1.00, 3.90) | age, ethnicity, education, parity, pp-BMI, family history of diabetes, serum vitamin B12 concentrations | 24–28 weeks gestation |
| 6 | Liu et al., 2020 [35] | RBC folate: ng/mL Q1: <224.7 Q2: 224.7–286.0 Q3: 286.0–380.7 Q4: ≥380.7 As continuous variable | Reference 1.35 (0.53, 3.45) 1.37 (0.54, 3.45) 2.47 (1.01, 6.03) 1.001(1.000, 1.002) | age, physical activity, BMI, parity, family history of diabetes, use of folic acid supplements, HOMA-IR, C-reactive protein, hemoglobin, vitamin B12, and serum homocysteine | early pregnancy |
| 7 | Saravanan et al., 2021 [32] | Serum folate As continuous variable | 1.11 (1.03, 1.18) | age, parity, smoking status, ethnicity, family history, household income and B12 status | early pregnancy |
| 8 | Chen et al., 2021 [34] | Serum folate: ng/mL Q1: <13.9 Q2: 13.9–16.0 Q3: ≥16.0 As continuous variable | Reference 0.91 (0.58, 1.44) 1.36 (0.94, 1.99) 1.01 (0.97, 1.05) | age, pre-conceptional BMI, family history of diabetes, smoking exposure, and drinking status. | Early pregnancy (9–13 weeks) |
| RBC folate: ng/mL Q1: <400 Q2: 400–600 Q3: ≥600 As continuous variable | Reference 1.39 (0.94, 2.04) 1.58 (1.03, 2.41) 1.07 (0.99, 1.15) | ||||
| 9 | Jankovic-Karasoulos et al., 2021 [42] | Serum folate effect for every 5-unit increase | 1.22 (0.93, 1.59) | maternal age, BMI, smoking status | 15 ± 1 weeks gestation |
| 10 | Liu et al., 2022 [30] | Serum folate: ng/mL Q1: 11.07 (8.82, 12.81) Q2: 17.14 (15.75, 18.44) Q3: 22.23 (20.71, 23.24) Q4: 24.85 (24.05, 25.25) As continuous variable | Reference 1.15 (1.04, 1.26) 1.40 (1.27, 1.54) 1.54 (1.40, 1.69) 1.16 (1.13, 1.19) | pre-pregnancy BMI status, fetal gender, parity, maternal age, vitamin B12 level and maternal education | before 24 weeks gestation |
| 11 | Yuan et al., 2022 [31] | Serum folate P5–P95 >P95 <P5 | Reference 1.23 (0.99, 1.53) 0.40 (0.23, 0.70) | maternal age, BMI, gravidity, parity, SVB12 levels | at delivery |
| 12 | Li et al., 2022 [48] | Serum folate: ng/mL Q1: <6.2 Q2: 6.2–9.4 Q3: 9.4–14.6 Q4: ≥14.6 As continuous variable | Reference 1.47 (0.99, 2.26) 1.61 (1.07, 2.49) 2.28 (1.49, 3.61) 1.59 (1.22, 2.13) | age, ethnicity, education, drinking, smoking, parity, family history of diabetes, pre-pregnancy BMI, serum B12 and Hcy concentrations | 24–28 weeks gestation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Liu, S.; Zhong, Z.; Guo, Y.; Xia, T.; Chen, Y.; Ding, L. The Influence of Maternal Folate Status on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2766. https://doi.org/10.3390/nu15122766
Xu R, Liu S, Zhong Z, Guo Y, Xia T, Chen Y, Ding L. The Influence of Maternal Folate Status on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(12):2766. https://doi.org/10.3390/nu15122766
Chicago/Turabian StyleXu, Ruhan, Shenhao Liu, Zhiqi Zhong, Yifei Guo, Tianqi Xia, Yanyan Chen, and Lingling Ding. 2023. "The Influence of Maternal Folate Status on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis" Nutrients 15, no. 12: 2766. https://doi.org/10.3390/nu15122766
APA StyleXu, R., Liu, S., Zhong, Z., Guo, Y., Xia, T., Chen, Y., & Ding, L. (2023). The Influence of Maternal Folate Status on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients, 15(12), 2766. https://doi.org/10.3390/nu15122766





