Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas
Abstract
1. Introduction
2. Methodology
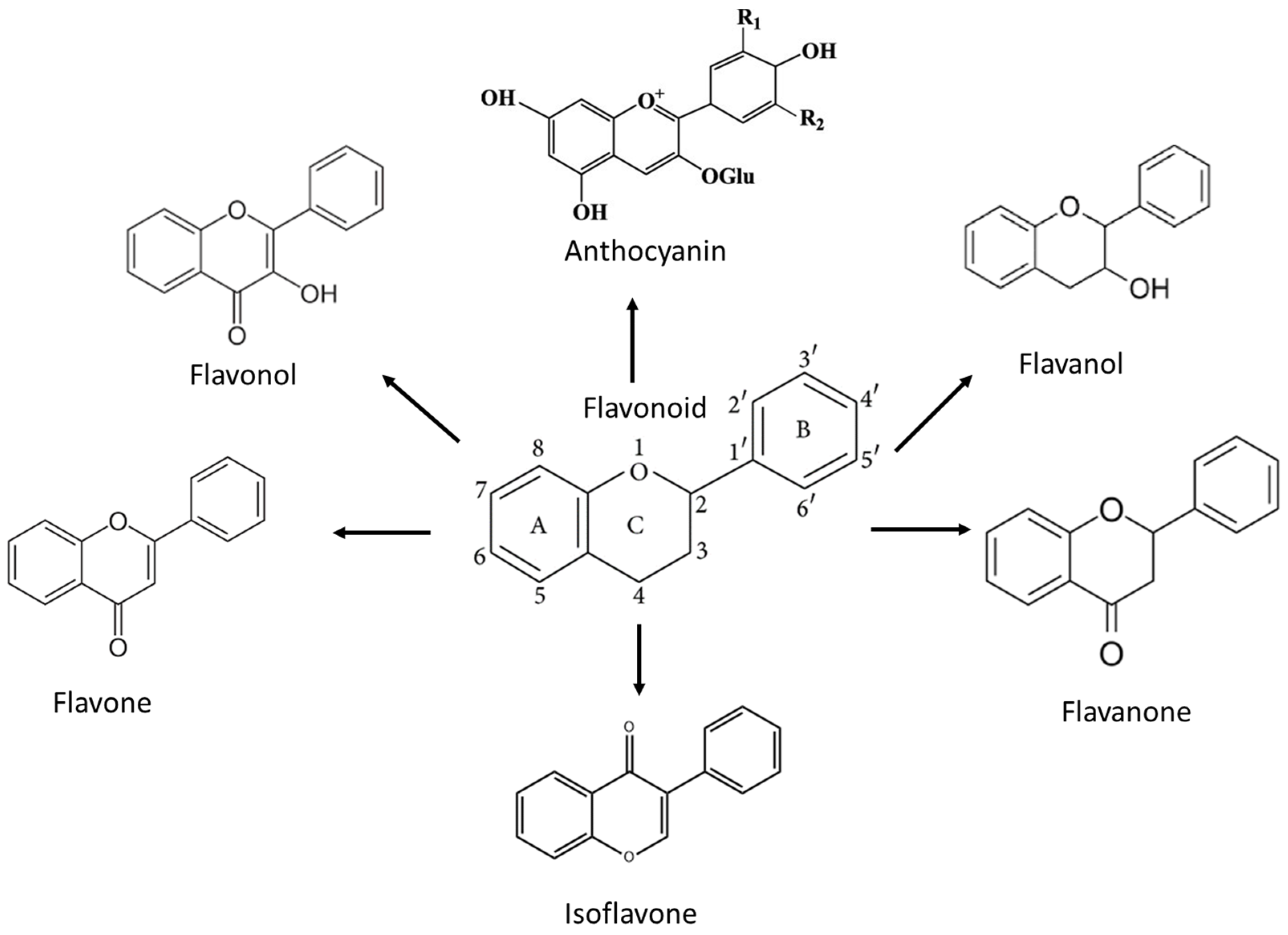
3. Flavonoids and the Mechanism of Action against High-Grade Adult-Type Diffuse Gliomas
3.1. Flavonol
3.1.1. Quercetin
3.1.2. Rutin
3.2. Flavone
3.2.1. Chrysin
3.2.2. Apigenin
3.3. Flavanone
3.3.1. Naringenin
3.3.2. Silibinin
3.4. Flavanol
EGCG
3.5. Isoflavone
3.5.1. Genistein
3.5.2. Biochanin A
3.6. Anthocyanin
Cyanidin-3-O-Glucoside
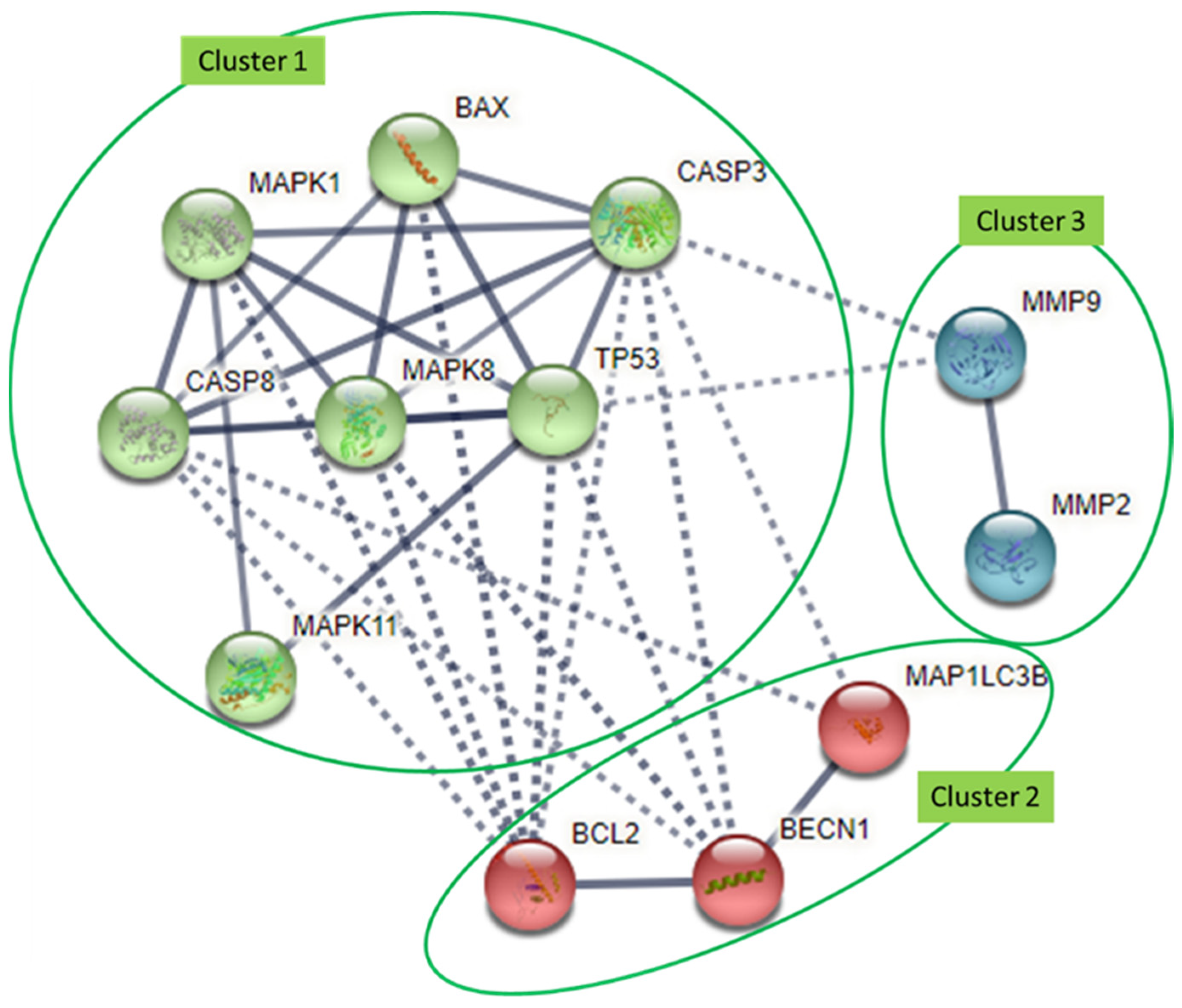
4. Interaction Analysis of Common Molecular Targets of Flavonoids
| Cellular Activities | Molecular Targets | Flavonols | Flavones | Flavanones | Flavanols | Isoflavones | Anthocyanidins | Glioma Model | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin | Rutin | Chrysin | Apigenin | Naringenin | Silibinin | EGCG | Genistein | Biochanin A | C3G | (In Vitro or In Vivo) | |||
| Autophagy | Atg7 | ↓ | ↓ | ↑ | U251, U87, GBM8901 | [57,78] | |||||||
| LC3B * | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | U373MG, T98, D54-MG, U87-MG, U251-MG, GBM8901 | [30,31,65,87] | ||||
| Beclin-1 * | ↓ | ↓ | ↑ | ↓ | ↓ | U373MG, T98, T98G, GBM8901 | [30,65,66,78] | ||||||
| p62 | ↓ | ↓ | ↓ | GBM8901 | [78] | ||||||||
| DNA repair | MGMT | ↓ | ↓ | ↓ | GBM8901, T98G, U87-MG, LN18/TR | [78,84,102,126] | |||||||
| PARP | ↓ | ↓ | ↓ | ↓ | T98G, U373, C6, U251 | [28,30,48,65] | |||||||
| Cell migration, invasion, adhesion | uPAR | ↓ | ↓ | ↓ | ↓ | U87-MG | [38,121] | ||||||
| MMP-2 * | ↓ | ↓ | ↓ | ↓ | ↓ | GL-15, T98G, U87MG | [81,85] | ||||||
| MMP-9 * | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | U87, T98G, U87MG | [58,59,60,85] | |||||
| Cell apoptosis | p53 * | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | T98G, U373, C6, U-118, LN-229, U251 | [27,30,48] | ||
| Bcl-2 * | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | A-172, T98G, U87MG | [28,84,85,90,91] | |||
| Bcl-xL | ↓ | ↓ | U-118, LN-229, A-172 | [27] | |||||||||
| Bax * | ↑ | ↑ | ↑ | ↑ | ↑ | U-118, LN-229, A-172 | [27,28,84,108,109] | ||||||
| Bad | ↑ | A-172 | [28] | ||||||||||
| Bak | ↑ | A-172 | [28]] | ||||||||||
| p65 | ↓ | U87-MG | [80] | ||||||||||
| p50 | ↓ | U87-MG | [80] | ||||||||||
| Caspsase-3 * | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | T98G, U373MG, U-118, LN-229 | [26,30,35,65,67,68,69] | |||||
| Caspase-7 | ↑ | U87-MG. A-172 | [110] | ||||||||||
| Caspase-8 * | ↑ | ↑ | ↑ | ↑ | ↑ | C6, U87-MG, LN228 | [90,91] | ||||||
| Caspase-9 | ↑ | ↑ | ↑ | T98G, U373, C6, U-118, LN-229 | [27,35,69] | ||||||||
| Rb | ↓ | U87MG, U373MG, T98G, | [23,70] | ||||||||||
| Cyclin A | ↓ | ||||||||||||
| Cell cycle arrest | Cyclin D | ↓ | ↓ | ↓ | ↓ | T98G, U87MG | [85] | ||||||
| CDK1 | ↓ | U373, GBM1207 | [116] | ||||||||||
| CDK2 | ↓ | U373, GBM1207 | [116] | ||||||||||
| Oxidative stress | Nrf2 | ↓ | U87 | [32] | |||||||||
| HIF-1 | ↓ | U251, GBM28, GBM37 | [79] | ||||||||||
| Cell proliferation, survival, differentiation, apoptosis | PDK1 | ↓ | U251, GBM28, GBM37 | [79] | |||||||||
| PDK3 | ↓ | U251, GBM28, GBM37 | [79] | ||||||||||
| Raf | ↓ | DBTRG-05, U251 | [62,63] | ||||||||||
| JNK * | ↑ | ↑ | U87-MG, D54-MG, U251-MG, T98G, U87MG | [30,74,84,127] | |||||||||
| Akt | ↓ | ↓ | ↓ | U87, GBM8901, T98G, U87MG | [58,63,78,85] | ||||||||
| Erk * | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | U87, U87-MG, U251, DBTRG-05MG, GL-15, C6, T98, A-172, U1242MG | [24,28,32,58,74,75,82] | ||||
| CK2 | ↓ | ↓ | |||||||||||
| p38 * | ↓ | ↑ | ↑ | ↓ | ↓ | C6, T98, U251, U87, A-172, T98G, U87MG | [28,32,62,63,82,84] | ||||||
| GLUT1 | ↓ | ↑ | ↓ | U251, GBM28, GBM37 | [79] | ||||||||
| IL-6 | ↓ | U251, TG1 | [64] | ||||||||||
| IL-10 | ↓ | U251, TG1 | [64 | ||||||||||
| STAT3 | ↓ | U87MG, U373MG, T98G | [23,70] | ||||||||||
| GRP78 | ↓ | U373Mg, U87MG | [36,111] | ||||||||||
| PDGF-Rbeta | ↓ | A-172 | [112,113] | ||||||||||
| Gli-1 | ↓ | C6 | [92] | ||||||||||
| Smo | ↓ | C6 | [92] | ||||||||||
5. Clinical Relevance of Flavonoid Molecular Targets in High-Grade Adult-Type Diffuse Glioma
6. Issues of Flavonoid Bioavailability and Methods to Overcome Them
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21 (Suppl. 5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; del Mar Álvarez-Torres, M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Whitfield, B.T.; Huse, J.T. Classification of adult-type diffuse gliomas: Impact of the World Health Organization 2021 update. Brain Pathol. 2022, 32, e13062. [Google Scholar] [CrossRef]
- Khan, M.N.; Sharma, A.M.; Pitz, M.; Loewen, S.K.; Quon, H.; Poulin, A.; Essig, M. High-grade glioma management and response assessment-recent advances and current challenges. Curr. Oncol. 2016, 23, e383–e391. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Kamiya-Matsuoka, C.; Hamza, M.A.; de Groot, J.F. Impact of adverse events of bevacizumab on survival outcomes of patients with recurrent glioblastoma. J. Clin. Neurosci. 2020, 74, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Somasundaram, K. Glioblastoma vs. temozolomide: Can the red queen race be won? Cancer Biol. Ther. 2019, 20, 1083–1090. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar]
- Michalska, M.; Gluba, A.; Mikhailidis, D.P.; Nowak, P.; Bielecka-Dabrowa, A.; Rysz, J.; Banach, M. The role of polyphenols in cardiovascular disease. Med. Sci. Monit. 2010, 16, Ra110–Ra119. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Ballard, C.R.; Maróstica, M.R. Chapter 10—Health Benefits of Flavonoids. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 185–201. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, J.; Nice, E.C.; Huang, C.; Shi, Z. The Multifaceted Role of Flavonoids in Cancer Therapy: Leveraging Autophagy with a Double-Edged Sword. Antioxidants 2021, 10, 1138. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef]
- Vidak, M.; Rozman, D.; Komel, R. Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells. Molecules 2015, 20, 19406–19432. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Hantke, B.; Lahmann, C.; Venzke, K.; Fischer, T.; Kocourek, A.; Windsor, L.J.; Bergemann, J.; Stäb, F.; Tschesche, H. Influence of flavonoids and vitamins on the MMP- and TIMP-expression of human dermal fibroblasts after UVA irradiation. Photochem. Photobiol. Sci. 2002, 1, 826–833. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, S.J.; Choi, Y.J.; Kim, M.J.; Kim, T.Y.; Ko, S.G. Quercetin Induces Apoptosis in Glioblastoma Cells by Suppressing Axl/IL-6/STAT3 Signaling Pathway. Am. J. Chin. Med. 2021, 49, 767–784. [Google Scholar] [CrossRef]
- Stump, T.A.; Santee, B.N.; Williams, L.P.; Kunze, R.A.; Heinze, C.E.; Huseman, E.D.; Gryka, E.J.; Simpson, D.S.; Amos, S. The antiproliferative and apoptotic effects of apigenin on glioblastoma cells. J. Pharm. Pharmacol. 2017, 69, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free. Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hao, Y.; Chen, S.; Jia, G.; Guo, Y.; Zhang, G.; Wang, C.; Cheng, R.; Hu, T.; Zhang, X.; et al. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl. Cancer Res. 2019, 8, 2005–2013. [Google Scholar] [CrossRef]
- Kamarudin, M.N.; Parhar, I. Chrysin Promotes Temozolomide-induced Apoptosis by Activating p38 MAPK and Suppressing Akt and ERK1/2 in Human Glioblastoma. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. Available online: https://www.mdpi.com/2227-9059/10/7/1686 (accessed on 1 August 2022). [PubMed]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, S.; Li, N.; Ho, A.S.W.; Kiang, K.M.Y.; Zhang, X.; Cheng, Y.S.; Poon, M.W.; Lee, D.; Pu, J.K.S.; et al. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J. Neuro-Oncol. 2017, 132, 393–400. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Sun, K.; Wang, X.; Pan, H.; Zhu, J.; Ji, X.; Li, X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des. Devel. Ther. 2018, 12, 721–733. [Google Scholar] [CrossRef]
- Dixit, D.; Sharma, V.; Ghosh, S.; Mehta, V.S.; Sen, E. Inhibition of Casein kinase-2 induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNFα)-induced apoptosis through SIRT1 inhibition. Cell Death Dis. 2012, 3, e271. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ray, S.K. Synergistic anti-tumor actions of luteolin and silibinin prevented cell migration and invasion and induced apoptosis in glioblastoma SNB19 cells and glioblastoma stem cells. Brain Res. 2015, 1629, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Brossard, M.; Pagé, M.; Gingras, D.; Béliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Et Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Chen, T.C.; Wang, W.; Golden, E.B.; Thomas, S.; Sivakumar, W.; Hofman, F.M.; Louise, S.G.; Schönthal, A.H. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011, 302, 100–108. [Google Scholar] [CrossRef]
- Khaw, A.K.; Yong, J.W.; Kalthur, G.; Hande, M.P. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Genes Chromosomes Cancer 2012, 51, 961–974. [Google Scholar] [CrossRef]
- Desai, V.; Jain, A.; Shaghaghi, H.; Summer, R.; Lai, J.; Bhushan, A. Combination of Biochanin A and Temozolomide Impairs Tumor Growth by Modulating Cell Metabolism in Glioblastoma Multiforme. Anticancer. Res. 2019, 39, 57–66. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerzsa, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PloS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Navarro-Núñez, L.; Castillo, J.; Lozano, M.L.; Martínez, C.; Benavente-García, O.; Vicente, V.; Rivera, J. Thromboxane A2 Receptor Antagonism by Flavonoids: Structure−Activity Relationships. J. Agric. Food Chem. 2009, 57, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the Antioxidant Activity of Flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Noureddine, H.; Hage-Sleiman, R.; Wehbi, B.; Fayyad-Kazan, H.; Hayar, S.; Traboulssi, M.; Alyamani, O.A.; Faour, W.H.; ElMakhour, Y. Chemical characterization and cytotoxic activity evaluation of Lebanese propolis. Biomed. Pharmacother. 2017, 95, 298–307. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant flavone apigenin: An emerging anticancer agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Deep, G.; Agarwal, R. Antimetastatic efficacy of silibinin: Molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010, 29, 447–463. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Fernández-Castillejo, S.; Pedret, A.; Llauradó, E.; Solà, R. Cyanidin-3-glucoside as a possible biomarker of anthocyanin-rich berry intake in body fluids of healthy humans: A systematic review of clinical trials. Nutr. Rev. 2019, 78, 597–610. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, J.; Guo, C.; Gao, X.; Chen, J.; Chang, C.; Han, X.; Wang, L. Characterization and optimization of hydrothermal extraction of quercetin from Quercus leaves using response surface methodology. Can. J. Chem. Eng. 2022, 100, 598–606. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro. J. Neuro-Oncol. 2016, 129, 39–45. [Google Scholar] [CrossRef]
- Pan, H.-C.; Jiang, Q.; Yu, Y.; Mei, J.-P.; Cui, Y.-K.; Zhao, W.-J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-T.; Shen, S.-C.; Chow, J.-M.; Lin, C.-W.; Shia, L.-T.; Chen, Y.-C. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE2 activation. Neurobiol. Dis. 2010, 37, 118–129. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.G.; Yang, J.Q.; Zhou, Y.; Meng, L.H.; Wang, H.; Li, C.L. Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Onco Targets Ther. 2017, 10, 4023–4028. [Google Scholar] [CrossRef]
- Saha, M.N.; Jiang, H.; Yang, Y.; Zhu, X.; Wang, X.; Schimmer, A.D.; Qiu, L.; Chang, H. Targeting p53 via JNK pathway: A novel role of RITA for apoptotic signaling in multiple myeloma. PloS ONE 2012, 7, e30215. [Google Scholar] [CrossRef]
- Souza, P.O.; Bianchi, S.E.; Figueiró, F.; Heimfarth, L.; Moresco, K.S.; Gonçalves, R.M.; Hoppe, J.B.; Klein, C.P.; Salbego, C.G.; Gelain, D.P.; et al. Anticancer activity of flavonoids isolated from Achyrocline satureioides in gliomas cell lines. Toxicol. Vitr. 2018, 51, 23–33. [Google Scholar] [CrossRef]
- Pozsgai, E.; Bellyei, S.; Cseh, A.; Boronkai, A.; Racz, B.; Szabo, A.; Sumegi, B.; Hocsak, E. Quercetin Increases the Efficacy of Glioblastoma Treatment Compared to Standard Chemoradiotherapy by the Suppression of PI-3-Kinase-Akt Pathway. Nutr. Cancer 2013, 65, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.B.; Cerqueira Coelho, P.L.; das Neves Oliveira, M.; Oliveira, J.L.; Oliveira Amparo, J.A.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; Dos Santos Souza, C.; de Faria Lopes, G.P.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef]
- Taylor, M.A.; Khathayer, F.; Ray, S.K. Quercetin and Sodium Butyrate Synergistically Increase Apoptosis in Rat C6 and Human T98G Glioblastoma Cells Through Inhibition of Autophagy. Neurochem. Res. 2019, 44, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox. Res. 2014, 26, 64–77. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin sensitizes glioblastoma to t-AUCB by dual inhibition of Hsp27 and COX-2 in vitro and in vivo. J. Exp. Clin. Cancer Res. 2016, 35, 61. [Google Scholar] [CrossRef] [PubMed]
- Bądziul, D.; Jakubowicz-Gil, J.; Langner, E.; Rzeski, W.; Głowniak, K.; Gawron, A. The effect of quercetin and imperatorin on programmed cell death induction in T98G cells in vitro. Pharmacol. Rep. 2014, 66, 292–300. [Google Scholar] [CrossRef]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell. Res. 2012, 318, 925–935. [Google Scholar] [CrossRef]
- Eldalawy, R. Quantitative estimation of rutin in rue (Ruta graveolens L.) cultivated in Iraq with the evaluation of its antioxidant activity. Asian J. Pharm. Clin. Res. 2017, 10, 353–355. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.-Y.; Li, H.-B.; Wu, D.-T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.-Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.-J.; Du, L.; Fei, L.; To, S.-T. Inhibitory Kinetics and Mechanism of Flavonoids Extracted from Cotinus coggygria Scop. Against Glioblastoma Cancer. Nutr. Cancer 2016, 68, 1357–1368. [Google Scholar] [CrossRef]
- Santos, B.L.; Silva, A.R.; Pitanga, B.P.S.; Sousa, C.S.; Grangeiro, M.S.; Fragomeni, B.O.; Coelho, P.L.C.; Oliveira, M.N.; Menezes-Filho, N.J.; Costa, M.F.D.; et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011, 127, 404–411. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Saul, R.C.; Saraí, C.H.; Karen, L.H.R.; Luis, A.C.C.; María, I.E.A.; Laura, E.G.O.; José de Jesús, O.-P.; Marco, A.L.M. Flavonoids: Important Biocompounds in Food. In Flavonoids; Goncalo, C.J., Ed.; Ch. 16.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Liao, C.-L.; Chen, C.-M.; Chang, Y.-Z.; Liu, G.-Y.; Hung, H.-C.; Hsieh, T.-Y.; Lin, C.-L. Pine (Pinus morrisonicola Hayata) Needle Extracts Sensitize GBM8901 Human Glioblastoma Cells to Temozolomide by Downregulating Autophagy and O6-Methylguanine-DNA Methyltransferase Expression. J. Agric. Food Chem. 2014, 62, 10458–10467. [Google Scholar] [CrossRef]
- Han, J.E.; Lim, P.W.; Na, C.M.; Choi, Y.S.; Lee, J.Y.; Kim, Y.; Park, H.W.; Moon, H.E.; Heo, M.S.; Park, H.R.; et al. Inhibition of HIF1α and PDK Induces Cell Death of Glioblastoma Multiforme. Exp. Neurobiol. 2017, 26, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Borawska, M.H.; Fiedorowicz, A.; Naliwajko, S.K.; Sawicka, D.; Car, H. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement. Altern. Med. 2013, 13, 50. [Google Scholar] [CrossRef]
- Santos, B.L.; Oliveira, M.N.; Coelho, P.L.C.; Pitanga, B.P.S.; da Silva, A.B.; Adelita, T.; Silva, V.D.A.; Costa, M.d.F.D.; El-Bachá, R.S.; Tardy, M.; et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem. -Biol. Interact. 2015, 242, 123–138. [Google Scholar] [CrossRef]
- Weng, M.-S.; Ho, Y.-S.; Lin, J.-K. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated protein kinase. Biochem. Pharmacol. 2005, 69, 1815–1827. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, M.; Sarwat, M.; Siddique, H.R. Apigenin in cancer prevention and therapy: A systematic review and meta-analysis of animal models. Crit. Rev. Oncol. Hematol. 2022, 176, 103751. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010, 116, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Z.; Dai, X.; Zhang, L.; Li, M. Apigenin and Temozolomide Synergistically Inhibit Glioma Growth Through the PI3K/AKT Pathway. Cancer Biother. Radiopharm. 2021. [Google Scholar] [CrossRef]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phytother. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef]
- Chen, L.J.; Hsu, T.C.; Yeh, P.J.; Yow, J.L.; Chang, C.L.; Lin, C.H.; Tzang, B.S. Differential Effects of Wedelia chinensis on Human Glioblastoma Multiforme Cells. Integr. Cancer Ther. 2021, 20, 15347354211000119. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chang, Y.-M.; Wang, K.-Y.; Chen, P.-N.; Hseu, Y.-C.; Chen, K.-M.; Yeh, K.-T.; Chen, C.-J.; Hsu, L.-S. Naringenin inhibited migration and invasion of glioblastoma cells through multiple mechanisms. Environ. Toxicol. 2019, 34, 233–239. [Google Scholar] [CrossRef]
- Aroui, S.; Aouey, B.; Chtourou, Y.; Meunier, A.-C.; Fetoui, H.; Kenani, A. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem. -Biol. Interact. 2016, 244, 195–203. [Google Scholar] [CrossRef]
- Daisy Precilla, S.; Kuduvalli, S.S.; Angeline Praveena, E.; Thangavel, S.; Anitha, T.S. Integration of synthetic and natural derivatives revives the therapeutic potential of temozolomide against glioma- an in vitro and in vivo perspective. Life Sci. 2022, 301, 120609. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Hao, G.; Wang, B.; Wang, J.; Liang, Y.; Liu, Y.; Zhen, E.; Feng, D.; Liang, G. Naringin suppresses the development of glioblastoma by inhibiting FAK activity. J. Drug Target. 2017, 25, 41–48. [Google Scholar] [CrossRef]
- Sargazi, M.L.; Juybari, K.B.; Tarzi, M.E.; Amirkhosravi, A.; Nematollahi, M.H.; Mirzamohammdi, S.; Mehrbani, M.; Mehrabani, M.; Mehrabani, M. Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: A possible role of hedgehog signaling pathway. Mol. Biol. Rep. 2021, 48, 6413–6421. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15. Available online: https://www.mdpi.com/1424-8247/15/5/538 (accessed on 12 August 2022). [CrossRef] [PubMed]
- Momeny, M.; Malehmir, M.; Zakidizaji, M.; Ghasemi, R.; Ghadimi, H.; Shokrgozar, M.A.; Emami, A.H.; Nafissi, S.; Ghavamzadeh, A.; Ghaffari, S.H. Silibinin inhibits invasive properties of human glioblastoma U87MG cells through suppression of cathepsin B and nuclear factor kappa B-mediated induction of matrix metalloproteinase 9. Anti-Cancer Drugs 2010, 21. Available online: https://journals.lww.com/anti-cancerdrugs/Fulltext/2010/03000/Silibinin_inhibits_invasive_properties_of_human.4.aspx (accessed on 3 August 2022).
- Dizaji, M.Z.; Malehmir, M.; Ghavamzadeh, A.; Alimoghaddam, K.; Ghaffari, S.H. Synergistic Effects of Arsenic Trioxide and Silibinin on Apoptosis and Invasion in Human Glioblastoma U87MG Cell Line. Neurochem. Res. 2012, 37, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.L.; Tay, V.; Guo, S.Z.; Ren, J.; Shu, M.G. Silibinin Induced Human Glioblastoma Cell Apoptosis Concomitant with Autophagy through Simultaneous Inhibition of mTOR and YAP. Biomed. Res. Int. 2018, 2018, 6165192. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2016, 21, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Nutritional Factors and Male Reproduction. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 458–464. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Hung, S.W.; Li, Y.; Chen, X.; Chu, K.O.; Zhao, Y.; Liu, Y.; Guo, X.; Man, G.C.; Wang, C.C. Green Tea Epigallocatechin-3-Gallate Regulates Autophagy in Male and Female Reproductive Cancer. Front. Pharmacol. 2022, 13, 906746. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.-R.; You, C.-G.; Zhang, N.; Sheng, H.-S.; Zheng, X.-S. Epigallocatechin Gallate Preferentially Inhibits O6-Methylguanine DNA-Methyltransferase Expression in Glioblastoma Cells Rather than in Nontumor Glial Cells. Nutr. Cancer 2018, 70, 1339–1347. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.-X.; Ma, J.-W.; Li, H.-Y.; Ye, J.-C.; Xie, S.-M.; Du, B.; Zhong, X.-Y. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J. Neuro-Oncol. 2015, 121, 41–52. [Google Scholar] [CrossRef]
- Djerir, D.; Iddir, M.; Bourgault, S.; Lamy, S.; Annabi, B. Biophysical evidence for differential gallated green tea catechins binding to membrane type-1 matrix metalloproteinase and its interactors. Biophys. Chem. 2018, 234, 34–41. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol. Rep. 2017, 37, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Annabi, B.; Lachambre, M.-P.; Bousquet-Gagnon, N.; Pagé, M.; Gingras, D.; Béliveau, R. Green tea polyphenol (−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2002, 1542, 209–220. [Google Scholar] [CrossRef]
- Djediai, S.; Gonzalez Suarez, N.; El Cheikh-Hussein, L.; Rodriguez Torres, S.; Gresseau, L.; Dhayne, S.; Joly-Lopez, Z.; Annabi, B. MT1-MMP Cooperates with TGF-β Receptor-Mediated Signaling to Trigger SNAIL and Induce Epithelial-to-Mesenchymal-like Transition in U87 Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 13006. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, V.; Tewari, R.; Koul, N.; Joseph, C.; Sen, E. Epigallocatechin-3-gallate exhibits anti-tumor effect by perturbing redox homeostasis, modulating the release of pro-inflammatory mediators and decreasing the invasiveness of glioblastoma cells. Mol. Med. Rep. 2008, 1, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Grube, S.; Ewald, C.; Kögler, C.; Lawson McLean, A.; Kalff, R.; Walter, J. Achievable Central Nervous System Concentrations of the Green Tea Catechin EGCG Induce Stress in Glioblastoma Cells in Vitro. Nutr. Cancer 2018, 70, 1145–1158. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Habel, A.; Gaiser, T. Epigalocatechin-3-gallate (EGCG) downregulates PEA15 and thereby augments TRAIL-mediated apoptosis in malignant glioma. Neurosci. Lett. 2008, 448, 161–165. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Devi, A.; Mishra, S. Molecular docking and molecular dynamics studies reveal structural basis of inhibition and selectivity of inhibitors EGCG and OSU-03012 toward glucose regulated protein-78 (GRP78) overexpressed in glioblastoma. J. Mol. Model. 2015, 21, 272. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Hadizadeh, K.R.; Seul, C.; Yun, Y.P.; Vetter, H.; Sachinidis, A. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172). Mol. Biol. Cell. 1999, 10, 1093–1104. [Google Scholar] [CrossRef]
- Sachinidis, A.; Seul, C.; Seewald, S.; Ahn, H.; Ko, Y.; Vetter, H. Green tea compounds inhibit tyrosine phosphorylation of PDGF beta-receptor and transformation of A172 human glioblastoma. FEBS Lett. 2000, 471, 51–55. [Google Scholar] [CrossRef]
- Zuiter, A.S. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Matthies, A.; Clavel, T.; Gütschow, M.; Engst, W.; Haller, D.; Blaut, M.; Braune, A. Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Appl. Environ. Microbiol. 2008, 74, 4847–4852. [Google Scholar] [CrossRef]
- Regenbrecht, C.R.; Jung, M.; Lehrach, H.; Adjaye, J. The molecular basis of genistein-induced mitotic arrest and exit of self-renewal in embryonal carcinoma and primary cancer cell lines. BMC Med. Genomics 2008, 1, 49. [Google Scholar] [CrossRef]
- Chen, X.; Hao, A.; Li, X.; Ye, K.; Zhao, C.; Yang, H.; Ma, H.; Hu, L.; Zhao, Z.; Hu, L.; et al. Activation of JNK and p38 MAPK Mediated by ZDHHC17 Drives Glioblastoma Multiforme Development and Malignant Progression. Theranostics 2020, 10, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Liu, B.; Zheng, X.; Li, P.; Zhao, T.; Jin, X.; Ye, F.; Zhang, P.; Chen, W.; et al. Genistein inhibits radiation-induced invasion and migration of glioblastoma cells by blocking the DNA-PKcs/Akt2/Rac1 signaling pathway. Radiother. Oncol. 2021, 155, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, P.; Hirayama, R.; Niu, Y.; Liu, X.; Chen, W.; Jin, X.; Zhang, P.; Ye, F.; Zhao, T.; et al. Genistein sensitizes glioblastoma cells to carbon ions via inhibiting DNA-PKcs phosphorylation and subsequently repressing NHEJ and delaying HR repair pathways. Radiother. Oncol. 2018, 129, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, D.; Li, L.; Wang, J.; Li, Q.; Duan, L.; Yin, H.; Wang, X.; Liu, Y.; Yuan, G.; et al. Biochanin A Sensitizes Glioblastoma to Temozolomide by Inhibiting Autophagy. Mol. Neurobiol. 2022, 59, 1262–1272. [Google Scholar] [CrossRef]
- Puli, S.; Lai, J.C.; Bhushan, A. Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J. Neurooncol. 2006, 79, 135–142. [Google Scholar] [CrossRef]
- Puli, S.; Jain, A.; Lai, J.C.; Bhushan, A. Effect of combination treatment of rapamycin and isoflavones on mTOR pathway in human glioblastoma (U87) cells. Neurochem. Res. 2010, 35, 986–993. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Hosseini, M.M.; Karimi, A.; Behroozaghdam, M.; Javidi, M.A.; Ghiasvand, S.; Bereimipour, A.; Aryan, H.; Nassiri, F.; Jangholi, E. Cytotoxic and Apoptogenic Effects of Cyanidin-3-Glucoside on the Glioblastoma Cell Line. World Neurosurg. 2017, 108, 94–100. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.; Ding, D.; Li, Z.; Cheng, L.; You, Q.; Zhang, S. Cyanidin-3-O-glucoside inhibits the β-catenin/MGMT pathway by upregulating miR-214-5p to reverse chemotherapy resistance in glioma cells. Sci. Rep. 2022, 12, 7773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Yadav, V.K.; Chu, Y.C.; Ong, J.R.; Huang, T.Y.; Lee, K.F.; Lee, K.H.; Yeh, C.T.; Lee, W.H. Hydroxychloroquine (HCQ) Modulates Autophagy and Oxidative DNA Damage Stress in Hepatocellular Carcinoma to Overcome Sorafenib Resistance via TLR9/SOD1/hsa-miR-30a-5p/Beclin-1 Axis. Cancers 2021, 13, 3227. [Google Scholar] [CrossRef] [PubMed]
- Biau, J.; Thivat, E.; Chautard, E.; Stefan, D.; Boone, M.; Chauffert, B.; Bourgne, C.; Richard, D.; Molnar, I.; Levesque, S.; et al. Phase 1 trial of ralimetinib (LY2228820) with radiotherapy plus concomitant temozolomide in the treatment of newly diagnosed glioblastoma. Radiother. Oncol. 2021, 154, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.R.; Ye, X.; Supko, J.G.; Desideri, S.; Grossman, S.A.; Brem, S.; Mikkelson, T.; Wang, D.; Chang, Y.C.; Hu, J.; et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 2014, 10, 1359–1368. [Google Scholar] [CrossRef]
- Compter, I.; Eekers, D.B.P.; Hoeben, A.; Rouschop, K.M.A.; Reymen, B.; Ackermans, L.; Beckervordersantforth, J.; Bauer, N.J.C.; Anten, M.M.; Wesseling, P.; et al. Chloroquine combined with concurrent radiotherapy and temozolomide for newly diagnosed glioblastoma: A phase IB trial. Autophagy 2021, 17, 2604–2612. [Google Scholar] [CrossRef]
- Levin, V.A.; Phuphanich, S.; Alfred Yung, W.K.; Forsyth, P.A.; Del Maestro, R.; Perry, J.R.; Fuller, G.N.; Bailet, Mark. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J. Neuro-Oncol. 2006, 78, 295–302. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Patil, C.G.; Nuño, M.; Elramsisy, A.; Mukherjee, D.; Carico, C.; Dantis, J.; Hu, J.; Yu, J.S.; Fan, X.; Black, K.L.; et al. High levels of phosphorylated MAP kinase are associated with poor survival among patients with glioblastoma during the temozolomide era. Neuro. Oncol. 2013, 15, 104–111. [Google Scholar] [CrossRef]
- Arvanitis, D.; Malliri, A.; Antoniou, D.; Linardopoulos, S.; Field, J.K.; Spandidos, D.A. Ras p21 expression in brain tumors: Elevated expression in malignant astrocytomas and glioblastomas multiforme. In Vivo 1991, 5, 317–321. [Google Scholar]
- Tsurushima, H.; Tsuboi, K.; Yoshii, Y.; Ohno, T.; Meguro, K.; Nose, T. Expression of N-ras gene in gliomas. Neurol. Med. Chir. 1996, 36, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, Y.; Liu, Z.; Liu, J.; Liu, X.; Chen, X.; Li, C.; Zeng, Y. p38γ overexpression in gliomas and its role in proliferation and apoptosis. Sci. Rep. 2013, 3, 2089. [Google Scholar] [CrossRef] [PubMed]
- Lyustikman, Y.; Momota, H.; Pao, W.; Holland, E.C. Constitutive activation of Raf-1 induces glioma formation in mice. Neoplasia 2008, 10, 501–510. [Google Scholar] [CrossRef]
- Qin, A.; Musket, A.; Musich, P.R.; Schweitzer, J.B.; Xie, Q. Receptor tyrosine kinases as druggable targets in glioblastoma: Do signaling pathways matter? Neuro-Oncol. Adv. 2021, 3, vdab133. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef]
- Hemann, M.T.; Lowe, S.W. The p53-Bcl-2 connection. Cell Death Differ. 2006, 13, 1256–1259. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.X.; Liu, J.P.; You, C.; Liu, Y.H.; Mao, Q. Gain of function of mutant TP53 in glioblastoma: Prognosis and response to temozolomide. Ann. Surg. Oncol. 2014, 21, 1337–1344. [Google Scholar] [CrossRef]
- Gluck, W.L.; Gounder, M.M.; Frank, R.; Eskens, F.; Blay, J.Y.; Cassier, P.A.; Soria, J.C.; Chawla, S.; De Weger, V.; Wagner, A.J.; et al. Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Invest. New Drugs. 2020, 38, 831–843. [Google Scholar] [CrossRef]
- Simpson, J.E.; Gammoh, N. The impact of autophagy during the development and survival of glioblastoma. Open Biol. 2020, 10, 200184. [Google Scholar] [CrossRef]
- Liu, L.-Q.; Wang, S.-B.; Shao, Y.-F.; Shi, J.-N.; Wang, W.; Chen, W.-Y.; Ye, Z.-Q.; Jiang, J.-Y.; Fang, Q.-X.; Zhang, G.-B.; et al. Hydroxychloroquine potentiates the anti-cancer effect of bevacizumab on glioblastoma via the inhibition of autophagy. Biomed. Pharmacother. 2019, 118, 10933. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.M.; Agnes, J.P.; Delgobo, M.; de Souza, P.O.; Thomé, M.P.; Heimfarth, L.; Lenz, G.; Moreira, J.C.F.; Zanotto-Filho, A. Late autophagy inhibitor chloroquine improves efficacy of the histone deacetylase inhibitor SAHA and temozolomide in gliomas. Biochem. Pharmacol. 2019, 163, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, Z.; Dai, S.; Qian, L.; Sun, L.; Gong, Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 2016, 35, 23. [Google Scholar] [CrossRef] [PubMed]
- Adamski, V.; Schmitt, C.; Ceynowa, F.; Adelung, R.; Lucius, R.; Synowitz, M.; Hattermann, K.; Held-Feindt, J. Effects of sequentially applied single and combined temozolomide, hydroxychloroquine and AT101 treatment in a long-term stimulation glioblastoma in vitro model. J. Cancer Res. Clin. Oncol. 2018, 144, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Saadat, F.; Zareighane, Z.; Safavifar, F.; Jalali, S.Z.; Berahmeh, A.; Khorramizadeh, M.R. The Repression of Matrix Metalloproteinases and Cytokine Secretion in Glioblastoma by Targeting K+ Channel. Basic Clin. Neurosci. 2021, 12, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S. Molecular mechanisms of glioma invasiveness: The role of proteases. Nat. Rev. Cancer 2003, 3, 489–501. [Google Scholar] [CrossRef]
- Sawaya, R.E.; Yamamoto, M.; Gokaslan, Z.L.; Wang, S.W.; Mohanam, S.; Fuller, G.N.; McCutcheon, I.E.; Stetler-Stevenson, W.G.; Nicolson, G.L.; Rao, J.S. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin. Exp. Metastasis 1996, 14, 35–42. [Google Scholar] [CrossRef]
- Rao, J.S.; Yamamoto, M.; Mohaman, S.; Gokaslan, Z.L.; Fuller, G.N.; Stetler-Stevenson, W.G.; Rao, V.H.; Liotta, L.A.; Nicolson, G.L.; Sawaya, R.E. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin. Exp. Metastasis 1996, 14, 12–18. [Google Scholar] [CrossRef]
- Zhang, K.; Li, C.; Liu, Y.; Li, L.; Ma, X.; Meng, X.; Feng, D. Evaluation of invasiveness of astrocytoma using 1H-magnetic resonance spectroscopy: Correlation with expression of matrix metalloproteinase-2. Neuroradiology 2007, 49, 913–919. [Google Scholar] [CrossRef]
- Ramachandran, R.K.; Sørensen, M.D.; Aaberg-Jessen, C.; Hermansen, S.K.; Kristensen, B.W. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. PLoS ONE 2017, 12, e0172234. [Google Scholar] [CrossRef]
- Lesueur, P.; Lequesne, J.; Grellard, J.M.; Dugué, A.; Coquan, E.; Brachet, P.E.; Geffrelot, J.; Kao, W.; Emery, E.; Berro, D.H.; et al. Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol. BMC Cancer 2019, 19, 198. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Nagase, H. Matrix metalloproteinases in cancer. Essays Biochem. 2002, 38, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Čvorović, J.; Ziberna, L.; Fornasaro, S.; Tramer, F.; Passamonti, S. Chapter 22—Bioavailability of Flavonoids: The Role of Cell Membrane Transporters. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 295–320. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, Alfonso; Quagliariello, Vincenzo. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Luo, J.; Wang, L.; Chen, X.; Zhang, L.; Jiang, S. PEG2000-DPSE-coated quercetin nanoparticles remarkably enhanced anticancer effects through induced programed cell death on C6 glioma cells. J. Biomed. Mater. Res. Part A 2013, 101, 3076–3085. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Chen, X.L.; Du, S.M.; Li, D.S.; Pei, Z.J.; Lan, H.; Wu, L.B. The JAK2/STAT3 and mitochondrial pathways are essential for quercetin nanoliposome-induced C6 glioma cell death. Cell Death Dis. 2013, 4, e746. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Wang, G.; Yao, Z.; Dang, X. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int. J. Mol. Med. 2016, 37, 690–702. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Yang, G.Y.; Du, S.M.; Zeng, N.; Li, D.S.; Li, R.M.; Chen, J.Y.; Feng, J.B.; Yuan, S.H.; et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int. J. Nanomedicine 2012, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Chen, X.L.; Du, L.; Li, F. Quercetin-loaded freeze-dried nanomicelles: Improving absorption and anti-glioma efficiency in vitro and in vivo. J. Control Release 2016, 235, 276–290. [Google Scholar] [CrossRef]
- Lou, M.; Zhang, L.N.; Ji, P.G.; Feng, F.Q.; Liu, J.H.; Yang, C.; Li, B.F.; Wang, L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 2016, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cruz Dos Santos, S.; Osti Silva, N.; Dos Santos Espinelli, J.B.J.; Germani Marinho, M.A.; Vieira Borges, Z.; Branco, N.B.C.; Faita, F.L.; Meira Soares, B.; Horn, A.P.; Parize, A.L.; et al. Molecular interactions and physico-chemical characterization of quercetin-loaded magnetoliposomes. Chem. Phys. Lipids 2019, 218, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Guan, R. Novel Phospholipid-Based Labrasol Nanomicelles Loaded Flavonoids for Oral Delivery with Enhanced Penetration and Anti-Brain Tumor Efficiency. Curr. Drug Deliv. 2020, 17, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol. 2020, 25, 757–766. [Google Scholar] [CrossRef]
- Liu, F.; Peng, B.; Li, M.; Ma, J.; Deng, G.; Zhang, S.; Sheu, W.C.; Zou, P.; Wu, H.; Liu, J.; et al. Targeted disruption of tumor vasculature via polyphenol nanoparticles to improve brain cancer treatment. Cell Rep. Phys. Sci. 2022, 3, 100691. [Google Scholar] [CrossRef]
- Paranthaman, S.; Uthaiah, C.A.; Osmani, R.A.M.; Hani, U.; Ghazwani, M.; Alamri, A.H.; Fatease, A.A.; Madhunapantula, S.V.; Gowda, D.V. Anti-Proliferative Potential of Quercetin Loaded Polymeric Mixed Micelles on Rat C6 and Human U87MG Glioma Cells. Pharmaceutics 2022, 14, 1643. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Shen, L.; Alrobaian, M.; Panda, S.K.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Ibrahim, I.A.A.; Singh, T.; et al. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed. Pharmacother. 2021, 138, 111461. [Google Scholar] [CrossRef]
- Alipour, M.; Bigdeli, M.R.; Aligholi, H.; Rasoulian, B.; Khaksarian, M. Sustained release of silibinin-loaded chitosan nanoparticle induced apoptosis in glioma cells. J. Biomed. Mater. Res. Part A 2020, 108, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.H.; Yu, H.J.; Cho, N.S.; Kim, T.H.; Kim, D.C.; Chung, C.B.; Hwang, Y.I.; Kim, K.H. Enhanced bioavailability of soy isoflavones by complexation with beta-cyclodextrin in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 10960. [Google Scholar] [CrossRef] [PubMed]
- Michala, A.-S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. Available online: https://www.mdpi.com/2079-9721/10/3/37 (accessed on 10 August 2022). [CrossRef]

| Flavonoid Subclasses | Examples | Common Source | References |
|---|---|---|---|
| Flavonol | (i) Quercetin | ||
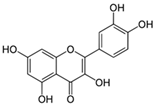 | Oak (Quercus) | [46] | |
| (ii) Rutin (flavonol glycoside) | |||
 | Rue (Ruta graveolens) | [47] | |
| Flavone | (i) Chrysin | ||
 | Passionflower (Passiflora) | [48] | |
| (ii) Apigenin | |||
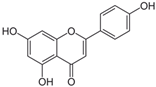 | Chamomile tea (Matricaria chamomilla) | [49] | |
| Flavanone | (i) Naringenin | ||
 | Citrus species | [50] | |
| (ii) Silibinin | |||
 | Milk thistle (Silybum marianum) | [51] | |
| Flavanol | (i) EGCG | ||
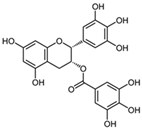 | Green and white tea | [52] | |
| Isoflavone | (i) Genistein | ||
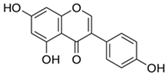 | Soy-based foods | [53] | |
| (ii) Biochanin A | |||
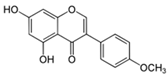 | Chickpea (Cicer arietinum) | [54] | |
| Anthocyanin | (i) Cyanidin 3-O-glucoside (C3G) | ||
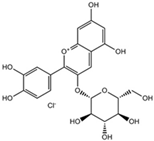 | Red- and blue-pigmented fruits | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients 2023, 15, 797. https://doi.org/10.3390/nu15040797
Wong SC, Kamarudin MNA, Naidu R. Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients. 2023; 15(4):797. https://doi.org/10.3390/nu15040797
Chicago/Turabian StyleWong, Shu Chyi, Muhamad Noor Alfarizal Kamarudin, and Rakesh Naidu. 2023. "Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas" Nutrients 15, no. 4: 797. https://doi.org/10.3390/nu15040797
APA StyleWong, S. C., Kamarudin, M. N. A., & Naidu, R. (2023). Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients, 15(4), 797. https://doi.org/10.3390/nu15040797





