The Potential Use of Honey as a Neuroprotective Agent for the Management of Neurodegenerative Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1H NMR | Proton Nuclear Magnetic Resonance |
| 5-LOX | 5-Lipoxygenase |
| 8-OHDG | 8-Hydroxy-2′-Deoxyguanosine |
| ACHE | Acetylcholinesterase |
| AD | Alzheimer’s Disease |
| AIF-1 | Allograft Inflammatory Factor 1 |
| ALS | Amyotrophic Lateral Sclerosis |
| APP | Amyloid Precursor Protein |
| BCHE | Butyrylcholinesterase |
| CAT | Catalase |
| COMT | Catechol-O-Methyltransferase |
| COX-2 | Cyclooxygenase-2 |
| CP | Carbonylated Protein |
| CSSI | Chronic Subclinical Systemic Inflammation |
| CT | Chimney Test |
| DHA | Docosahexaenoic Acid |
| dUBQN | Ubiquilin |
| FDA | U.S. Food and Drug Administration |
| FJC | Fluoro-Jade C |
| GFAP | Glial Fibrillary Acidic Protein |
| GWASs | Genome-Wide Associations |
| HD | Huntington’s Disease |
| HDACs | Histone Deacetylases |
| IFN-G | Interferon Gamma |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin 6 |
| KA | Kainic Acid |
| L-DOPA | Levodopa |
| LPS | Lipopolysaccharide |
| MAO-B | Monoamine Oxidase B |
| MDA | Malondialdehyde |
| MFF | Mixed Functional Food |
| mPFC | Medial Prefrontal Cortex |
| MS | Multiple Sclerosis |
| NF-ΚB P65 | Nuclear Factor kappa B p6 |
| NMDA | N-methyl-D-aspartase |
| NMJS | Neuromuscular Junctions |
| NO | Nitric Oxide |
| NRF2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| OFT | Open-field Test |
| P38 MAPK | P38 Mitogen-Activated Protein Kinase |
| PCA | Principal Component Analysis |
| PD | Parkinson’s Disease |
| PON2 | Paraoxonase 2 |
| ROS | Reactive Oxygen Species |
| SBH | Stingless Bee Honey |
| SIRT1 | Sirtuins |
| t-BuOOH | Tert-butyl Hydroperoxide |
| TAS | Total Antioxidant Status |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TUH | Tualang Honey |
| THH | Thyme Honey |
| TNF-A | Tumor Necrosis Factor Alpha |
References
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef]
- Sarrafchi, A.; Bahmani, M.; Shirzad, H.; Rafieian-Kopaei, M. Oxidative stress and Parkinson’s disease: New hopes in treatment with herbal antioxidants. Curr. Pharm. Des. 2015, 22, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Essa, M.; Braidy, N.; Bridge, W.; Subash, S.; Manivasagam, T.; Vijayan, R.; Al-Adawi, S.; Guillemin, G. Review of natural products on Parkinson’s disease pathology. J. Aging Res. Clin. Pract. 2014, 3, 127. [Google Scholar] [CrossRef]
- Eratne, D.; Loi, S.M.; Farrand, S.; Kelso, W.; Velakoulis, D.; Looi, J.C. Alzheimer’s disease: Clinical update on epidemiology, pathophysiology and diagnosis. Australas. Psychiatry 2018, 26, 347–357. [Google Scholar] [CrossRef]
- Dubey, H.; Gulati, K.; Ray, A. Recent studies on cellular and molecular mechanisms in Alzheimer’s disease: Focus on epigenetic factors and histone deacetylase. Rev. Neurosci. 2018, 29, 241–260. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2019, 36, 1–12. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Sivanandy, P.; Leey, T.C.; Xiang, T.C.; Ling, T.C.; Han, S.A.W.; Semilan, S.L.A.; Hong, P.K. Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms. Int. J. Environ. Res. Public Health 2021, 19, 364. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic Acid Composition, Antiatherogenic and Anticancer Potential of Honeys Derived from Various Regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Vandamme, L.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Monstrey, S. Honey in modern wound care: A systematic review. Burns 2013, 39, 1514–1525. [Google Scholar] [CrossRef]
- Thomas, C.; Mayegowda Shilpa, B.; Babu Mythri, R. Disease Modifying Potential of Functional Foods for Neurodegenerative Disorders: Status Update on Regulatory Compliance. In Functional Foods-Phytochemicals and Health Promoting Potential; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Maurya, H.; Kumar, S. Current medication trends and global impact on neurodegenerative disorders. Ann. Clin. Pharmacol. Ther. 2018, 6, 9. [Google Scholar]
- Food and Agriculture Organization/World Health Organization. The Standard for Honey CXS 12-19811 was Adopted in 1981. Revised in 1987, 2001. Amended in 2019. Codex Alimentarius. 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/shproxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 20 September 2022).
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honeybees and stingless bees: A comparative review. Rev. Bras. De Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- European Commission. European Commission Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, 10–47. Available online: http://data.europa.eu/eli/dir/2001/110/oj (accessed on 20 September 2022).
- Di Rosa, A.R.; Marino, A.M.F.; Leone, F.; Corpina, G.G.; Giunta, R.P.; Chiofalo, V. Characterization of Sicilian Honeys Pollen Profiles Using a Commercial E-Tongue and Melissopalynological Analysis for Rapid Screening: A Pilot Study. Sensors 2018, 18, 4065. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; El Midaoui, A.; Bourkhis, B.; Ennassir, J.; Alem, C.; Filali-Zegzouti, Y. Physicochemical properties of eleven monofloral honey samples produced in Morocco. Arab. J. Basic Appl. Sci. 2019, 26, 476–487. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Pia Manna, P.; Zhang, J.; Lamas, L.B.; Martínez Flórez, S.; Toyos, P.A.; et al. Phenolic com-pounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Ye, Y.-L.; Lin, T.-Y.; Wang, Y.-W.; Peng, C.-C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Bilan, A.R.; Freyssin, A.; Page, G.; Fauconneau, B. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen. Res. 2018, 13, 955–961. [Google Scholar] [CrossRef]
- Godoy, L.D.; Lucas, J.E.; Bender, A.J.; Romanick, S.S.; Ferguson, B.S. Targeting the epigenome: Screening bioactive compounds that regulate histone deacetylase activity. Mol. Nutr. Food Res. 2017, 61, 1600744. [Google Scholar] [CrossRef]
- Al-Abd, N.M.; Kassim, M.; Zajmi, A. The inhibitory effect of Galangin on cytokines and nitric oxide in microglia BV2 cell line. Malays. J. Sci. 2017, 36, 145–156. [Google Scholar] [CrossRef]
- Namsi, A.; Nury, T.; Hamdouni, H.; Yammine, A.; Vejux, A.; Vervandier-Fasseur, D.; Latruffe, N.; Masmoudi-Kouki, O.; Lizard, G. Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases 2018, 6, 67. [Google Scholar] [CrossRef]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Serralheiro, M.L.; Eduarda Araujo, M. Biological properties of phenolic compound extracts in selected Algerian honey—The inhibition of acetylcholinesterase (AChE) and α-glucosidase activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Honey as the Potential Natural Source of Cholinesterase Inhibitors in Alzheimer’s Disease. Plant Foods Hum. Nutr. 2020, 75, 30–32. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. [Google Scholar] [CrossRef] [PubMed]
- Elamine, Y.; Lyoussi, B.; Miguel, M.G.; Anjos, O.; Estevinho, L.; Alaiz, M.; Girón-Calle, J.; Martín, J.; Vioque, J. Physicochemical characteristics and antiproliferative and antioxidant activities of Moroccan Zantaz honey rich in methyl syringate. Food Chem. 2020, 339, 128098. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. The effects of Tualang Honey sup-plement on medial prefrontal cortex morphology and cholinergic system in stressed ovariectomized rats. Int. J. Appl. Res. Nat. Prod. 2014, 7, 28–36. [Google Scholar]
- Rosli, N.H.M.; Yahya, H.M.; Ibrahim, F.W.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Rajab, N.F. Serum Metabolomics Profiling of Commercially Mixed Functional Foods—Effects in Beta-Amyloid Induced Rats Measured Using 1H NMR Spectroscopy. Nutrients 2020, 12, 3812. [Google Scholar] [CrossRef]
- Aameri, R.; Ghorbani, H.; Bazrafshan, H.R.; Gharib, F.Z.; Korani, B. Iranian thyme honey plays be-havioral, cellular, and molecular vital roles as an excellent preventive and therapeutic agent in the brain of Alz-heimer’s rat model. Neurosci. Lett. 2022, 783, 136702. [Google Scholar] [CrossRef] [PubMed]
- Phokasem, P.; Jantrapirom, S.; Karinchai, J.; Yoshida, H.; Masamitsu Yamaguchi, M.; Chantawannakul, P. Honeybee products and edible insect powders improve the locomotive and learning abilities of Ubiquilinknockdown Drosophila. BMC Complement. Med. Ther. 2020, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Sairazi, N.S.M.; Asari, M.A.; Mummedy, S.; Muzaimi, M.; Sulaiman, S.A. Effect of tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complement. Altern. Med. 2017, 17, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Asari, M.A.; Zulkaflee, M.H.; Sirajudeen, K.N.S.; Mohd Yusof, N.A.; Mohd Sairazi, N.S. Tualang Honey and DHA-rich fish oil reduce the production of pro-inflammatory cytokines in the rat brain following exposure to chronic stress. J. Taibah Univ. Med. Sci. 2019, 14, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Akim, A.M.; Ab Hamid, H.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 15–33. [Google Scholar] [CrossRef]
- Sairazi, N.S.M.; Sirajudeen, K.N.S.; Mustapha, M.; Mummedy, S.; Asari, M.A.; Sulaiman, S.A. Tualang Honey Reduced Neuroinflammation and Caspase-3 Activity in Rat Brain after Kainic Acid-Induced Status Epilepticus. Evidence-Based Complement. Altern. Med. 2018, 2018, 7287820. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; Moreira, L.K.D.S.; Neri, H.F.D.S.; da Silva, C.R.B.; Pruccoli, L.; dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology 2021, 465, 153033. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.; Devgan, N.; Mani, S. Effect of cinnamon and honey on on-time and off-time in Parkinson’s Disease: A case report. J. Gujarat Res. Soc. 2019, 21, 650–654. [Google Scholar]
- Zhou, S.; Huang, G. The biological activities of butyrylcholinesterase inhibitors. Biomed. Pharmacother. 2021, 146, 112556. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomás-Barberán, F.A. Flavonoids in Monospecific Eucalyptus Honeys from Australia. J. Agric. Food Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic Compounds Prevent Alzheimer’s Pathology through Different Effects on the Amyloid-β Aggregation Pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neu-ronal stress responses. Trends Neurosci. 2006, 29, 632–639. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the anti-oxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic acid disruption of Aβ1–42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2018, 124, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Khair, S.B.A.; Kazim, A.S.; Minhas, S.T.; Al-Tel, T.H.; Al-Hayani, A.A.; Haque, M.E.; Eliezer, D.; et al. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.; Mishra, D. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for protection against Scopolamine-induced Amnesia. Drug Deliv. 2013, 20, 112–119. [Google Scholar] [CrossRef] [PubMed]
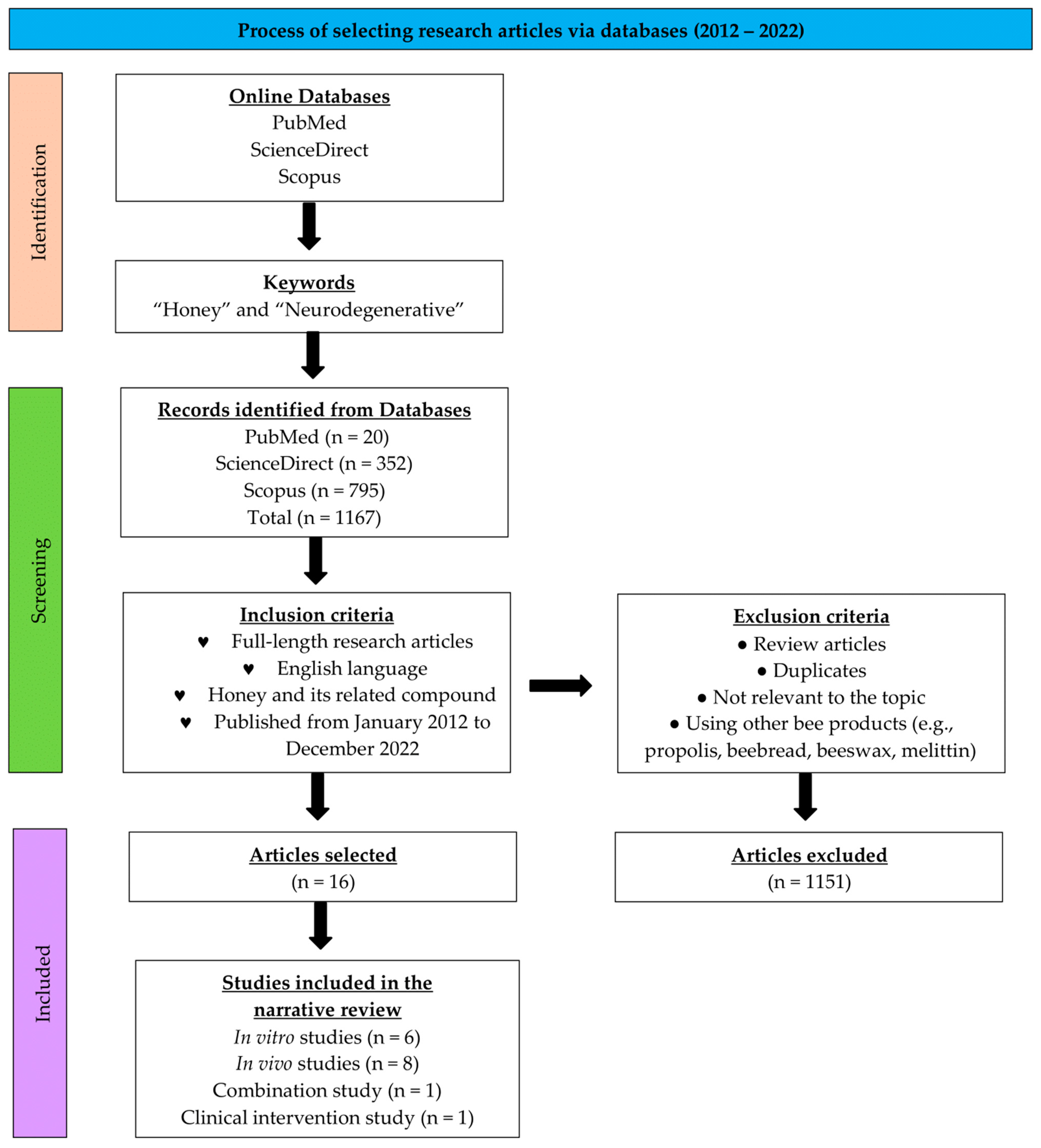
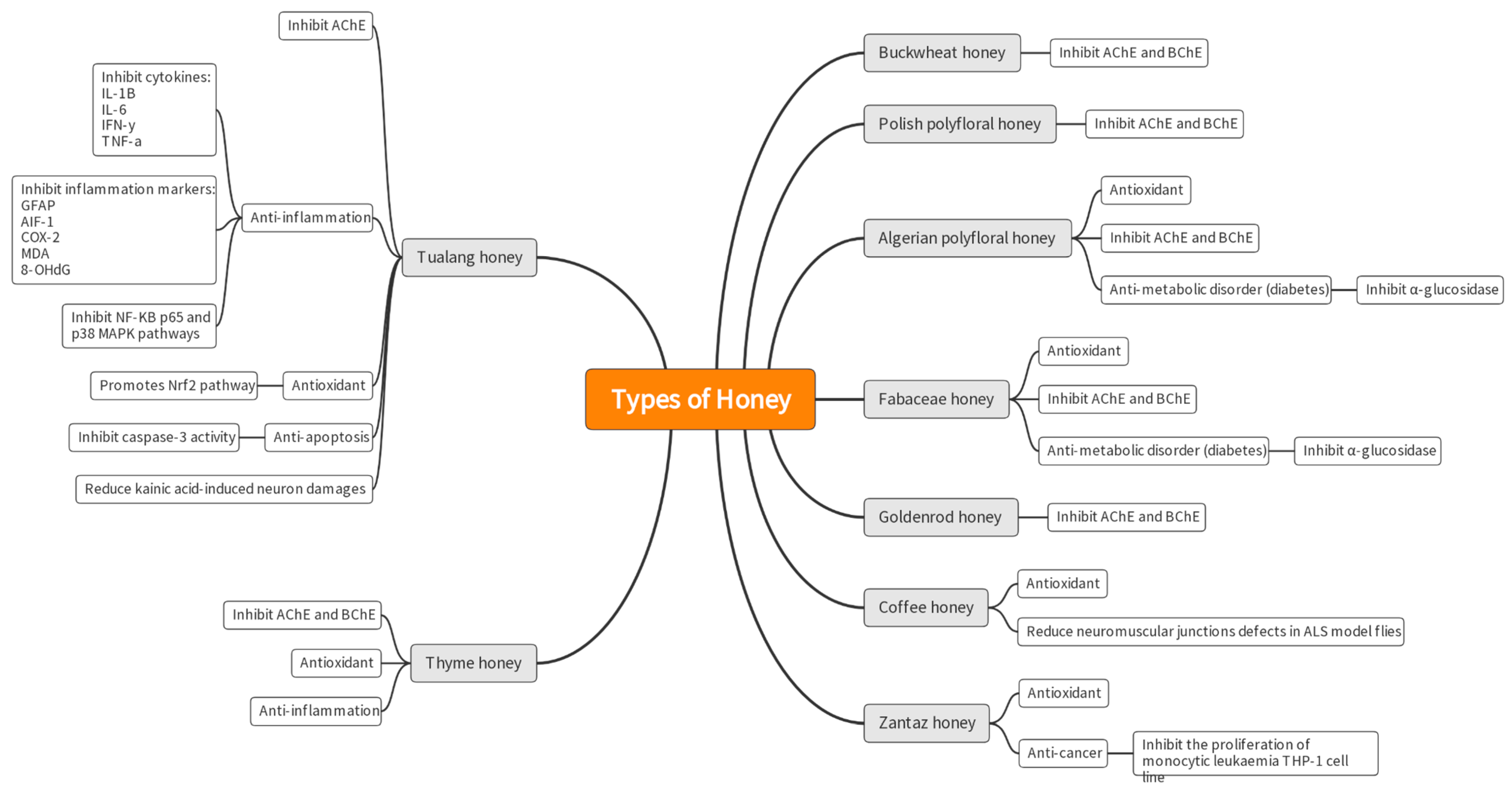
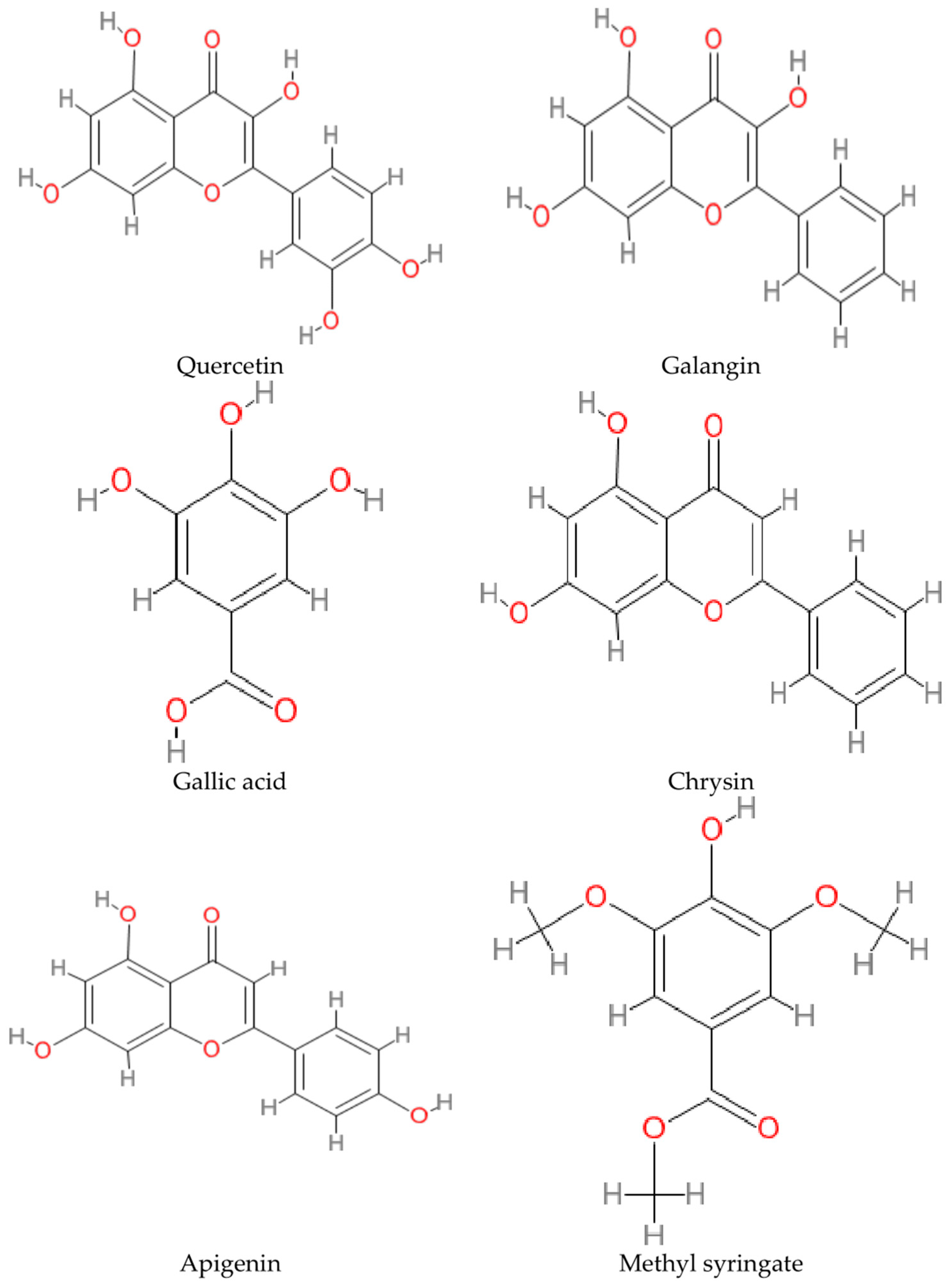
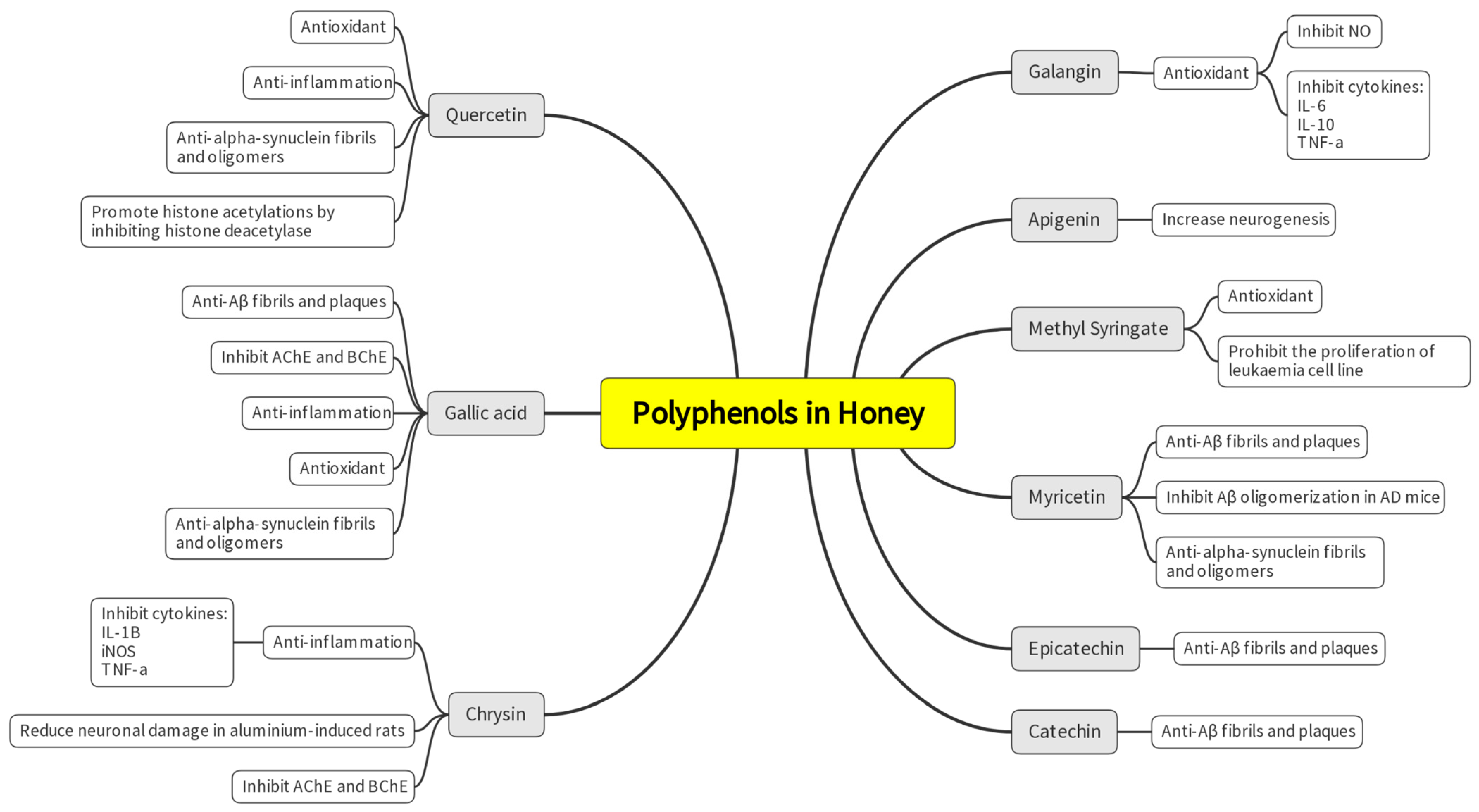
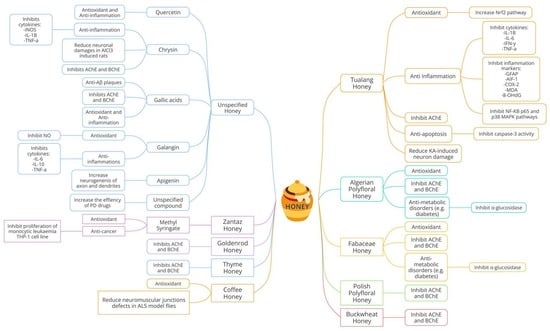
| No. | Targeted Neurodegenerative Disease(s) | Key Findings | Reference |
|---|---|---|---|
| 1. | AD, PD | -Galangin (flavonoid) inhibits nitric oxide (NO), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin 10 (IL-10) synthesis in lipopolysaccharide (LPS)-stimulated BV2 macroglia which modulates neuroinflammatory activity. -Galangin prevents neuroinflammation and could be a promising pharmacological agent or food supplement. | [29] |
| 2. | AD, PD | -Apigenin is present in honey, parsley, rosemary, and olive oil, which are standard in the Mediterranean diet. -Resveratrol and apigenin were discovered to promote neuron development and imitate neurotrophic activity. | [30] |
| 3. | AD | -Neurological problems in AD can be prohibited by inhibiting acetylcholinesterase (AChE). -Associations were found between antioxidants with total phenolic compounds and flavonoids content in selected Algerian honey samples. The samples also show anti-inflammatory, anti-AChE and anti-α-glucosidase activities. -The inhibition of AChE was reported to be in the range of 20.69 to 76.04% with IC50 values of 0.367 to 0.629 mg/mL. -Galantamine was used as a control for AChE inhibition and showed IC50 of 0.210 mg/mL, comparable to the honey test groups. | [31] |
| 4. | AD | -This study used the colorimetric method to analyze 47 varieties of Polish honey as a source of AChE and butyrylcholinesterase (BChE) inhibitors. Buckwheat honey had the best potential to inhibit AChE (39.51% inhibition), while multifloral honey had the highest potential to inhibit BChE (39.76%). No IC50 values or drug control were mentioned in the article. | [32] |
| 5. | Unspecified neurodegenerative disorder | -Zantaz honey contains methyl syringate (more than 50% of the total polyphenols), gallic acid, epicatechin, syringic acid, and catechin. -Using principal component analysis (PCA), methyl syringate and gallic acid showed the strongest positive correlation with antioxidant activities and cell proliferation inhibition in Caco-2 and THP-1 cells. | [34] |
| 6. | AD | -Thyme, linden, bean, and heather honey showed high inhibition of AChE compared to the other 15 types of honey tested. Inhibition ranged from 18.31% to 21.17%. -Goldenrod, thyme, heather, buckwheat, and acacia honey inhibited BChE at a percentage ranging between 26% and 34%. There were no IC50 values, and no drug control was reported. -Positive correlations were shown between TPC and anti-AChE and anti-BChE activities. | [33] |
| No. | Targeted Neurodegenerative Disease(s) | Methods/Key Findings | Reference |
|---|---|---|---|
| 1. | AD | -Two months after ovariectomy, the rats were treated with 18 days of TUH or 17 β-estradiol as the control. During the last 15 days, the rats were subjected to stress routines and finally sacrificed. -The treatment of either TUH or 17 β-estradiol attenuated the reduction in acetylcholine and elevation of AChE in the brain homogenates of stressed ovariectomized rats. -The treatment of either TUH or 17 β-estradiol also showed a healthier order and number of Nissl-positive cells in medial prefrontal cortex (mPFC) neurons compared to untreated stressed ovariectomized rats. | [35] |
| 2. | Unspecified neurodegenerative disorder | -Five groups of rats—normal saline, KA (kainic acid)-induced, TUH with KA-induced, anti-inflammation aspirin with KA-induced, and anti-epileptics topiramate with KA-induced. Five treatments were given for two and a half days. After the last treatment, KA was administered. -Pre-treatment of TUH reduced the locomotor hyperactivity, thiobarbituric acid, and neuronal degeneration induced by KA in the piriform cortex. -The TUH pre-treatment also reduced the weakening of the antioxidant status post-KA. | [39] |
| 3. | Unspecified neurodegenerative disorder | -Rats were treated with TUH five times for the duration of two and half days. After the last treatment, the rats were induced with KA. Anti-epileptics topiramate was used as a control. -Pre-treatment with TUH for KA-induced status epilepticus rats significantly (N = 72, p < 0.05) attenuated the elevation of neuroinflammation markers such as TNF-α, interleukin-1 beta (IL-1β), glial fibrillary acidic protein (GFAP), allograft inflammatory factor 1 (AIF-1), and cyclooxygenase-2 (COX-2) level in the cerebral cortex, cerebellum, and brainstems. -The TUH pre-treatment also weakened the caspase-3 activity in the cerebral cortex. | [42] |
| 4. | Unspecified neurodegenerative disorder | -Rats were subjected to stress routines and induced with TUH or docosahexaenoic acid (DHA)-rich oil or both. The duration was 28 days. -TNF-a, IL6, and interferon-gamma (IFN-y) concentrations in the brain homogenates of rats treated with DHA-rich fish oil, TUH, and TUH-DHA were lower than those in the control and stress-only-exposed groups (p < 0.05), but there was no difference across groups that received treatments. | [40] |
| 5. | Unspecified neurodegenerative disorder | -LPS-induced-chronic subclinical systemic inflammation (CSSI) rats were treated with SBH. -Rats were administered LPS thrice every week for four weeks. Then, the rats were treated with SBH for one month. -The treatment significantly reduced inflammatory markers, MDA, 8-hydroxy-2′-deoxyguanosine (8-OHdG), NF-κB p65, p38 mitogen-activated protein kinase (p38 MAPK), and organ damage. -The treatment also enhanced antioxidants and nuclear factor erythroid 2–related factor 2 (Nrf2) expression. | [41] |
| 6. | AD | --Rats were induced with Aβ-42 for 14 days to model AD. The rats were then given mixed functional food (MFF) treatment for 30 days. N-acetylcysteine was used as a control. -Aβ-induced rats were treated with MFF containing honey, dates, and pomegranate, which later improved the rats’ spatial memory and learning in the Y-maze test. -Metabolomic analysis using 1H NMR spectroscopy showed 12 metabolites that portrayed significant differences. Metabolic pathway analysis revealed that the MFF improved amino acid and energy metabolism, thus providing a neuroprotective effect. | [36] |
| 7. | ALS | -ALS is associated with the human ubiquilin two genes. Ubiquilin (dUbqn) knockdown flies were treated with coffee honey. -Flies were cultured using standard fly-medium spiked with coffee honey (1% v/v). -Coffee honey (1% v/v) treatment proved the recovery of structural defects in neuromuscular junctions (NMJs), increased learning potential, and decreased the accumulation of ROS caused by dUbqn depletion in the brain. | [38] |
| 8. | AD | -One group of rats received THH treatment for two weeks and then four weeks of THH accompanied by AlCl3 treatment to induce AD. Another group received six weeks of THH and AlCl3 treatment. Rivastigmine and tap water were used as control. -AD rats showed neurodegeneration, hippocampal damage, and increased malondialdehyde (MDA) and performed poorly in the Y-maze test. -Iranian THH increases the total oxidant, frequency of healthy cells, and normal neurons in all parts of the cortex and hippocampus. The behavior evaluation showed no significant difference between the effects of honey and the rivastigmine control group. | [37] |
| No. | Targeted Neurodegenerative Disease(s) | Key Findings | Reference |
|---|---|---|---|
| 1. | Unspecified neurodegenerative disorder | -In the in vitro study, neuronal SH-SY5Y cell lines treated with chrysin showed the capacity to mitigate the early oxidative stress induced by the oxidant tert-butyl hydroperoxide (t-BuOOH) which mimics lipid peroxidation. The IC50 for the ROS inhibition in neuronal SH-SY5Y cells were 4 μM chrysin/100 μM H2O2 and 4 μM chrysin/100 μM t-BuOOH. The treatment also counteracted the Fenton reaction in the presence of AlCl3 and the late necrotic death triggered by the reaction. -In the in vivo part, mouse models of neurotoxicity induced by chronic exposure to AlCl3 for 90 days were treated with chrysin. The mouse showed reduced cognitive impairment, and the hippocampus’s AChE and BChE activities were regulated. The treatment also reduced oxidative damage and necrotic cell frequency in the brain cortex and hippocampus. | [43] |
| No. | Targeted Neurodegenerative Disease(s) | Key Findings | Reference |
|---|---|---|---|
| 1. | PD | -A 71-year-old female subject diagnosed with PD more than 15 years prior is the topic of this case report. Clinical improvement was seen with the cinnamon and honey therapy when it was shown to increase “on-time” with oral pharmaceutical medication. “On-time” was defined by the investigators as the duration when the PD drugs consumed by the patient could negate PD symptoms from re-emerging. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadzil, M.A.M.; Mustar, S.; Rashed, A.A. The Potential Use of Honey as a Neuroprotective Agent for the Management of Neurodegenerative Diseases. Nutrients 2023, 15, 1558. https://doi.org/10.3390/nu15071558
Fadzil MAM, Mustar S, Rashed AA. The Potential Use of Honey as a Neuroprotective Agent for the Management of Neurodegenerative Diseases. Nutrients. 2023; 15(7):1558. https://doi.org/10.3390/nu15071558
Chicago/Turabian StyleFadzil, Mohammad Adi Mohammad, Suraiami Mustar, and Aswir Abd Rashed. 2023. "The Potential Use of Honey as a Neuroprotective Agent for the Management of Neurodegenerative Diseases" Nutrients 15, no. 7: 1558. https://doi.org/10.3390/nu15071558
APA StyleFadzil, M. A. M., Mustar, S., & Rashed, A. A. (2023). The Potential Use of Honey as a Neuroprotective Agent for the Management of Neurodegenerative Diseases. Nutrients, 15(7), 1558. https://doi.org/10.3390/nu15071558