Combined Citrulline and Glutathione Supplementation Improves Endothelial Function and Blood Pressure Reactivity in Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Measurements
2.3.1. Flow-Mediated Dilation
2.3.2. Pulse Wave Velocity
2.3.3. Brachial and Aortic Blood Pressures at Rest and During the CPT
2.3.4. Serum Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Flow-Mediated Dilation and Arterial Stiffness
3.3. Blood Pressure at Rest and During the Cold Pressor Test
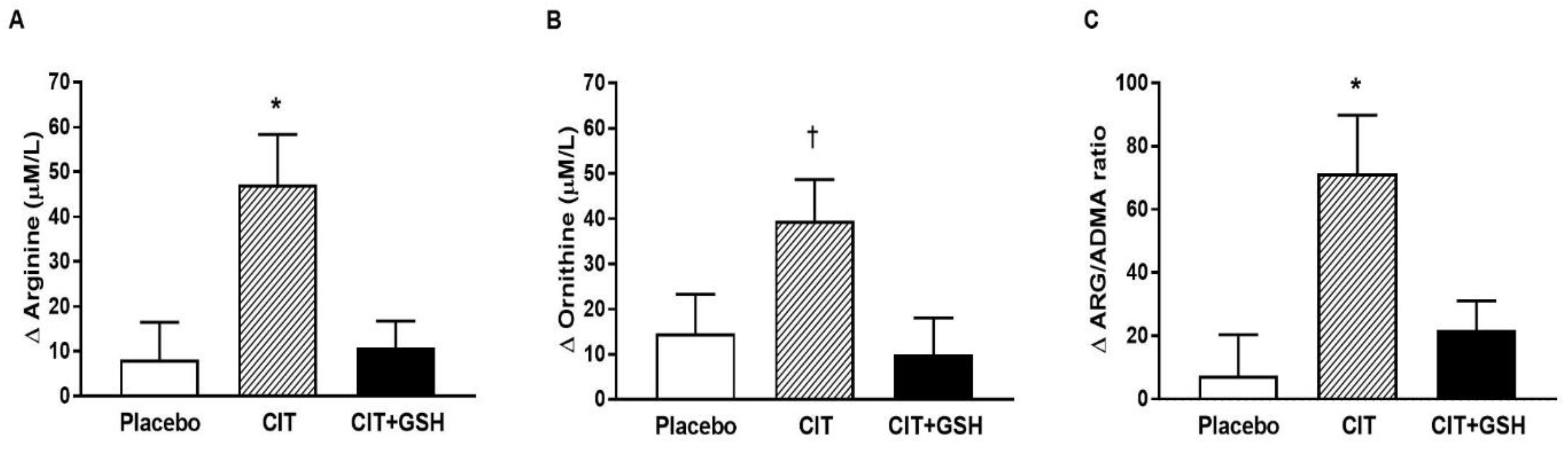
3.4. Serum Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| ARG | L-Arginine |
| baPWV | Brachial-ankle pulse wave velocity |
| BP | Blood pressure |
| cdPWV | Carotid-distal pulse wave velocity |
| cfPWV | Carotid-femoral pulse wave velocity |
| CIT | L-citrulline |
| CPT | Cold pressor test |
| crPWV | Carotid-radial pulse wave velocity |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| eNOS | Endothelial nitric oxide synthase |
| faPWV | Femoral-ankle pulse wave velocity |
| FBG | Fasting blood glucose |
| FMD | Flow-mediated dilation |
| GSH | Glutathione |
| MAP | Mean arterial pressure |
| NO | Nitric oxide |
| NOx | NO metabolites |
| ORN | Ornithine |
| PWV | Pulse wave velocity |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SE | Standard error |
References
- Rossi, G.P.; Seccia, T.M.; Nussdorfer, G.G. Reciprocal regulation of endothelin-1 and nitric oxide: Relevance in the physiology and pathology of the cardiovascular system. Int. Rev. Cytol. 2001, 209, 241–272. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2011, 33, 829–837. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef]
- Pernow, J.; Jung, C. Arginase as a potential target in the treatment of cardiovascular disease: Reversal of arginine steal? Cardiovasc. Res. 2013, 98, 334–343. [Google Scholar] [CrossRef]
- Berkowitz, D.E.; White, R.; Li, D.C.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef]
- Bode-Boger, S.M.; Scalera, F.; Ignarro, L.J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H.; Bode-Boger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- Sydow, K.; Schwedhelm, E.; Arakawa, N.; Bode-Boger, S.M.; Tsikas, D.; Hornig, B.; Frolich, J.C.; Boger, R.H. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: Effects of L-arginine and B vitamins. Cardiovasc. Res. 2003, 57, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Deane, K.D.; Meditz, A.L.; Kohrt, W.M. Tumor necrosis factor-alpha inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 2013, 230, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]
- Tomiyama, H.; Ishizu, T.; Kohro, T.; Matsumoto, C.; Higashi, Y.; Takase, B.; Suzuki, T.; Ueda, S.; Yamazaki, T.; Furumoto, T.; et al. Longitudinal association among endothelial function, arterial stiffness and subclinical organ damage in hypertension. Int. J. Cardiol. 2018, 253, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Chiurlia, E.; Nuzzo, A.; Cioni, E.; Origliani, G.; Modena, M.G. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J. Am. Coll. Cardiol. 2004, 44, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Wenner, M.M.; Greaney, J.L.; Matthews, E.L.; McGinty, S.; Kaur, J.; Vongpatanasin, W.; Fadel, P.J. Influence of Age and Estradiol on Sympathetic Nerve Activity Responses to Exercise in Women. Med. Sci. Sports Exerc. 2022, 54, 408–416. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef]
- Keller-Ross, M.L.; Cunningham, H.A.; Carter, J.R. Impact of age and sex on neural cardiovascular responsiveness to cold pressor test in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R288–R295. [Google Scholar] [CrossRef]
- Baker, S.E.; Limberg, J.K.; Ranadive, S.M.; Joyner, M.J. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1271–R1275. [Google Scholar] [CrossRef]
- Menkes, M.S.; Matthews, K.A.; Krantz, D.S.; Lundberg, U.; Mead, L.A.; Qaqish, B.; Liang, K.Y.; Thomas, C.B.; Pearson, T.A. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension 1989, 14, 524–530. [Google Scholar] [CrossRef]
- Han, Y.; Du, J.; Wang, J.; Liu, B.; Yan, Y.L.; Deng, S.B.; Zou, Y.; Jing, X.D.; Du, J.L.; Liu, Y.J.; et al. Cold Pressor Test in Primary Hypertension: A Cross-Sectional Study. Front. Cardiovasc. Med. 2022, 9, 860322. [Google Scholar] [CrossRef]
- Parker, B.A.; Smithmyer, S.L.; Jarvis, S.S.; Ridout, S.J.; Pawelczyk, J.A.; Proctor, D.N. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1148–H1156. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Boger, R.H.; Frolich, J.C. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef]
- Huang, J.; Ladeiras, D.; Yu, Y.; Ming, X.F.; Yang, Z. Detrimental Effects of Chronic L-Arginine Rich Food on Aging Kidney. Front. Pharmacol. 2020, 11, 582155. [Google Scholar] [CrossRef]
- Shatanawi, A.; Momani, M.S.; Al-Aqtash, R.; Hamdan, M.H.; Gharaibeh, M.N. L-Citrulline Supplementation Increases Plasma Nitric Oxide Levels and Reduces Arginase Activity in Patients with Type 2 Diabetes. Front. Pharmacol. 2020, 11, 584669. [Google Scholar] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. L-citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of l-citrulline and l-arginine rapidly increases plasma l-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of l-citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole body vibration training and L-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metabol. 2016, 41, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Alvarez-Alvarado, S.; Ormsbee, M.J.; Madzima, T.A.; Campbell, J.C.; Wong, A. Impact of L-citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Exp. Gerontol. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kalfon, R. l-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br. J. Nutr. 2016, 116, 279–285. [Google Scholar] [CrossRef]
- Figueroa, A.; Trivino, J.A.; Sanchez-Gonzalez, M.A.; Vicil, F. Oral L-citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am. J. Hypertens. 2010, 23, 12–16. [Google Scholar] [CrossRef]
- Jaime, S.J.; Nagel, J.; Maharaj, A.; Fischer, S.M.; Schwab, E.; Martinson, C.; Radtke, K.; Mikat, R.P.; Figueroa, A. L-Citrulline supplementation attenuates aortic pulse pressure and wave reflection responses to cold stress in older adults. Exp. Gerontol. 2022, 159, 111685. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Yabuki, Y.; Fukunaga, K. Combined l-citrulline and glutathione administration prevents neuronal cell death following transient brain ischemia. Brain Res. 2017, 1663, 123–131. [Google Scholar] [CrossRef] [PubMed]
- McKinley-Barnard, S.; Andre, T.; Morita, M.; Willoughby, D.S. Combined L-citrulline and glutathione supplementation increases the concentration of markers indicative of nitric oxide synthesis. J. Int. Soc. Sport. Nutr. 2015, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Klatsky, A.L. Alcohol and cardiovascular diseases: Where do we stand today? J. Intern. Med. 2015, 278, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Kim, W.; Shin, J.H.; Lee, C.J.; Kim, H.C.; Kang, S.H.; Jung, M.H.; Kim, D.H.; Lee, J.H.; Kim, H.L.; et al. Office Blood Pressure Range and Cardiovascular Events in Patients With Hypertension: A Nationwide Cohort Study in South Korea. J. Am. Heart Assoc. 2021, 10, e017890. [Google Scholar] [CrossRef]
- Envelope, S. Create a Blocked Randomisation List. Obtenido de Sealed Envelope. 2019. Available online: https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed on 20 June 2020).
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Ezaki, H.; Morishita, K.; Miyake, T. Effects of oral L-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol. Endocrin. Metab. Agent. Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 77–84. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Bapir, M.; Skene, S.S.; Sies, H.; Kelm, M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc. Res. 2022, 119, 283–293. [Google Scholar] [CrossRef]
- Yeboah, J.; Crouse, J.R.; Hsu, F.-C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Shai, I.; Tekes-Manova, D.; Israeli, E.; Pereg, D.; Shochat, T.; Kochba, I.; Rudich, A.; Israeli Diabetes Research, G. Normal fasting plasma glucose levels and type 2 diabetes in young men. N. Engl. J. Med. 2005, 353, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, X.M.; Sun, Y.M.; Jin, H.B.; Fu, R.; Wang, Y.Y.; Wu, Y.; Luan, Y. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int. J. Clin. Pract. 2008, 62, 877–882. [Google Scholar] [CrossRef]
- DeVan, A.E.; Eskurza, I.; Pierce, G.L.; Walker, A.E.; Jablonski, K.L.; Kaplon, R.E.; Seals, D.R. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin. Sci. 2013, 124, 325–331. [Google Scholar] [CrossRef]
- McEniery, C.M.; Yasmin; Hall, I.R.; Qasem, A.; Wilkinson, I.B.; Cockcroft, J.R. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT). J. Am. Coll. Cardiol. 2005, 46, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, A.; Tomiyama, H.; Takeda, K.; Tsuda, H.; Arai, T.; Hirose, K.; Koji, Y.; Hori, S.; Yamamoto, Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 2002, 25, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.K.; Tweddel, A.C.; Doshi, S.N.; Goodfellow, J.; Henderson, A.H. Flow-mediated changes in pulse wave velocity: A new clinical measure of endothelial function. Eur. Heart J. 2006, 27, 302–309. [Google Scholar] [CrossRef]
- Kamran, H.; Salciccioli, L.; Ko, E.H.; Qureshi, G.; Kazmi, H.; Kassotis, J.; Lazar, J. Effect of reactive hyperemia on carotid-radial pulse wave velocity in hypertensive participants and direct comparison with flow-mediated dilation: A pilot study. Angiology 2010, 61, 100–106. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Narita, K.; Chiba, K.; Takemoto, H.; Morita, M.; Morishita, K. Effects of L-citrulline diet on stress-induced cold hypersensitivity in mice. Pharmacogn. Res. 2014, 6, 297–302. [Google Scholar] [CrossRef]
- Choi, H.M.; Stebbins, C.L.; Nho, H.; Kim, K.A.; Kim, C.; Kim, J.K. Skeletal muscle metaboreflex is enhanced in postmenopausal women. Eur. J. Appl. Physiol. 2012, 112, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef]
- Celik, M.; Unal, H.U. The l-Arginine/Asymmetric Dimethylarginine (ADMA) Ratio in Health and Disease: An Overview. In L-Arginine in Clinical Nutrition; Patel, V.B., Preedy, V.R., Rajendram, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 225–238. [Google Scholar]
- Luneburg, N.; Xanthakis, V.; Schwedhelm, E.; Sullivan, L.M.; Maas, R.; Anderssohn, M.; Riederer, U.; Glazer, N.L.; Vasan, R.S.; Boger, R.H. Reference intervals for plasma L-arginine and the L-arginine:asymmetric dimethylarginine ratio in the Framingham Offspring Cohort. J. Nutr. 2011, 141, 2186–2190. [Google Scholar] [CrossRef]
- Ellger, B.; Richir, M.C.; van Leeuwen, P.A.; Debaveye, Y.; Langouche, L.; Vanhorebeek, I.; Teerlink, T.; Van den Berghe, G. Glycemic control modulates arginine and asymmetrical-dimethylarginine levels during critical illness by preserving dimethylarginine-dimethylaminohydrolase activity. Endocrinology 2008, 149, 3148–3157. [Google Scholar] [CrossRef]
- Bogle, R.G.; MacAllister, R.J.; Whitley, G.S.; Vallance, P. Induction of NG-monomethyl-L-arginine uptake: A mechanism for differential inhibition of NO synthases? Am. J. Physiol. 1995, 269, C750–C756. [Google Scholar] [CrossRef] [PubMed]
- Palloshi, A.; Fragasso, G.; Piatti, P.; Monti, L.D.; Setola, E.; Valsecchi, G.; Galluccio, E.; Chierchia, S.L.; Margonato, A. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. Am. J. Cardiol. 2004, 93, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Casiraghi, M.C.; Setola, E.; Galluccio, E.; Pagani, M.A.; Quaglia, L.; Bosi, E.; Piatti, P. L-arginine enriched biscuits improve endothelial function and glucose metabolism: A pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 2013, 62, 255–264. [Google Scholar] [CrossRef]
- Blum, A.; Hathaway, L.; Mincemoyer, R.; Schenke, W.H.; Kirby, M.; Csako, G.; Waclawiw, M.A.; Panza, J.A.; Cannon, R.O., III. Effects of oral L-arginine on endothelium-dependent vasodilation and markers of inflammation in healthy postmenopausal women. J. Am. Coll. Cardiol. 2000, 35, 271–276. [Google Scholar] [CrossRef]
- Esen, O.; Eser, M.C.; Abdioglu, M.; Benesova, D.; Gabrys, T.; Karayigit, R. Eight Days of L-Citrulline or L-Arginine Supplementation Did Not Improve 200-m and 100-m Swimming Time Trials. Int. J. Environ. Res. Public Health 2022, 19, 4462. [Google Scholar] [CrossRef]
- Flam, B.R.; Eichler, D.C.; Solomonson, L.P. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide 2007, 17, 115–121. [Google Scholar] [CrossRef]
- Yu, E.; Ruiz-Canela, M.; Hu, F.B.; Clish, C.B.; Corella, D.; Salas-Salvado, J.; Hruby, A.; Fito, M.; Liang, L.; Toledo, E.; et al. Plasma Arginine/Asymmetric Dimethylarginine Ratio and Incidence of Cardiovascular Events: A Case-Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Campolo, J.; Bernardi, S.; Cozzi, L.; Rocchiccioli, S.; Dellanoce, C.; Cecchettini, A.; Tonini, A.; Parolini, M.; De Chiara, B.; Micheloni, G.; et al. Medium-term effect of sublingual l-glutathione supplementation on flow-mediated dilation in subjects with cardiovascular risk factors. Nutrition 2017, 38, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Bradley, R.D. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 2011, 17, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263. [Google Scholar] [CrossRef]
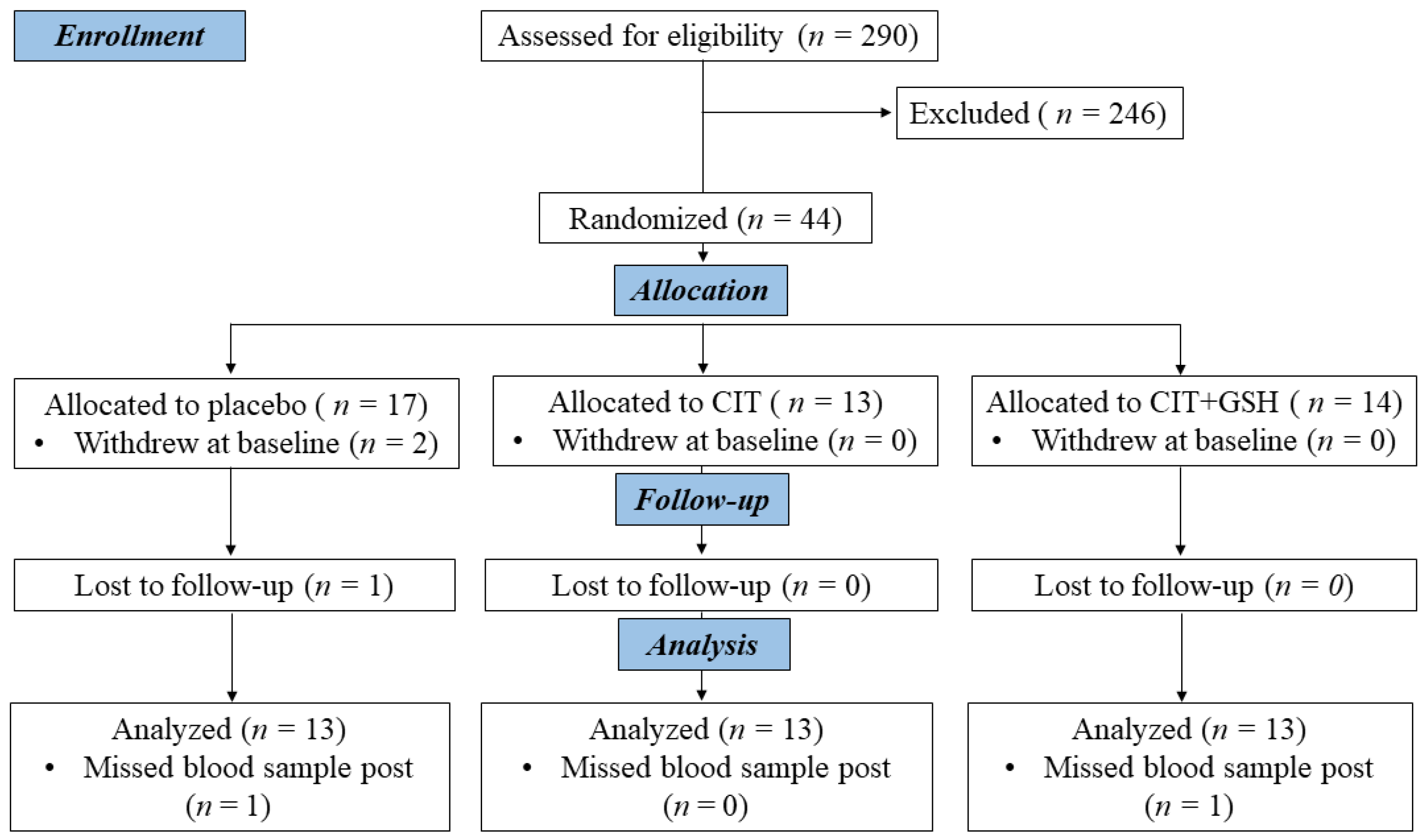
| Measures and Medication | Placebo (n = 13) | CIT (n = 13) | CIT+GSH (n = 13) | p |
|---|---|---|---|---|
| Age (years) | 60 ± 5 | 58 ± 4 | 58 ± 6 | 0.60 |
| Height (m) | 1.58 ± 0.06 | 1.59 ± 0.08 | 1.59 ± 0.10 | 0.92 |
| Body weight (kg) | 73.3 ± 9.3 | 72.1 ± 11.2 | 75.5 ± 15.7 | 0.94 |
| BMI (kg/m2) | 29.3 ± 3.4 | 29.0 ± 4.8 | 29.5 ± 4.2 | 0.91 |
| WC (cm) | 93.3 ± 8.5 | 93.1 ± 12.6 | 91.5 ± 11.0 | 0.65 |
| Total fat mass (%) | 45 ± 5 | 42 ± 7 | 46 ± 5 | 0.18 |
| Total lean mass (%) | 54 ± 5 | 55 ± 5 | 56 ± 6 | 0.18 |
| FBG (mg/dL) | 87 ± 7 | 90 ± 6 | 94 ± 7 † | 0.04 |
| Medication (n) | ||||
| ARB/ACE inhibitors | 2 | 1 | 3 | |
| Diuretics | 1 | 0 | 2 | |
| Ca2+ channel blockers | 1 | 0 | 0 |
| Measure | Placebo | CIT | CIT+GSH | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | p | |
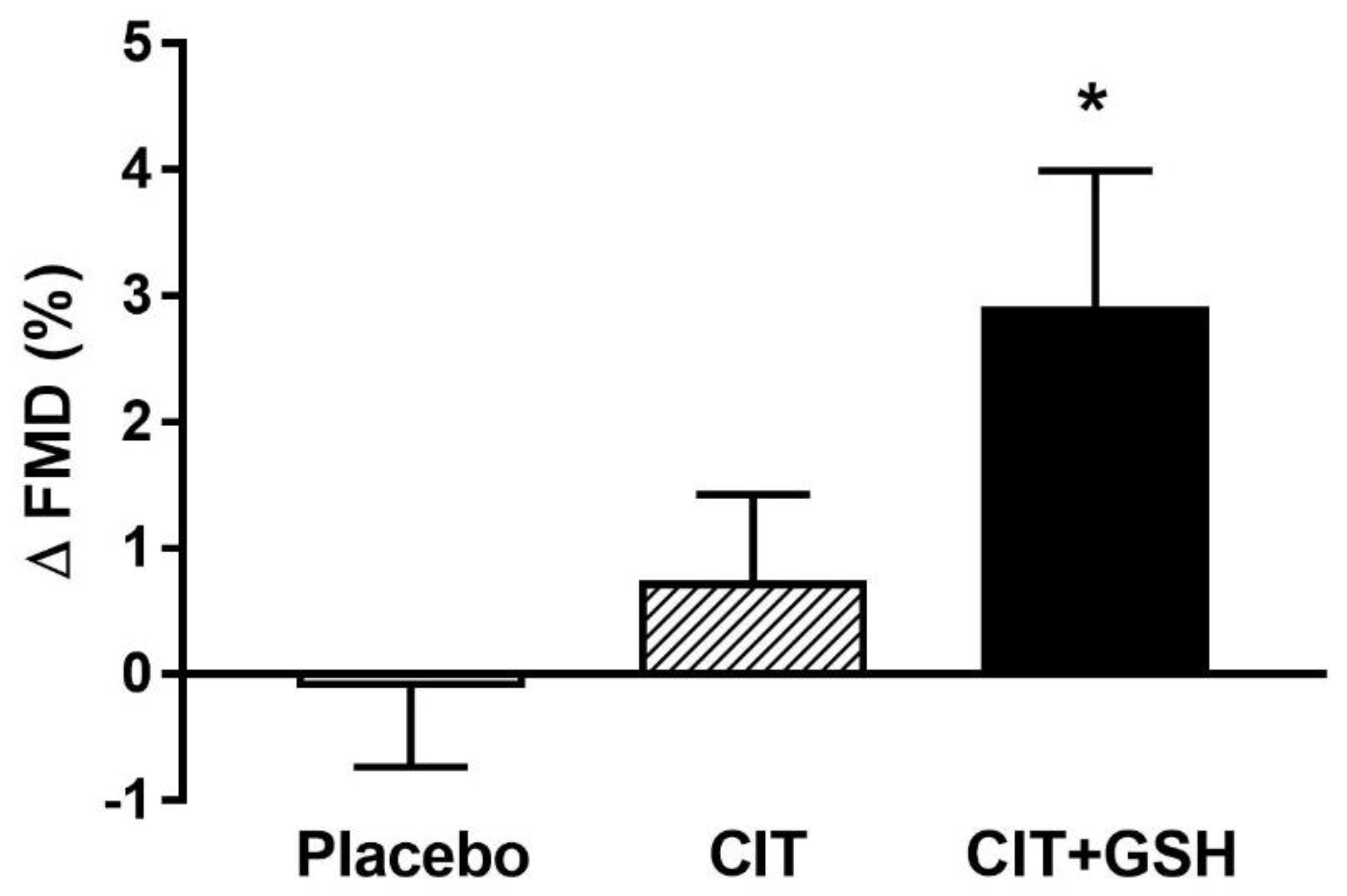
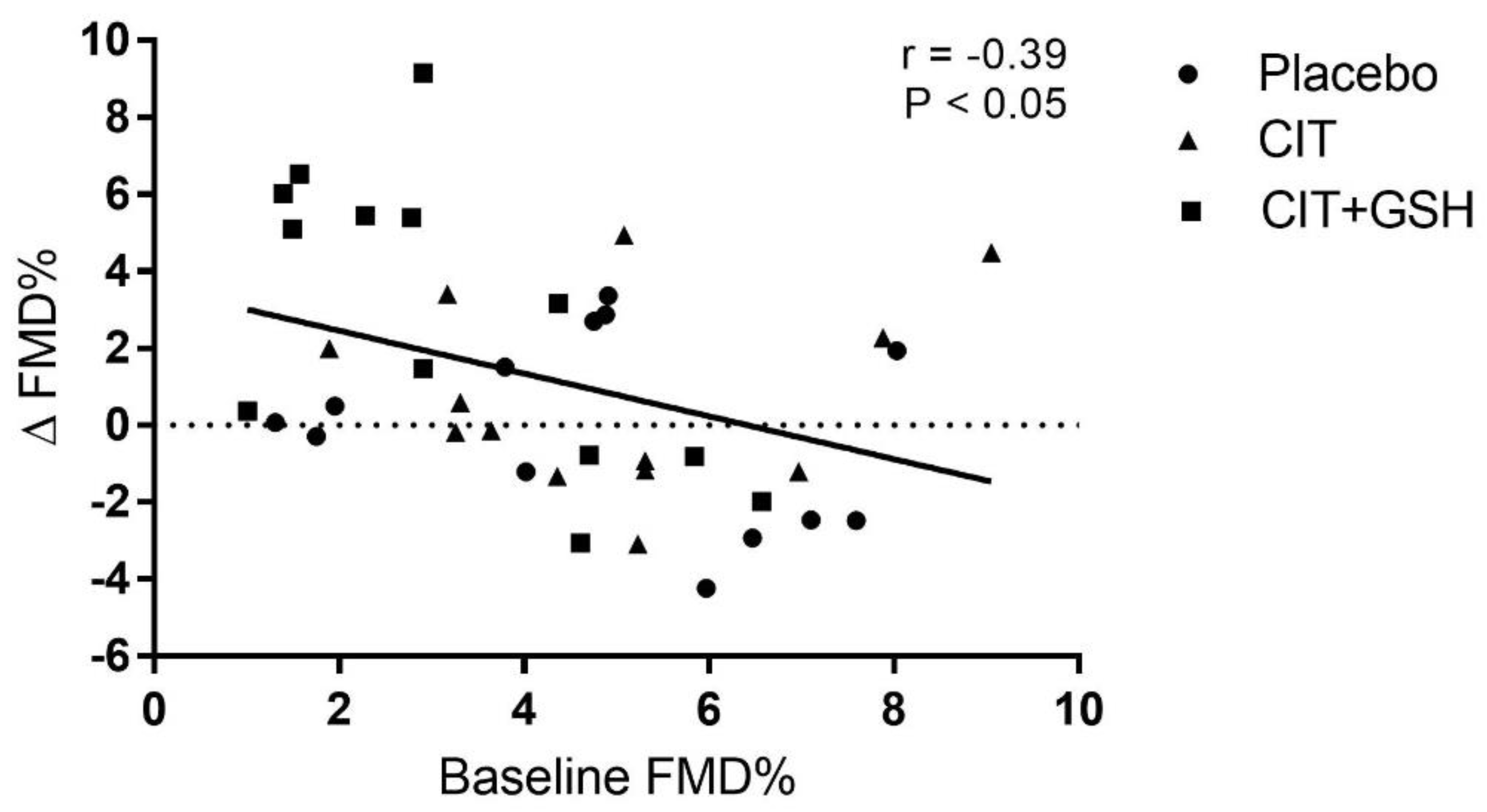
| FMD (%) | 4.8 ± 2.2 | 4.8 ± 2.9 | 5.0 ± 2.0 | 5.7 ± 3.5 | 3.1 ± 1.8 | 6.0 ± 3.0 *‡ | 0.04 |
| cfPWV (m/s) | 7.6 ± 1.4 | 7.6 ± 1.1 | 6.9 ± 0.8 | 7.3 ± 0.8 | 7.3 ± 0.7 | 7.3 ± 1.3 | 0.28 |
| crPWV (m/s) | 8.3 ± 1.7 | 8.3 ± 1.2 | 8.5 ± 1.2 | 8.5 ± 1.8 | 8.5 ± 0.9 | 7.9 ± 0.9 * | 0.51 |
| cdPWV (m/s) | 8.9 ± 1.5 | 8.2 ± 1.1 | 8.8 ± 0.8 | 8.4 ± 0.9 | 8.2 ± 0.9 | 8.2 ± 1.1 | 0.23 |
| faPWV (m/s) | 8.5 ± 0.3 | 8.7 ± 0.3 | 9.0 ± 0.3 | 9.3 ± 0.3 | 9.0 ± 0.3 | 8.8 ± 0.3 | 0.69 |
| Measure | Condition | Placebo | CIT | CIT+GSH | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | p | ||
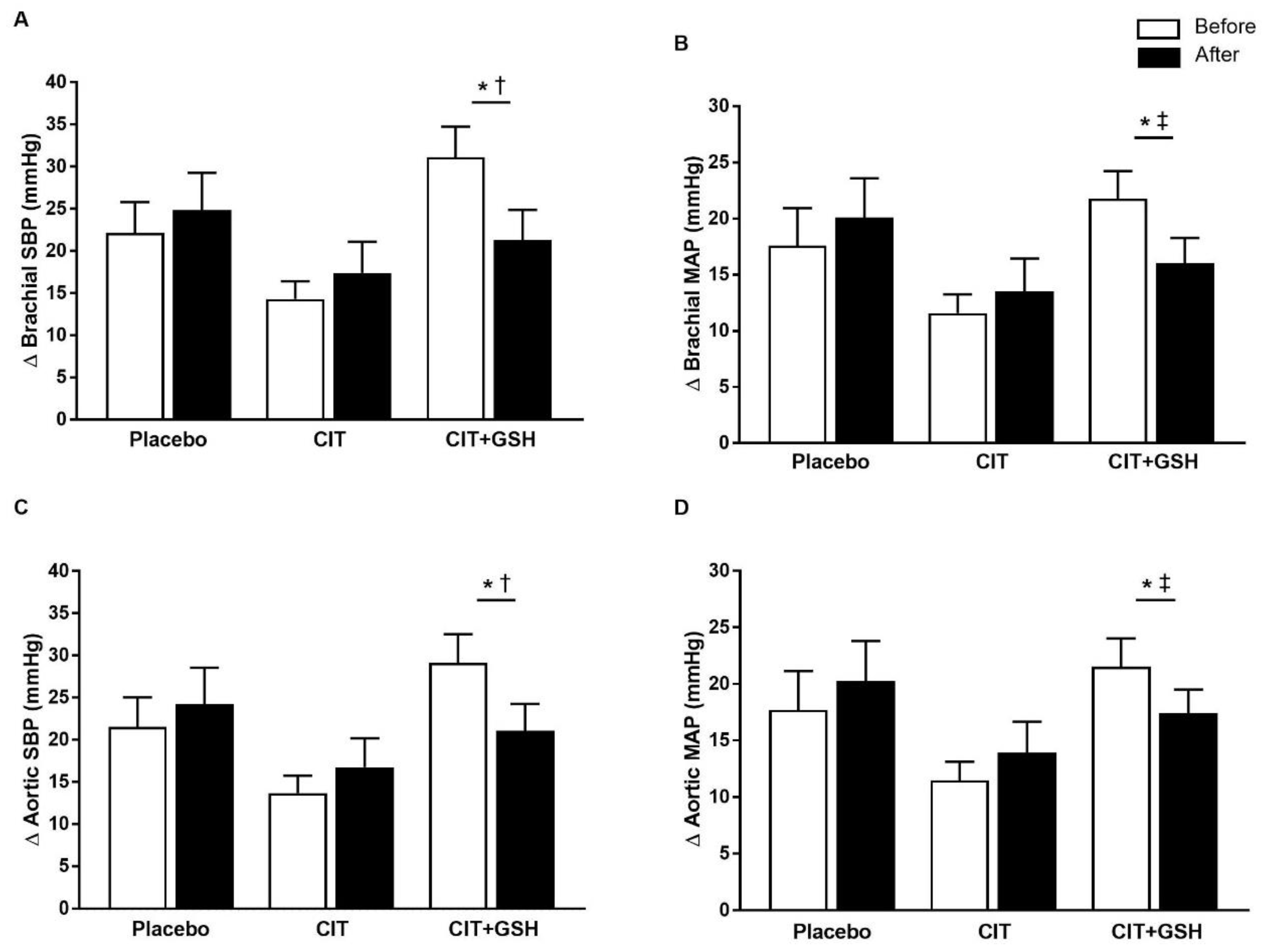
| Brachial SBP (mmHg) | Rest | 113 ± 13 | 111 ± 12 | 113 ± 13 | 114 ± 11 | 110 ± 12 | 115 ± 12 | 0.27 |
| CPT | 135 ± 15 | 136 ± 12 | 127 ± 13 | 132 ± 14 | 141 ± 18 | 135 ± 16 | ||
| Brachial DBP (mmHg) | Rest | 75 ± 8 | 75 ± 8 | 71 ± 9 | 71 ± 9 | 71 ± 11 | 73 ± 8 | 0.69 |
| CPT | 91 ± 11 | 93 ± 8 | 81 ± 9 | 83 ± 8 | 88 ± 14 | 89 ± 10 | ||
| Brachial MAP (mmHg) | Rest | 88 ± 9 | 87 ± 9 | 85 ± 10 | 85 ± 7 | 84 ± 11 | 87 ± 7 | 0.40 |
| CPT | 105 ± 11 | 107 ± 9 | 96 ± 9 | 99 ± 9 | 106 ± 14 | 103 ± 11 | ||
| Aortic SBP (mmHg) | Rest | 107 ± 13 | 105 ± 11 | 108 ± 13 | 109 ± 10 | 104 ± 11 | 107 ± 9 | 0.35 |
| CPT | 129 ± 15 | 129 ± 12 | 121 ± 14 | 125 ± 13 | 133 ± 18 | 128 ± 16 | ||
| Aortic DBP (mmHg) | Rest | 76 ± 8 | 76 ± 8 | 72 ± 9 | 72 ± 9 | 72 ± 11 | 74 ± 8 | 0.66 |
| CPT | 92 ± 11 | 94 ± 8 | 82 ± 9 | 83 ± 8 | 90 ± 14 | 90 ± 10 | ||
| Aortic MAP (mmHg) | Rest | 87 ± 9 | 86 ± 9 | 84 ± 10 | 84 ± 8 | 83 ± 11 | 85 ± 7 | 0.70 |
| CPT | 104 ± 11 | 106 ± 9 | 95 ± 10 | 97 ± 9 | 104 ± 14 | 102 ± 11 | ||
| AIx75 (%) | Rest | 27 ± 6 | 28 ± 7 | 30 ± 8 | 29 ± 8 | 27 ± 5 | 27 ± 6 | 0.88 |
| CPT | 33 ± 6 | 31 ± 7 | 32 ± 8 | 32 ± 6 | 33 ± 8 | 30 ± 7 | ||
| Tr (ms) | Rest | 137 ± 6 | 138 ± 7 | 135 ± 8 | 135 ± 8 | 138 ± 9 | 136 ± 7 | 0.29 |
| CPT | 135 ± 8 | 139 ± 11 | 135 ± 8 | 136 ± 10 | 138 ± 8 | 139 ± 8 | ||
| Measure | Placebo | CIT | CIT+GSH | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | p | |
| Arginine (µM/L) | 127 ± 21 | 135 ± 32 | 121 ± 21 | 168 ± 36 *† | 117 ± 25 | 128 ± 19 | 0.005 |
| Citrulline (µM/L) | 41 ± 10 | 41 ± 13 | 40 ± 8 | 49 ± 13 * | 38 ± 10 | 39 ± 7 | 0.09 |
| Ornithine (µM/L) | 106 ± 28 | 120 ± 34 | 110 ± 20 | 149 ± 38 ‡# | 104 ± 25 | 114 ± 24 | 0.043 |
| ADMA (mM/L) | 0.54 ± 0.05 | 0.55 ± 0.05 | 0.54 ± 0.06 | 0.56 ± 0.06 * | 0.55 ± 0.07 | 0.54 ± 0.08 | 0.27 |
| Arginine/ADMA | 240 ± 50 | 248 ± 61 | 228 ± 50 | 299 ± 48 *† | 215 ± 47 | 237 ± 37 * | 0.007 |
| Arginase-I (ng/mL) | 87 ± 2 | 87 ± 2 | 88 ± 3 | 87 ± 3 ‡ | 90 ± 3 | 89 ± 3 | 0.32 |
| Glucose (mg/dL) | 87 ± 7 | 87 ± 7 | 90 ± 6 | 91 ± 10 | 94 ± 7 | 94 ± 8 | 0.89 |
| Insulin (mIU/mL) | 15 ± 4 | 14 ± 4 | 15 ± 6 | 15 ± 5 | 12 ± 4 | 12 ± 5 | 0.75 |
| HOMA-IR | 3.1 ± 1.0 | 3.1 ± 1.1 | 3.4 ± 1.5 | 3.4 ± 1.3 | 2.7 ± 1.1 | 2.9 ± 1.1 | 0.82 |
| GPx (µmol/L) | 393 ± 44 | 399 ± 44 | 396 ± 43 | 400 ± 43 | 435 ± 35 | 439 ± 33 * | 0.75 |
| SOD (ng/mL) | 20 ± 3 | 20 ± 2 | 21 ± 3 | 20 ± 2 | 21 ± 4 | 20 ± 3 | 0.42 |
| Ox-LDL (U/L) | 65 ± 13 | 65 ± 14 | 69 ± 12 | 68 ± 12 | 72 ± 12 | 72 ± 12 | 0.53 |
| MDA (µm/L) | 1.0 ± 0.37 | 1.1 ± 0.39 | 0.94 ± 0.39 | 0.99 ± 0.38 | 1.1 ± 0.45 | 1.0 ± 0.38 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa, A.; Maharaj, A.; Kang, Y.; Dillon, K.N.; Martinez, M.A.; Morita, M.; Nogimura, D.; Fischer, S.M. Combined Citrulline and Glutathione Supplementation Improves Endothelial Function and Blood Pressure Reactivity in Postmenopausal Women. Nutrients 2023, 15, 1557. https://doi.org/10.3390/nu15071557
Figueroa A, Maharaj A, Kang Y, Dillon KN, Martinez MA, Morita M, Nogimura D, Fischer SM. Combined Citrulline and Glutathione Supplementation Improves Endothelial Function and Blood Pressure Reactivity in Postmenopausal Women. Nutrients. 2023; 15(7):1557. https://doi.org/10.3390/nu15071557
Chicago/Turabian StyleFigueroa, Arturo, Arun Maharaj, Yejin Kang, Katherine N. Dillon, Mauricio A. Martinez, Masahiko Morita, Dai Nogimura, and Stephen M. Fischer. 2023. "Combined Citrulline and Glutathione Supplementation Improves Endothelial Function and Blood Pressure Reactivity in Postmenopausal Women" Nutrients 15, no. 7: 1557. https://doi.org/10.3390/nu15071557
APA StyleFigueroa, A., Maharaj, A., Kang, Y., Dillon, K. N., Martinez, M. A., Morita, M., Nogimura, D., & Fischer, S. M. (2023). Combined Citrulline and Glutathione Supplementation Improves Endothelial Function and Blood Pressure Reactivity in Postmenopausal Women. Nutrients, 15(7), 1557. https://doi.org/10.3390/nu15071557







