Acute Administration of Ojeok-san Ameliorates Pain-like Behaviors in Pre-Clinical Models of Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Animals
2.1.1. Experiment #1
2.1.2. Experiment #2
2.1.3. Experiment #3
2.1.4. Experiment #4
2.2. Dextran Sulphate Sodium (DSS) Colitis Development
2.3. Ojeok-san Administration
2.4. Disease Activity Index, Histology, and Complete Blood Count
2.5. Real-Time PCR
2.6. Behavioral Tests
2.7. Data Analysis
3. Results
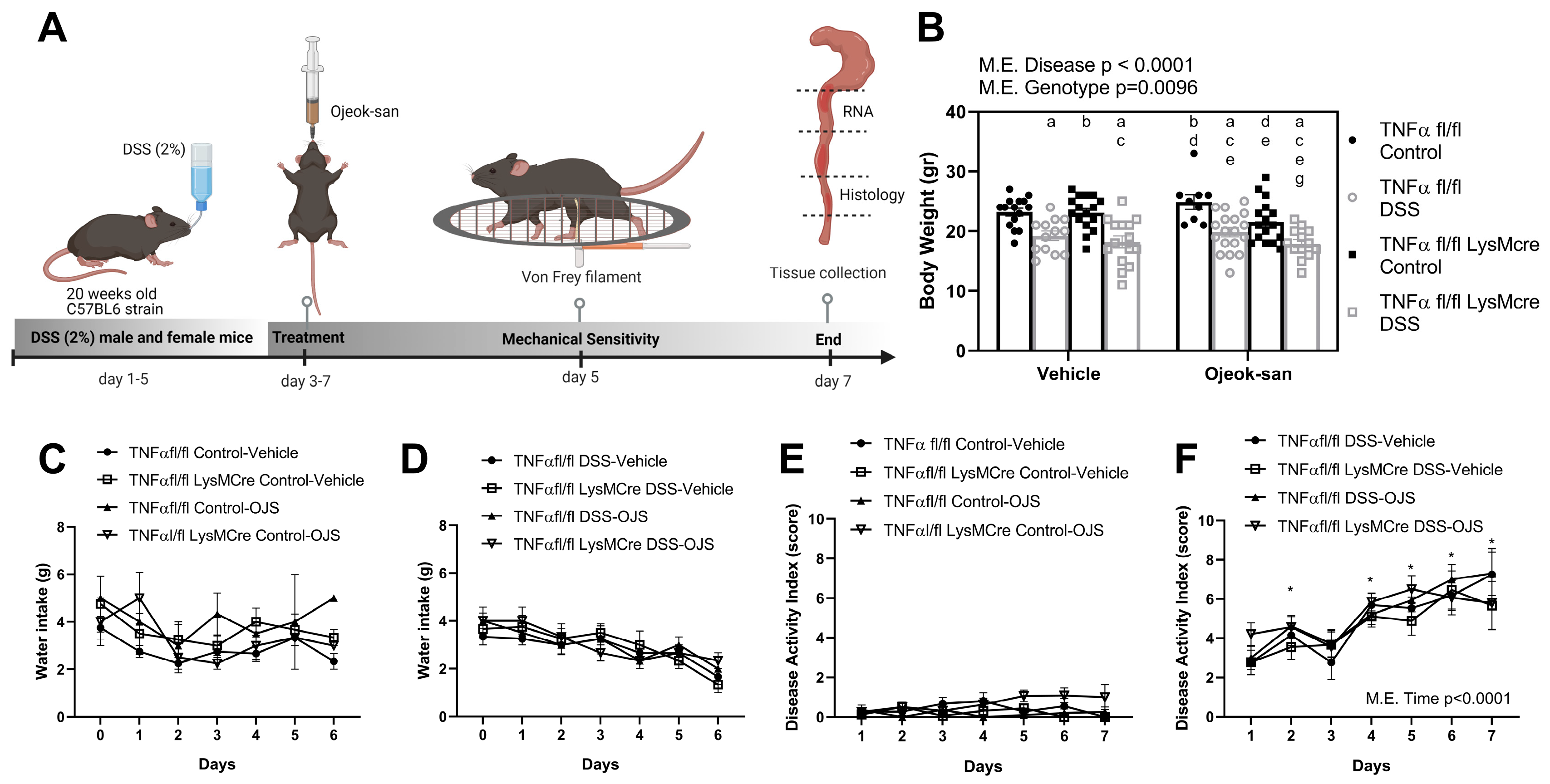
3.1. Macrophage TNFα Deletion and Ojeok-san Administration Do Not Impact Disease Progression in a Chemical Model of Acute Colitis
3.2. Macrophage Deletion of TNFα Does Not Impact the Blood Profile but Ojeok-san Administration Diminishes Lymphocyte Levels in Mice with Colitis
3.3. Deletion of Macrophage TNFα Does Not Impact Mechanical Sensitivity but Ojeok-san Administration Improves Mechanical Hyperalgesia in Mice with Colitis
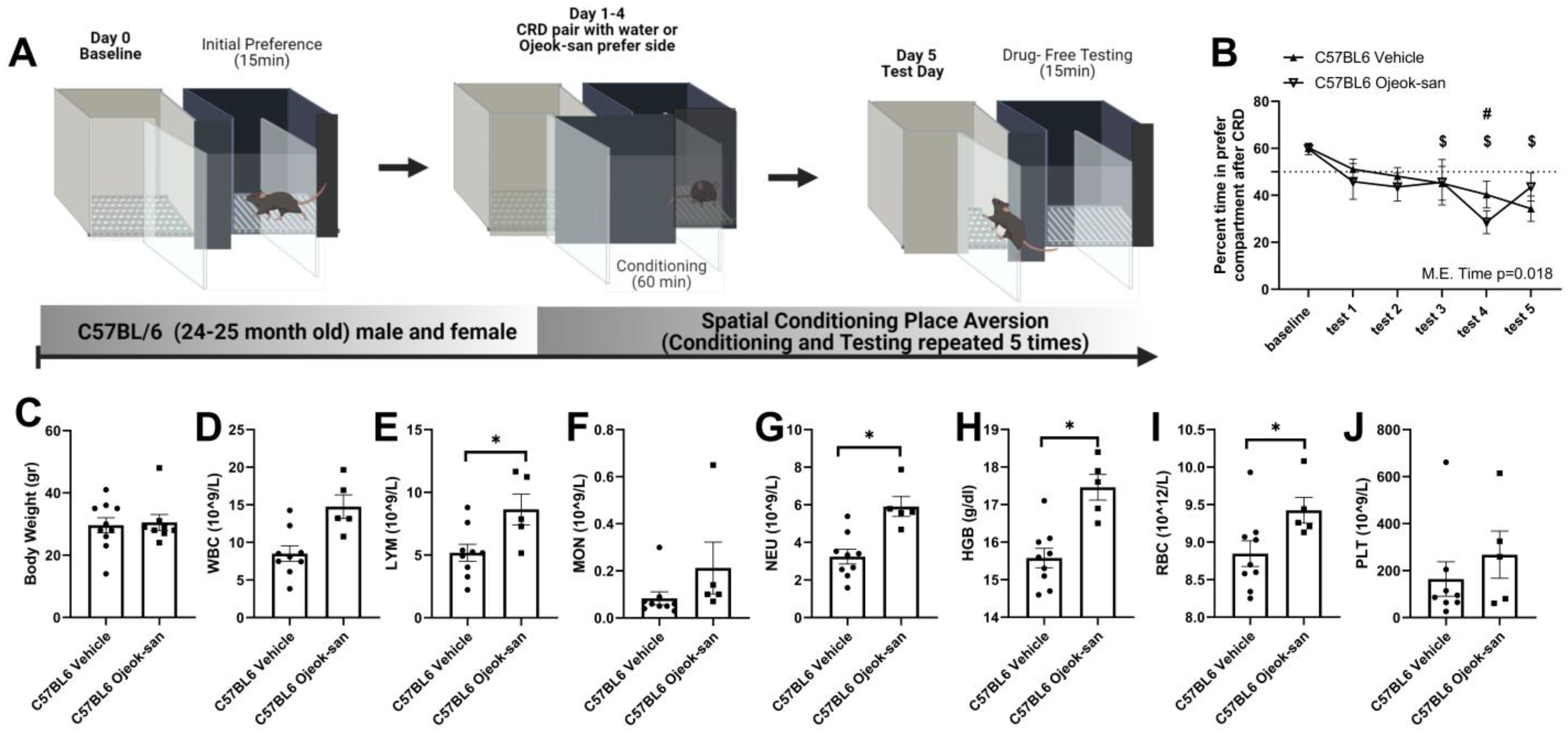
3.4. Four Weeks of Ojeok-san Administration Does Not Impact Disease Progression nor Show Rewarding Properties in the mdr1a KO Mouse Model of Spontaneous Colitis
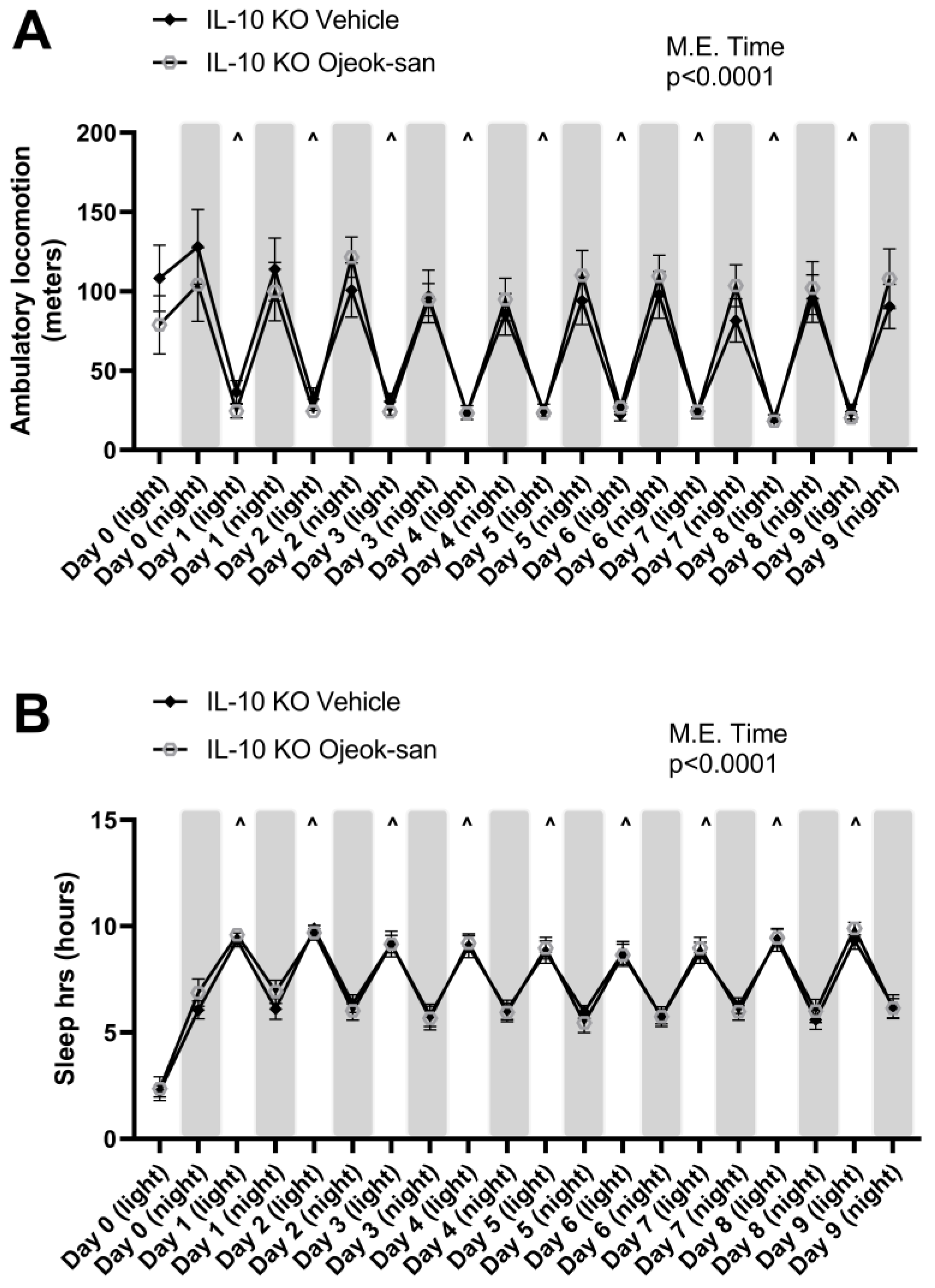
3.5. Five Weeks of Ojeok-san Supplementation Does Not Impact Disease Progression but Improves Mechanical Hyperalgesia without Promoting Rewarding Behavior in an IL-10 KO Mouse Model of Spontaneous Colitis
3.6. Ojeok-san Reduces Aversive Behavior Produced by the Colorectal Balloon Distension in C57BL6 Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlhamer, J.M.; Zammitti, E.P.; Ward, B.W.; Wheaton, A.G.; Croft, J.B. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr. Update on the Incidence and Prevalence of Inflammatory Bowel Disease in the United States. Gastroenterol. Hepatol. 2016, 12, 704–707. [Google Scholar]
- Aghazadeh, R.; Zali, M.R.; Bahari, A.; Amin, K.; Ghahghaie, F.; Firouzi, F. Inflammatory bowel disease in Iran: A review of 457 cases. J. Gastroenterol. Hepatol. 2005, 20, 1691–1695. [Google Scholar] [CrossRef]
- Wagtmans, M.J.; Verspaget, H.W.; Lamers, C.B.; van Hogezand, R.A. Crohn’s disease in the elderly: A comparison with young adults. J. Clin. Gastroenterol. 1998, 27, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Hardy, P.Y.; Fikri, J.; Libbrecht, D.; Louis, E.; Joris, J. Pain Characteristics in Patients with Inflammatory Bowel Disease: A Monocentric Cross-Sectional Study. J. Crohns Colitis 2022, 16, 1363–1371. [Google Scholar] [CrossRef]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef]
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489.e3. [Google Scholar] [CrossRef]
- Vilar, P.; de Carpi, J.M.; Acuna, C.E.; Masiques, M.A. Infliximab in paediatric inflammatory bowel disease. J. Crohns Colitis 2007, 1, 2–9. [Google Scholar] [CrossRef]
- van Staa, T.P.; Card, T.; Logan, R.F.; Leufkens, H.G. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: A large epidemiological study. Gut 2005, 54, 1573–1578. [Google Scholar] [CrossRef]
- Matuk, R.; Crawford, J.; Abreu, M.T.; Targan, S.R.; Vasiliauskas, E.A.; Papadakis, K.A. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 352–356. [Google Scholar] [CrossRef]
- Takeuchi, K.; Smale, S.; Premchand, P.; Maiden, L.; Sherwood, R.; Thjodleifsson, B.; Bjornsson, E.; Bjarnason, I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Edwards, J.T.; Radford-Smith, G.L.; Florin, T.H. Chronic narcotic use in inflammatory bowel disease patients: Prevalence and clinical characteristics. J. Gastroenterol. Hepatol. 2001, 16, 1235–1238. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Korelitz, B.I. Narcotic dependence in inflammatory bowel disease. J. Clin. Gastroenterol. 1988, 10, 275–278. [Google Scholar] [CrossRef]
- Cross, R.K.; Wilson, K.T.; Binion, D.G. Narcotic use in patients with Crohn’s disease. Am. J. Gastroenterol. 2005, 100, 2225–2229. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Chen, D.M.; Pritchard, M.L.; Sandborn, W.J. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin. Gastroenterol. Hepatol. 2006, 4, 621–630. [Google Scholar] [CrossRef]
- Nathan, C.F. Secretory products of macrophages. J. Clin. Investig. 1987, 79, 319–326. [Google Scholar] [CrossRef]
- Salvo, E.; Tu, N.H.; Scheff, N.N.; Dubeykovskaya, Z.A.; Chavan, S.A.; Aouizerat, B.E.; Ye, Y. TNFalpha promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Al Obeed, O.A.; Alkhayal, K.A.; Al Sheikh, A.; Zubaidi, A.M.; Vaali-Mohammed, M.A.; Boushey, R.; McKerrow, J.H.; Abdulla, M.H. Increased expression of tumor necrosis factor-alpha is associated with advanced colorectal cancer stages. World J. Gastroenterol. 2014, 20, 18390–18396. [Google Scholar] [CrossRef] [PubMed]
- Enos, R.T.; Velazquez, K.T.; McClellan, J.L.; Cranford, T.L.; Nagarkatti, M.; Nagarkatti, P.S.; Davis, J.M.; Murphy, E.A. High-fat diets rich in saturated fat protect against azoxymethane/dextran sulfate sodium-induced colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G906–G919. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, K.T.; Enos, R.T.; McClellan, J.L.; Cranford, T.L.; Chatzistamou, I.; Singh, U.P.; Nagarkatti, M.; Nagarkatti, P.S.; Fan, D.; Murphy, E.A. MicroRNA-155 deletion promotes tumorigenesis in the azoxymethane-dextran sulfate sodium model of colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G347–G358. [Google Scholar] [CrossRef]
- Watson, A.J.; Hughes, K.R. TNF-alpha-induced intestinal epithelial cell shedding: Implications for intestinal barrier function. Ann. N. Y. Acad. Sci. 2012, 1258, 1–8. [Google Scholar] [CrossRef]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic functions of TNF-alpha in the regulation of the intestinal epithelial response to inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef]
- Sands, B.E.; Kaplan, G.G. The role of TNFalpha in ulcerative colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Ha, H.; Lee, J.K.; Lee, H.Y.; Seo, C.S.; Lee, M.Y.; Huh, J.I.; Shin, H.K. Genotoxicity assessment of a herbal formula, Ojeok-san. J. Ethnopharmacol. 2011, 135, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R. (Eds.) Botanical Medicine in Clinical Practice; CABI: Oxfordshire, UK, 2008; p. 915. [Google Scholar]
- Ha, H.; Lee, J.K.; Lee, H.Y.; Seo, C.S.; Kim, J.H.; Lee, M.Y.; Koh, W.S.; Shin, H.K. Evaluation of safety of the herbal formula Ojeok-san: Acute and sub-chronic toxicity studies in rats. J. Ethnopharmacol. 2010, 131, 410–416. [Google Scholar] [CrossRef]
- Jeong, S.J.; Huh, J.I.; Shin, H.K. Cytotoxicity and sub-acute toxicity in Crl:CD (SD) rats of traditional herbal formula Ojeok-san. BMC Complement. Altern. Med. 2015, 15, 38. [Google Scholar] [CrossRef]
- Kim, E.J.; Nam, D.; Ahn, B.J.; Lee, S.D.; Lee, J.D.; Kim, K.S. Study to establish Ojeok-San (Five Accumulation Powder: Wu ji san) administration criteria and a questionnaire to evaluate the holistic effects of Ojeok-San on patients with low back pain. J. Altern. Complement. Med. 2013, 19, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seo, C.S.; Kim, S.S.; Shin, H.K. Compositional Differences of Ojeok-san (Wuji-san) Decoctions Using Pressurized or Non-pressurized Methods for Variable Extraction Times. J. Pharmacopunct. 2012, 15, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Park, H.; Yang, H.J.; Ma, J.Y.; Ma, C.J. Simultaneous quantification of marker components in Ojeok-san by HPLC-DAD. J. Nat. Med. 2011, 65, 375–380. [Google Scholar] [CrossRef]
- Cunningham, P.; Sumal, A.; Patton, E.; Helms, H.; Noneman, M.T.; Martinez-Muniz, G.; Bader, J.E.; Chatzistamou, I.; Aladhami, A.; Unger, C.; et al. Ojeok-san ameliorates visceral and somatic nociception in a mouse model of colitis induced colorectal cancer. PLoS ONE 2022, 17, e0270338. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, W.-H.; Cha, Y.-Y.; Lee, E. Effects of Ojeoksangamibang Extract on the Recovery of Liver Function in CCl 4-exposed Rats. J. Korean Med. Rehabil. 2013, 23, 45–53. [Google Scholar]
- Lee, M.J.; Hwang, D.S.; Lee, J.M.; Jang, J.B.; Lee, K.S.; Lee, C.H. Activation of Immune System & Antimetastatic Effects of Ojeok-san by Oral Administration. J. Korean Obstet. Gynecol. 2014, 27, 34–45. [Google Scholar]
- Yoo, S.R.; Jeong, S.J.; Kim, Y.J.; Lim, H.S.; Jin, S.E.; Jeon, W.Y.; Shin, I.S.; Shin, N.R.; Kim, S.S.; Ha, H.K.; et al. Effects of water and ethanol extracts from Ojeok-san on inflammation and its related diseases. J. Intern. Korean Med. 2012, 33, 418–428. [Google Scholar]
- Han, B.H.; Seo, C.S.; Yoon, J.J.; Kim, H.Y.; Ahn, Y.M.; Eun, S.Y.; Hong, M.H.; Lee, J.G.; Shin, H.K.; Lee, H.S.; et al. The Inhibitory Effect of Ojeoksan on Early and Advanced Atherosclerosis. Nutrients 2018, 10, 1256. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.H.; Kim, J.E.; Jung, S.E.; Ham, S.H.; Yang, W.M.; Kwon, B.I. Anti-inflammatory Effects of Ojeok-san in LPS-induced Inflammatory Rat Model. J. Korean Med. 2021, 42, 21–30. [Google Scholar] [CrossRef]
- Sawa, Y.; Oshitani, N.; Adachi, K.; Higuchi, K.; Matsumoto, T.; Arakawa, T. Comprehensive analysis of intestinal cytokine messenger RNA profile by real-time quantitative polymerase chain reaction in patients with inflammatory bowel disease. Int. J. Mol. Med. 2003, 11, 175–179. [Google Scholar] [CrossRef]
- van Heel, D.A.; Udalova, I.A.; De Silva, A.P.; McGovern, D.P.; Kinouchi, Y.; Hull, J.; Lench, N.J.; Cardon, L.R.; Carey, A.H.; Jewell, D.P.; et al. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-kappa)B transcription factors. Hum. Mol. Genet. 2002, 11, 1281–1289. [Google Scholar] [CrossRef]
- Masuda, H.; Iwai, S.; Tanaka, T.; Hayakawa, S. Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative colitis, particularly in patients with inactive phase. J. Clin. Lab. Immunol. 1995, 46, 111–123. [Google Scholar]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed]
- Aladhami, A.K.; Unger, C.A.; Ennis, S.L.; Altomare, D.; Ji, H.; Hope, M.C., 3rd; Velazquez, K.T.; Enos, R.T. Macrophage tumor necrosis factor-alpha deletion does not protect against obesity-associated metabolic dysfunction. FASEB J. 2021, 35, e21665. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Tumanov, A.V.; Liepinsh, D.J.; Kruglov, A.A.; Marakusha, B.I.; Shakhov, A.N.; Murakami, T.; Drutskaya, L.N.; Forster, I.; Clausen, B.E.; et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: Protective and deleterious effects. Immunity 2005, 22, 93–104. [Google Scholar] [CrossRef]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Smit, J.J.; van Tellingen, O.; Beijnen, J.H.; Wagenaar, E.; van Deemter, L.; Mol, C.A.; van der Valk, M.A.; Robanus-Maandag, E.C.; te Riele, H.P.; et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994, 77, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Panwala, C.M.; Jones, J.C.; Viney, J.L. A novel model of inflammatory bowel disease: Mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J. Immunol. 1998, 161, 5733–5744. [Google Scholar] [CrossRef]
- Sufka, K.J. Conditioned place preference paradigm: A novel approach for analgesic drug assessment against chronic pain. Pain 1994, 58, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Lohler, J.; Rennick, D.; Rajewsky, K.; Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Vanderwall, A.G.; Noor, S.; Sun, M.S.; Sanchez, J.E.; Yang, X.O.; Jantzie, L.L.; Mellios, N.; Milligan, E.D. Effects of spinal non-viral interleukin-10 gene therapy formulated with d-mannose in neuropathic interleukin-10 deficient mice: Behavioral characterization, mRNA and protein analysis in pain relevant tissues. Brain Behav. Immun. 2018, 69, 91–112. [Google Scholar] [CrossRef]
- da Silva, M.D.; Bobinski, F.; Sato, K.L.; Kolker, S.J.; Sluka, K.A.; Santos, A.R. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol. Neurobiol. 2015, 51, 19–31. [Google Scholar] [CrossRef]
- Mietto, B.S.; Kroner, A.; Girolami, E.I.; Santos-Nogueira, E.; Zhang, J.; David, S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J. Neurosci. 2015, 35, 16431–16442. [Google Scholar] [CrossRef]
- Krukowski, K.; Eijkelkamp, N.; Laumet, G.; Hack, C.E.; Li, Y.; Dougherty, P.M.; Heijnen, C.J.; Kavelaars, A. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J. Neurosci. 2016, 36, 11074–11083. [Google Scholar] [CrossRef]
- Kamp, E.H.; Jones, R.C., 3rd; Tillman, S.R.; Gebhart, G.F. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G434–G444. [Google Scholar] [CrossRef]
- Boue, J.; Basso, L.; Cenac, N.; Blanpied, C.; Rolli-Derkinderen, M.; Neunlist, M.; Vergnolle, N.; Dietrich, G. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4(+) T cells in mice. Gastroenterology 2014, 146, 166–175. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Viennois, E.; Chen, F.; Laroui, H.; Baker, M.T.; Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: Lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res. Notes 2013, 6, 360. [Google Scholar] [CrossRef]
- Ness, T.J.; Gebhart, G.F. Colorectal distension as a noxious visceral stimulus: Physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988, 450, 153–169. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Tramullas, M.; Fitzgerald, P.; Cryan, J.F. Rodent models of colorectal distension. Curr. Protoc. Neurosci. 2012, 61, 9–40. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.A.; Gebhart, G.F. Assessment of colon sensitivity by luminal distension in mice. Nat. Protoc. 2007, 2, 2624–2631. [Google Scholar] [CrossRef]
- Arvidsson, S.; Larsson, M.; Larsson, H.; Lindstrom, E.; Martinez, V. Assessment of visceral pain-related pseudo-affective responses to colorectal distension in mice by intracolonic manometric recordings. J. Pain 2006, 7, 108–118. [Google Scholar] [CrossRef]
- Ness, T.J.; Metcalf, A.M.; Gebhart, G.F. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain 1990, 43, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef]
- Szigethy, E. Pain Management in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 53–56. [Google Scholar]
- Zeitz, J.; Ak, M.; Muller-Mottet, S.; Scharl, S.; Biedermann, L.; Fournier, N.; Frei, P.; Pittet, V.; Scharl, M.; Fried, M.; et al. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS ONE 2016, 11, e0156666. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.K.; Takakura, W.; Bresee, C.; Melmed, G.Y. Pain in Inflammatory Bowel Disease Is Not Improved During Hospitalization: The Impact of Opioids on Pain and Healthcare Utilization. Dig. Dis. Sci. 2020, 65, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.; Zhong, F.; Gebhart, G.F.; Bielefeldt, K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G451–G457. [Google Scholar] [CrossRef]
- Delafoy, L.; Gelot, A.; Ardid, D.; Eschalier, A.; Bertrand, C.; Doherty, A.M.; Diop, L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 2006, 55, 940–945. [Google Scholar] [CrossRef]
- Eijkelkamp, N.; Kavelaars, A.; Elsenbruch, S.; Schedlowski, M.; Holtmann, G.; Heijnen, C.J. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: Spinal cord c-Fos expression and behavior. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G749–G757. [Google Scholar] [CrossRef]
- Gross, V.; Andus, T.; Leser, H.G.; Roth, M.; Scholmerich, J. Inflammatory mediators in chronic inflammatory bowel diseases. Klin. Wochenschr. 1991, 69, 981–987. [Google Scholar] [CrossRef]
- Powrie, F.; Leach, M.W. Genetic and spontaneous models of inflammatory bowel disease in rodents: Evidence for abnormalities in mucosal immune regulation. Ther. Immunol. 1995, 2, 115–123. [Google Scholar]
- Jin, X.; Gereau, R.W.T. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006, 26, 246–255. [Google Scholar] [CrossRef]
- Tu, H.; Juelich, T.; Smith, E.M.; Tyring, S.K.; Rady, P.L.; Hughes, T.K., Jr. Evidence for endogenous interleukin-10 during nociception. J. Neuroimmunol. 2003, 139, 145–149. [Google Scholar] [CrossRef]
- Larsson, M.H.; Rapp, L.; Lindstrom, E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol. Motil. 2006, 18, 144–152. [Google Scholar] [CrossRef]
- Basso, L.; Benamar, M.; Mas-Orea, X.; Deraison, C.; Blanpied, C.; Cenac, N.; Saoudi, A.; Dietrich, G. Endogenous control of inflammatory visceral pain by T cell-derived opioids in IL-10-deficient mice. Neurogastroenterol. Motil. 2020, 32, e13743. [Google Scholar] [CrossRef]
- Ibeakanma, C.; Vanner, S. TNFalpha is a key mediator of the pronociceptive effects of mucosal supernatant from human ulcerative colitis on colonic DRG neurons. Gut 2010, 59, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [PubMed]
- Yu, M.; Wen, S.; Wang, M.; Liang, W.; Li, H.H.; Long, Q.; Guo, H.P.; Liao, Y.H.; Yuan, J. TNF-alpha-secreting B cells contribute to myocardial fibrosis in dilated cardiomyopathy. J. Clin. Immunol. 2013, 33, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Chieppa, M.; Salucci, V.; Avogadri, F.; Sonzogni, A.; Sampietro, G.M.; Nespoli, A.; Viale, G.; Allavena, P.; Rescigno, M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005, 6, 507–514. [Google Scholar] [CrossRef]
- Jung, H.C.; Eckmann, L.; Yang, S.K.; Panja, A.; Fierer, J.; Morzycka-Wroblewska, E.; Kagnoff, M.F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 1995, 95, 55–65. [Google Scholar] [CrossRef]
- Martinus, R.D.; Goldsbury, J. Endothelial TNF-alpha induction by Hsp60 secreted from THP-1 monocytes exposed to hyperglycaemic conditions. Cell Stress Chaperones 2018, 23, 519–525. [Google Scholar] [CrossRef]
- Jevnikar, A.M.; Brennan, D.C.; Singer, G.G.; Heng, J.E.; Maslinski, W.; Wuthrich, R.P.; Glimcher, L.H.; Kelley, V.E. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int. 1991, 40, 203–211. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Chowers, Y. Tailoring anti-TNF therapy in IBD: Drug levels and disease activity. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 243–255. [Google Scholar]
- Guerra, I.; Bermejo, F. Management of inflammatory bowel disease in poor responders to infliximab. Clin. Exp. Gastroenterol. 2014, 7, 359–367. [Google Scholar]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol. 2016, 7, 289–300. [Google Scholar] [CrossRef]
- De Santis, S.; Kunde, D.; Galleggiante, V.; Liso, M.; Scandiffio, L.; Serino, G.; Pinto, A.; Campiglia, P.; Sorrentino, R.; Cavalcanti, E.; et al. TNFalpha deficiency results in increased IL-1beta in an early onset of spontaneous murine colitis. Cell Death Dis. 2017, 8, e2993. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Handa, O.; Ishikawa, T.; Nakagawa, S.; Yamaguchi, T.; Yoshida, N.; Minami, M.; Kita, M.; Imanishi, J.; et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J. Gastroenterol. Hepatol. 2003, 18, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.E.; Callister, R.J.; Keely, S. Understanding and targeting centrally mediated visceral pain in inflammatory bowel disease. Front. Pharmacol. 2014, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Black, M.M.; Schuler, M.; Keane, V.; Snow, L.; Rigney, B.A.; Magder, L. Risk factors for disruption in primary caregiving among infants of substance abusing women. Child Abus. Negl. 1997, 21, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Bartho, L.; Matusak, O.; Bauer, V. Calcitonin gene-related peptide action on intestinal circular muscle. Am. J. Physiol. 1989, 256 Pt 1, G546–G552. [Google Scholar] [CrossRef]
- Eysselein, V.E.; Reinshagen, M.; Cominelli, F.; Sternini, C.; Davis, W.; Patel, A.; Nast, C.C.; Bernstein, D.; Anderson, K.; Khan, H.; et al. Calcitonin gene-related peptide and substance P decrease in the rabbit colon during colitis. A time study. Gastroenterology 1991, 101, 1211–1219. [Google Scholar] [CrossRef]
- Clifton, M.S.; Hoy, J.J.; Chang, J.; Idumalla, P.S.; Fakhruddin, H.; Grady, E.F.; Dada, S.; Corvera, C.U.; Bhargava, A. Role of calcitonin receptor-like receptor in colonic motility and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G36–G44. [Google Scholar] [CrossRef] [PubMed]
- Mazelin, L.; Theodorou, V.; Fioramonti, J.; Bueno, L. Vagally dependent protective action of calcitonin gene-related peptide on colitis. Peptides 1999, 20, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, L.; Huynh, J.; Duraffourd, C.; Jaramillo, I.; Vegezzi, G.; Saccani, F.; Boschetti, E.; Brecha, N.C.; De Giorgio, R.; Sternini, C. Activation of mu opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol. Motil. 2015, 27, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Philippe, D.; Chakass, D.; Thuru, X.; Zerbib, P.; Tsicopoulos, A.; Geboes, K.; Bulois, P.; Breisse, M.; Vorng, H.; Gay, J.; et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: Implications for homeostatic intestinal inflammation. Gut 2006, 55, 815–823. [Google Scholar] [CrossRef]
- Bechara, A.; van der Kooy, D. Kappa receptors mediate the peripheral aversive effects of opiates. Pharmacol. Biochem. Behav. 1987, 28, 227–233. [Google Scholar] [CrossRef]
- Chitnavis, M.V.; Baray, M.; Northup, P.G.; Tuskey, A.G.; Behm, B.W. Opioid use and misuse in ulcerative colitis. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Nugent, Z.; Singh, H.; Bugden, S.; Bernstein, C.N. The prevalence and predictors of opioid use in inflammatory bowel disease: A population-based analysis. Am. J. Gastroenterol. 2014, 109, 1613–1620. [Google Scholar] [CrossRef]
- Matthes, H.W.; Maldonado, R.; Simonin, F.; Valverde, O.; Slowe, S.; Kitchen, I.; Befort, K.; Dierich, A.; Le Meur, M.; Dolle, P.; et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 1996, 383, 819–823. [Google Scholar] [CrossRef]
- Hnasko, T.S.; Sotak, B.N.; Palmiter, R.D. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J. Neurosci. 2007, 27, 12484–12488. [Google Scholar] [CrossRef]
- Gerdjikov, T.V.; Beninger, R.J. Place preference induced by nucleus accumbens amphetamine is impaired by local blockade of Group II metabotropic glutamate receptors in rats. BMC Neurosci. 2006, 7, 43. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patton, E.A.; Cunningham, P.; Noneman, M.; Helms, H.P.; Martinez-Muniz, G.; Sumal, A.S.; Dhameja, M.K.; Unger, C.A.; Alahdami, A.K.; Enos, R.T.; et al. Acute Administration of Ojeok-san Ameliorates Pain-like Behaviors in Pre-Clinical Models of Inflammatory Bowel Diseases. Nutrients 2023, 15, 1559. https://doi.org/10.3390/nu15071559
Patton EA, Cunningham P, Noneman M, Helms HP, Martinez-Muniz G, Sumal AS, Dhameja MK, Unger CA, Alahdami AK, Enos RT, et al. Acute Administration of Ojeok-san Ameliorates Pain-like Behaviors in Pre-Clinical Models of Inflammatory Bowel Diseases. Nutrients. 2023; 15(7):1559. https://doi.org/10.3390/nu15071559
Chicago/Turabian StylePatton, Emma A., Patrice Cunningham, Matthew Noneman, Henry P. Helms, Gustavo Martinez-Muniz, Aman S. Sumal, Milan K. Dhameja, Christian A. Unger, Ahmed K. Alahdami, Reilly T. Enos, and et al. 2023. "Acute Administration of Ojeok-san Ameliorates Pain-like Behaviors in Pre-Clinical Models of Inflammatory Bowel Diseases" Nutrients 15, no. 7: 1559. https://doi.org/10.3390/nu15071559
APA StylePatton, E. A., Cunningham, P., Noneman, M., Helms, H. P., Martinez-Muniz, G., Sumal, A. S., Dhameja, M. K., Unger, C. A., Alahdami, A. K., Enos, R. T., Chatzistamou, I., & Velázquez, K. T. (2023). Acute Administration of Ojeok-san Ameliorates Pain-like Behaviors in Pre-Clinical Models of Inflammatory Bowel Diseases. Nutrients, 15(7), 1559. https://doi.org/10.3390/nu15071559





