Nutritional and Behavioral Countermeasures as Medication Approaches to Relieve Motion Sickness: A Comprehensive Review
Abstract
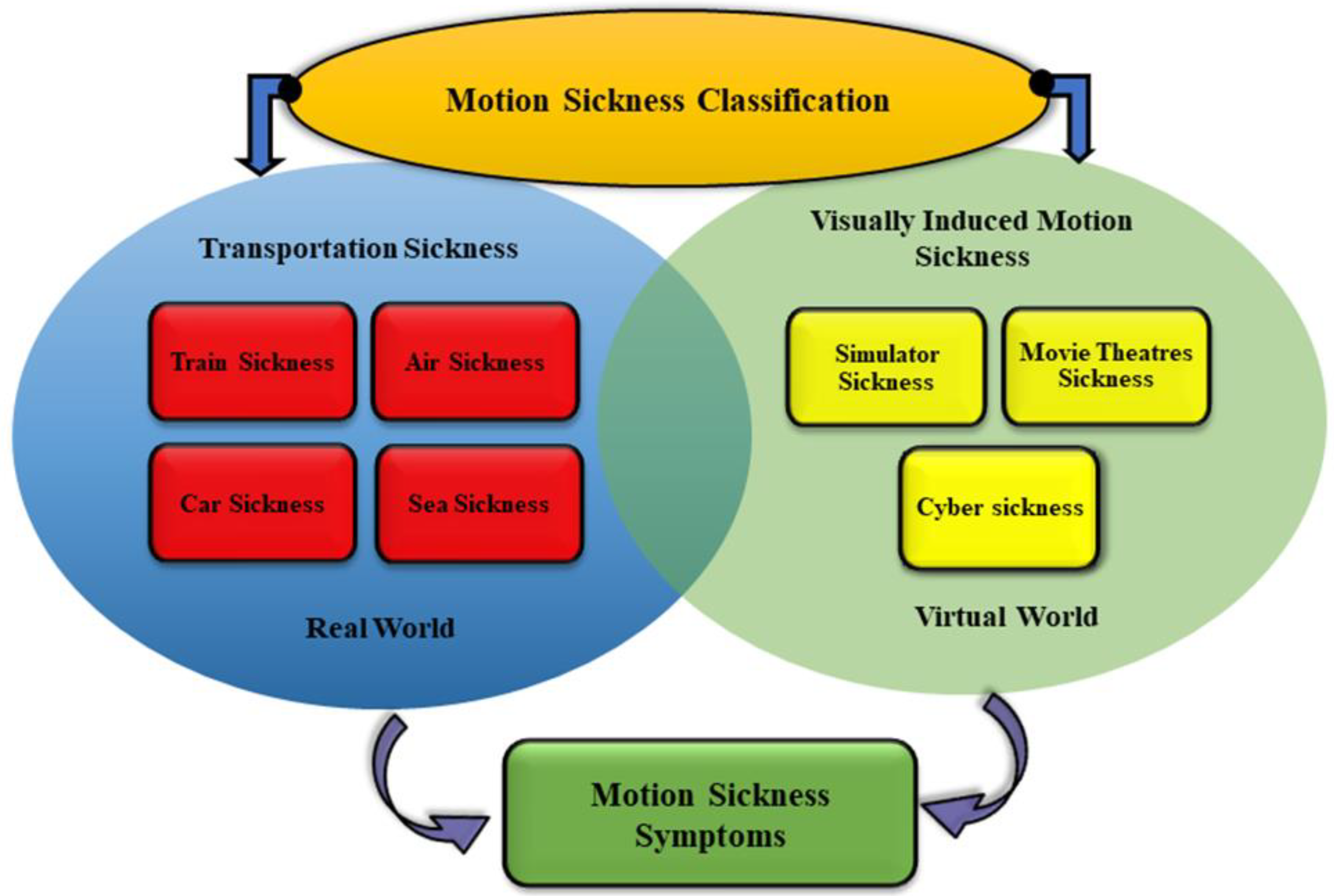
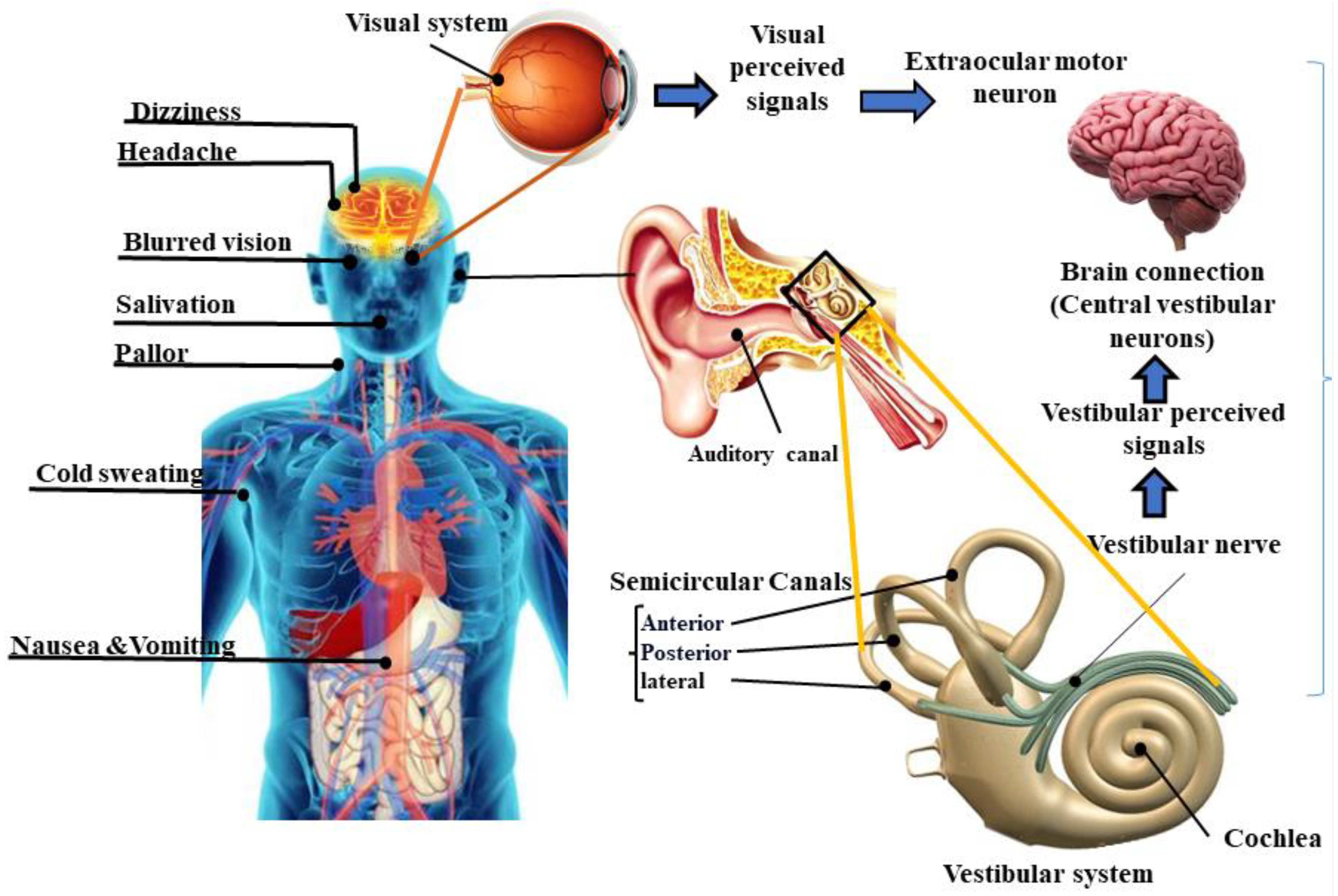
1. Introduction
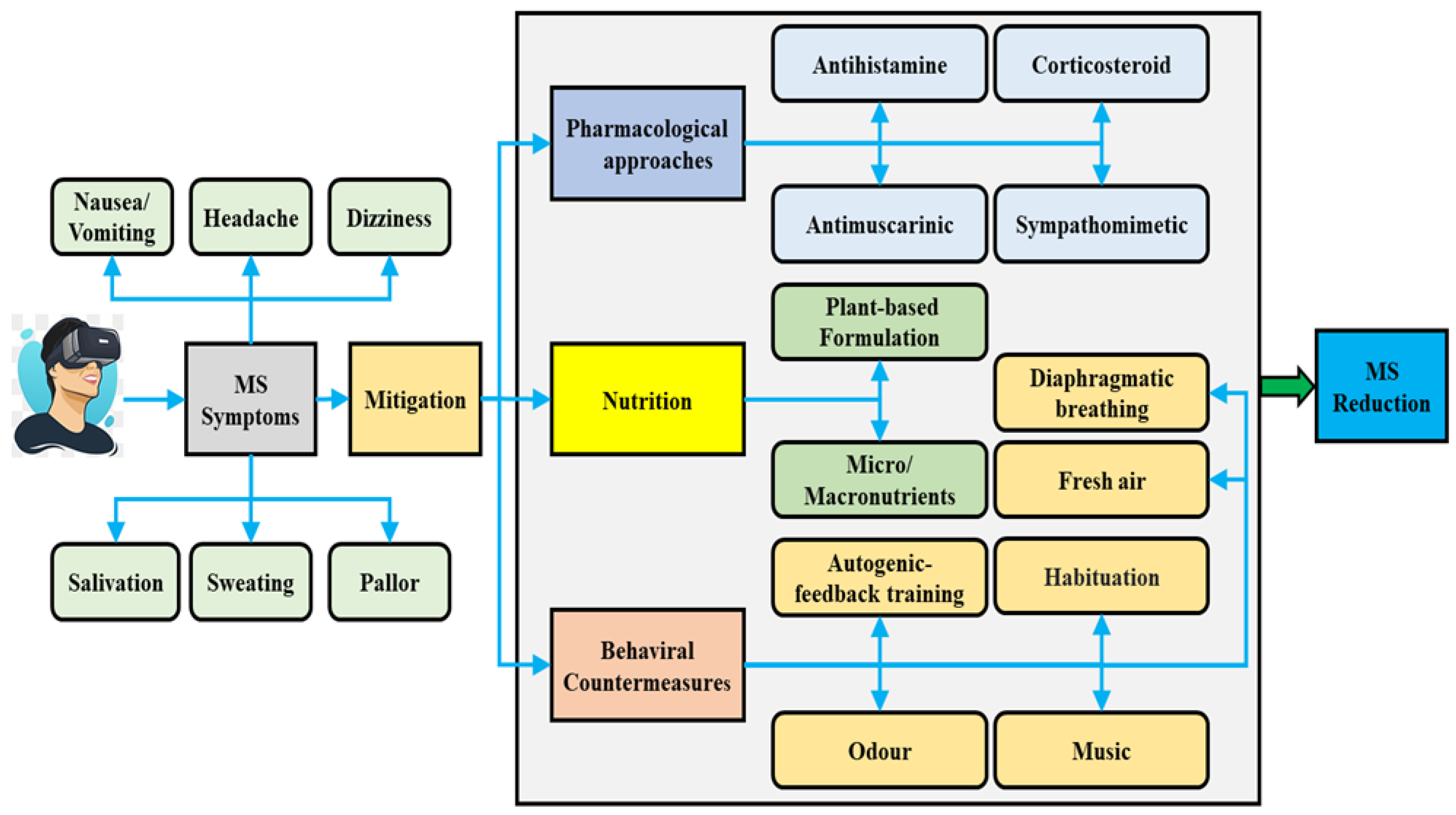
2. Interventions for Mitigating Motion Sickness
2.1. Pharmacological Approaches for Mitigating Motion Sickness
2.1.1. Antihistamines
2.1.2. Antimuscarinics
2.1.3. Sympathomimetics
2.1.4. Corticosteroids
2.2. Non-Pharmacological Interventions for Mitigating Motion Sickness
2.2.1. Effects of Nutrition on Motion Sickness Syndrome
Plant-Based Interventions
2.2.2. Effects of Music on Motion Sickness
2.2.3. Effects of Diaphragmatic Breathing on Motion Sickness
2.2.4. Effects of Odor on Motion Sickness
2.2.5. Effects of Administration of Fresh Air on Motion Sickness
2.2.6. Effects of Autogenic-Feedback Training on Motion Sickness
2.2.7. Effects of Habituation Program on Motion Sickness
3. Discussion
3.1. Limitations
3.1.1. Data Collection Impact
3.1.2. Neuronal Mechanism of MS
3.1.3. Clinical Intervention
3.2. Research Gaps and Future Directions
- Most complementary and alternative treatments have not been tested in clinical trials, and research is needed to determine their impact on human physiological and biochemical metabolites during MS occurrence.
- Based on our literature review, a significant gap in the existing research has been identified with respect to the potential influence of taste, food texture, and flavor on MS levels. Despite the critical role that these factors play in determining our dietary choices, their impact on MS levels has not been investigated previously. Therefore, there is a pressing need for further rigorous research to investigate the potential role of taste, food texture, and flavor in modulating MS levels.
- It is important to note that the impact of nutrition may vary based on multiple factors, including the person’s overall diet and health, as well as the specific type and amount of protein and carbohydrates consumed. Further research is necessary to fully comprehend the connection between protein, carbohydrates, and MS.As physiological signals are affected by MS, a comprehensive study of physiological responses to MS in both real and virtual environments would be a valuable contribution to our understanding of this phenomenon. It may help develop effective preventive and therapeutic strategies.
- Along with the effect of music and DB, the effect of distraction should be investigated extensively. Lin et al. [199] distinguished four main challenges to driving cognitive research, including distraction, drowsiness, navigation, and MS. Based on their recommendation, these four main challenges are key to studying brain activity in different experimental paradigms.
- Some research studies have suggested that a combination of multiple techniques could more successfully minimize MS symptoms. Therefore, future studies should consider attempting to combine methods such as the effect of both favorite music, DB, and favorite odor on MS.
- Given that the impact of vestibular deficiency has been studied in real-world environments, it is worth investigating in future research whether vestibular system impairment can affect the severity of VIMS.
- The impact of artificial intelligence (AI) technology (machine learning (ML) and deep learning (DL) as well-known AI technologies) is a gap in MS research that should be investigated more comprehensively. Several studies have been conducted on the use of traditional ML and DL methods for MS. Based on the results, we found that traditional ML techniques are most frequently used compared to DL methods. As we discussed limitations in data collection above, DL methods require huge amounts of data to provide meaningful results. Therefore, we highly recommend applying different ML and DL approaches to find hidden patterns in MS data as well as providing an automated detection/reduction framework. On the other hand, our findings show that there is no study on uncertainty quantification (UQ) of ML and DL methods. Even though ML and DL can provide excellent results, the issue of dealing with their uncertainty remains unresolved. In Table 1, we list and discuss the most relevant studies on MS with the application of ML and DL methods.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazloumi Gavgani, A.; Walker, F.R.; Hodgson, D.M.; Nalivaiko, E. A comparative study of cybersickness during exposure to virtual reality and “classic” motion sickness: Are they different? J. Appl. Physiol. 2018, 125, 1670–1680. [Google Scholar] [CrossRef]
- Keshavarz, B.; Hecht, H.; Lawson, B. Visually induced motion sickness: Characteristics, causes, and countermeasures. In Handbook of Virtual Environments: Design, Implementation, and Applications; CRC Press: Boca Raton, FL, USA, 2014; pp. 648–697. [Google Scholar]
- Bos, J.E.; Bles, W.; Groen, E.L. A theory on visually induced motion sickness. Displays 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Levine, M.; Muth, E.; Williamson, M.; Stern, R. Protein-predominant meals inhibit the development of gastric tachyarrhythmia, nausea and the symptoms of motion sickness. Aliment. Pharmacol. Ther. 2004, 19, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lindseth, G.; Lindseth, P.D. The relationship of diet to airsickness. Aviat. Space Environ. Med. 1995, 66, 537–541. [Google Scholar] [PubMed]
- Keshavarz, B. Exploring behavioral methods to reduce visually induced motion sickness in virtual environments. In Proceedings of the Virtual, Augmented and Mixed Reality: 8th International Conference, VAMR 2016, Held as Part of HCI International 2016, Toronto, ON, Canada, 17–22 July 2016; pp. 147–155. [Google Scholar]
- Liu, R.; Peli, E.; Hwang, A.D. Measuring visually induced motion sickness using wearable devices. Electron. Imaging 2017, 2017, 218–223. [Google Scholar] [CrossRef]
- Tu, M.-Y.; Chu, H.; Lai, C.-Y.; Chiang, K.-T.; Huang, C.-C.; Chin, H.-C.; Wen, Y.-H.; Chen, C.-L. Effect of Standardized Yelling on Subjective Perception and Autonomic Nervous System Activity in Motion Sickness. Int. J. Environ. Res. Public Health 2021, 18, 12854. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Hu, S.; Wang, J. Correlation of phasic and tonic skin-conductance responses with severity of motion sickness induced by viewing an optokinetic rotating drum. Percept. Mot. Ski. 2003, 97, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Recenti, M.; Ricciardi, C.; Aubonnet, R.; Picone, I.; Jacob, D.; Svansson, H.Á.; Agnarsdóttir, S.; Karlsson, G.H.; Baeringsdóttir, V.; Petersen, H. Toward predicting motion sickness using virtual reality and a moving platform assessing brain, muscles, and heart signals. Front. Bioeng. Biotechnol. 2021, 9, 635661. [Google Scholar] [CrossRef]
- Yates, B.J.; Catanzaro, M.F.; Miller, D.J.; McCall, A.A. Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: Potential contributions to motion sickness. Exp. Brain Res. 2014, 232, 2455–2469. [Google Scholar] [CrossRef]
- Schmidt, E.A.; Kuiper, O.X.; Wolter, S.; Diels, C.; Bos, J.E. An international survey on the incidence and modulating factors of carsickness. Transp. Res. Part F Traffic Psychol. Behav. 2020, 71, 76–87. [Google Scholar] [CrossRef]
- Chinn, H. Evaluation of drugs for protection against motion sickness aboard transport ships. J. Am. Med. Assn 1956, 160, 755–760. [Google Scholar]
- Irwin, J. The Pathology of Sea-Sickness. Lancet 1881, 118, 907–909. [Google Scholar] [CrossRef]
- Paillard, A.C.; Quarck, G.; Paolino, F.; Denise, P.; Paolino, M.; Golding, J.F.; Ghulyan-Bedikian, V. Motion sickness susceptibility in healthy subjects and vestibular patients: Effects of gender, age and trait-anxiety. J. Vestib. Res. 2013, 23, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Oman, C.M. Motion sickness: A synthesis and evaluation of the sensory conflict theory. Can. J. Physiol. Pharmacol. 1990, 68, 294–303. [Google Scholar] [CrossRef]
- Yates, B.J.; Bolton, P.S.; Macefield, V.G. Vestibulo-sympathetic responses. Compr. Physiol. 2011, 4, 851–887. [Google Scholar]
- Bertolini, G.; Straumann, D. Moving in a moving world: A review on vestibular motion sickness. Front. Neurol. 2016, 7, 14. [Google Scholar] [CrossRef]
- Stern, R.M.; Koch, K.L. Motion sickness and differential susceptibility. Curr. Dir. Psychol. Sci. 1996, 5, 115–120. [Google Scholar] [CrossRef]
- Golding, J.F.; Gresty, M.A. Pathophysiology and treatment of motion sickness. Curr. Opin. Neurol. 2015, 28, 83–88. [Google Scholar] [CrossRef]
- Reason, J.T.; Brand, J.J. Motion Sickness; Academic Press: Washington, DC, USA, 1975. [Google Scholar]
- Chang, E.; Kim, H.T.; Yoo, B. Virtual reality sickness: A review of causes and measurements. Int. J. Hum.-Comput. Interact. 2020, 36, 1658–1682. [Google Scholar] [CrossRef]
- Golding, J.F. Predicting individual differences in motion sickness susceptibility by questionnaire. Personal. Individ. Differ. 2006, 41, 237–248. [Google Scholar] [CrossRef]
- Wang, J.; Lewis, R.F. Contribution of intravestibular sensory conflict to motion sickness and dizziness in migraine disorders. J. Neurophysiol. 2016, 116, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Warwick-Evans, L.; Symons, N.; Fitch, T.; Burrows, L. Evaluating sensory conflict and postural instability. Theories of motion sickness. Brain Res. Bull. 1998, 47, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Morita, M.; Horii, A.; Nishiike, S.; Kitahara, T.; Uno, A. Neural mechanisms of motion sickness. J. Med. Investig. 2001, 48, 44–59. [Google Scholar]
- Li–gui, H.; En–tong, W.; Wei, C.; Wei–xi, G. Role of Histamine H1 Receptors in Vestibular Nucleus in Motion Sickness. J. Otol. 2011, 6, 20–25. [Google Scholar] [CrossRef]
- Gordon, C.R.; Doweck, I.; Nachum, Z.; Gonen, A.; Spitzer, O.; Shupak, A. Evaluation of betahistine for the prevention of seasickness: Effect on vestibular function, psychomotor performance and efficacy at sea. J. Vestib. Res. 2003, 13, 103–111. [Google Scholar] [CrossRef]
- Schmäl, F. Neuronal mechanisms and the treatment of motion sickness. Pharmacology 2013, 91, 229–241. [Google Scholar] [CrossRef]
- Gordon, C.; Gonen, A.; Nachum, Z.; Doweck, I.; Spitzer, O.; Shupak, A. The effects of dimenhydrinate, cinnarizine and transdermal scopolamine on performance. J. Psychopharmacol. 2001, 15, 167–172. [Google Scholar] [CrossRef]
- Money, K. Motion sickness. Physiol. Rev. 1970, 50, 1–39. [Google Scholar] [CrossRef]
- Motamed, H.; Moezzi, M.; Rooyfard, A.D.; Angali, K.A.; Izadi, Z. A Comparison of the Effects and Side Effects of Oral Betahistine with Injectable Promethazine in the Treatment of Acute Peripheral Vertigo in Emergency. J. Clin. Med. Res. 2017, 9, 994. [Google Scholar] [CrossRef]
- Wood, C.D.; Kennedy, R.E.; Graybiel, A.; Trumbull, R.; Wherry, R.J. Clinical Effectiveness of Anti-motion-Sickness Drugs: Computer Review of the Literature. JAMA 1966, 198, 1155–1158. [Google Scholar] [CrossRef]
- Valoti, M.; Frosini, M.; Dragoni, S.; Fusi, F.; Sgaragli, G. Pharmacokinetics of diphenhydramine in healthy volunteers with a dimenhydrinate 25 mg chewing gum formulation. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Matsuki, N.; Saito, H. Suncusmurinus as a new experimental model for motion sickness. Life Sci. 1988, 43, 413–420. [Google Scholar] [CrossRef]
- Brown, J.; Sigmundson, H. Delirium from misuse of dimenhydrinate. Can. Med. Assoc. J. 1969, 101, 49. [Google Scholar]
- Shupak, A.; Gordon, C.R. Motion sickness: Advances in pathogenesis, prediction, prevention, and treatment. Aviat. Space Environ. Med. 2006, 77, 1213–1223. [Google Scholar]
- Matsnev, E.; Sigaleva, E. Efficacy of histaminergic drugs in experimental motion sickness. J. Vestib. Res. 2007, 17, 313–321. [Google Scholar] [CrossRef]
- Sugimoto, K.; Abe, K.; Lee, T.-H.; Sakurai, E.; Yanai, K.; Kogure, K.; Itoyama, Y.; Watanabe, T. Histamine depletion in brain caused by treatment with (S) α-fluoromethylhistidine enhances ischemic damage of gerbil hippocampal CA2 neurons. Brain Res. 1994, 666, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Granerus, G.; Olafsson, J.; Roupe, G. Treatment of two mastocytosis patients with a histidine decarboxylase inhibitor. Agents Actions 1985, 16, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Newby, D. Community Pharmacy ANZ-eBook: Symptoms, Diagnosis and Treatment; Elsevier Health Science: Chatswood, NSW, Australia, 2015. [Google Scholar]
- Wood, C.D.; Graybiel, A. Evaluation of Antimotion Sickness Drugs: A New Effective Remedy Revealed; Naval Aerospace Medical Inst.: Pensacola, FL, USA, 1970. [Google Scholar]
- Masso, J.M.; Obeso, J.; Carrera, N.; Martinez-Lage, J. Aggravation of Parkinson’s disease by cinnarizine. J. Neurol. Neurosurg. Psychiatry 1987, 50, 804–805. [Google Scholar] [CrossRef]
- Cowings, P.S.; Toscano, W.B.; DeRoshia, C.; Miller, N.E. Promethazine as a motion sickness treatment: Impact on human performance and mood states. Aviat. Space Environ. Med. 2000, 71, 1013–1022. [Google Scholar]
- Cantisani, C.; Ricci, S.; Grieco, T.; Paolino, G.; Faina, V.; Silvestri, E.; Calvieri, S. Topical promethazine side effects: Our experience and review of the literature. BioMed Res. Int. 2013, 2013, 151509. [Google Scholar] [CrossRef]
- Alhasso, A.A.; McKinlay, J.; Patrick, K.; Stewart, L. Anticholinergic drugs versus non-drug active therapies for overactive bladder syndrome in adults. Cochrane Database Syst. Rev. 2006, 12, CD003193. [Google Scholar]
- Weinstein, S.E.; Stern, R.M. Comparison of marezine and dramamine in preventing symptoms of motion sickness. Aviat. Space Environ. Med. 1997, 68, 890–894. [Google Scholar] [PubMed]
- Dumasia, M.; Grainger, L.; Houghton, E. Biotransformation of cyclizine in greyhounds. 1: Identification and analysis of cyclizine and some basic metabolites in canine urine by gas chromatography-mass spectrometry. Xenobiotica 2002, 32, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Pendse, V.; Madan, B. Some pharmacological actions of cyclizine, chlorcyclizine and homchlorcyclizine. Indian J. Physiol. Pharmacol. 1969, 13, 31. [Google Scholar] [PubMed]
- Wood, C.D. Pharmacological countermeasures against motion sickness. Motion Space Sick. 1990, 17, 343–352. [Google Scholar]
- Gohil, V.M.; Offner, N.; Walker, J.A.; Sheth, S.A.; Fossale, E.; Gusella, J.F.; MacDonald, M.E.; Neri, C.; Mootha, V.K. Meclizine is neuroprotective in models of Huntington’s disease. Hum. Mol. Genet. 2010, 20, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, I.; Ito, J.; Takahashi, H.; Sasa, M.; Takaori, S. Experimental vestibular pharmacology: A minireview with special reference to neuroactive substances and antivertigo drugs. Acta Oto-Laryngol. 1985, 98, 62–70. [Google Scholar] [CrossRef]
- Wood, C.D.; Graybiel, A. A theory of motion sickness based on pharmacological reactions. Clin. Pharmacol. Ther. 1970, 11, 621–629. [Google Scholar] [CrossRef]
- Yang, T.; Pei, J.; Yang, S.; Liu, Z.; Sun, R. Medical prevention of space motion sickness—Animal model of therapeutic effect of a new medicine on motion sickness. Adv. Space Res. 2002, 30, 751–755. [Google Scholar] [CrossRef]
- Parrott, A. Transdermal scopolamine: A review of its effects upon motion sickness, psychological performance, and physiological functioning. Aviat. Space Environ. Med. 1989, 60, 1–9. [Google Scholar]
- Migirov, A.; Yusupov, A. Antiemetic Antimuscarinics. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Murray, J.B. Psychophysiological aspects of motion sickness. Percept. Mot. Ski. 1997, 85, 1163–1167. [Google Scholar] [CrossRef]
- Kono, T.; Tokumaru, O.; Mizumoto, C.; Tatsuno, J.; Chen, J. Impaired gastric slow waves induced by spatial disorientation and effect of domperidone. Am. J. Gastroenterol. 1999, 94, 1224–1229. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.-L.; Mo, F.-F.; Li, M. Dexamethasone alleviates motion sickness in rats in part by enhancing the endocannabinoid system. Eur. J. Pharmacol. 2014, 727, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hempen, C.; Weiss, E.; Hess, C.F. Dexamethasone treatment in patients with brain metastases and primary brain tumors: Do the benefits outweigh the side-effects? Support. Care Cancer 2002, 10, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Polderman, J.A.; Farhang-Razi, V.; Van Dieren, S.; Kranke, P.; DeVries, J.H.; Hollmann, M.W.; Preckel, B.; Hermanides, J. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 2018, 11, CD011940. [Google Scholar] [PubMed]
- Russell, M.E.B.; Hoffman, B.; Stromberg, S.; Carlson, C.R. Use of controlled diaphragmatic breathing for the management of motion sickness in a virtual reality environment. Appl. Psychophysiol. Biofeedback 2014, 39, 269–277. [Google Scholar] [CrossRef]
- Sauder, K.A.; Johnston, E.R.; Skulas-Ray, A.C.; Campbell, T.S.; West, S.G. Effect of meal content on heart rate variability and cardiovascular reactivity to mental stress. Psychophysiology 2012, 49, 470–477. [Google Scholar] [CrossRef]
- Hayashi, K.; Suekuni, M.; Sugiyama, K. Effect of food intake on respiratory chemosensitivity to CO2 in young adults. J. Physiol. Anthropol. 2019, 38, 8. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Ratnaparkhi, A.; Sudhakaran, J. Neural pathways in nutrient sensing and insulin signaling. Front. Physiol. 2022, 13, 1002183. [Google Scholar] [CrossRef]
- Tan, R.; Li, W.; Hu, F.; Xiao, X.; Li, S.; Xing, Y.; Wang, H.; Cao, D. Motion sickness detection for intelligent vehicles: A wearable-device-based approach. In Proceedings of the 2022 IEEE 25th International Conference on Intelligent Transportation Systems (ITSC), Macau, China, 8–12 October 2022; pp. 4355–4362. [Google Scholar]
- Dennison, M.S.; Wisti, A.Z.; D’Zmura, M. Use of physiological signals to predict cybersickness. Displays 2016, 44, 42–52. [Google Scholar] [CrossRef]
- Marx, J.; Hockberger, R.; Walls, R. Rosen’s Emergency Medicine-Concepts and Clinical Practice E-Book: 2-Volume Set; Elsevier Health Sciences: Philadelphia, PA, USA, 2013. [Google Scholar]
- Van Ryckeghem, F. Corticosteroids, the oldest agent in the prevention of chemotherapy-induced nausea and vomiting: What about the guidelines? J. Transl. Int. Med. 2016, 4, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Xu, L.-H.; Chang, L.; Yin, P.; Jiang, Z.-L. Reduction of motion sickness through targeting histamine N-methyltransferase in the dorsal vagal complex of the brain. J. Pharmacol. Exp. Ther. 2018, 364, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular regulation of histamine synthesis. Front. Immunol. 2018, 9, 1392. [Google Scholar] [CrossRef]
- Gay, L.N.; Carliner, P.E. The prevention and treatment of motion sickness I. seasickness. Science 1949, 109, 359. [Google Scholar] [CrossRef] [PubMed]
- Strickland Jr, B.A.; Hahn, G.L. The effectiveness of Dramamine in the prevention of airsickness. Science 1949, 109, 359–360. [Google Scholar] [CrossRef]
- Seibel, K.; Schaffler, K.; Reitmeir, P. A randomised, placebo-controlled study comparing two formulations of dimenhydrinate with respect to efficacy in motion sickness and sedation. Arzneimittelforschung 2002, 52, 529–536. [Google Scholar] [CrossRef]
- Day, B.L.; Fitzpatrick, R.C. The vestibular system. Curr. Biol. 2005, 15, R583–R586. [Google Scholar] [CrossRef]
- Gutner, L.B.; Gould, W.J.; Batterman, R.C. Action of dimenhydrinate (Dramamine) and other drugs on vestibular function. AMa Arch. Otolaryngol. 1951, 53, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Brainard, A.; Gresham, C. Prevention and treatment of motion sickness. Am. Fam. Phys. 2014, 90, 41–46. [Google Scholar]
- Southard, B.T.; Al Khalili, Y. Promethazine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jennings, R.T. Managing space motion sickness. J. Vestib. Res. 1998, 8, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, I.; Shamsi, Z.; Stanley, N.; Fairweather, D. A double-blind, placebo-controlled investigation of the effects of fexofenadine, loratadine and promethazine on cognitive and psychomotor function. Br. J. Clin. Pharmacol. 1999, 48, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.; LeDuc, P.A.; Curry, I.P.; Phelps, S.E.; Fuller, D.R. Airsickness prevention in helicopter passengers. Aviat. Space Environ. Med. 2007, 78, 408–413. [Google Scholar]
- Cowings, P.S. Autogenic-Feedback Training Exercise (AFTE) Method and System. U.S. Patent US5694939A, 9 December 1997. [Google Scholar]
- Cowings, P.S.; Toscano, W.B. Autogenic-feedback training exercise is superior to promethazine for control of motion sickness symptoms. J. Clin. Pharmacol. 2000, 40, 1154–1165. [Google Scholar] [CrossRef]
- Arrang, J.-M.; Garbarg, M.; Quach, T.T.; Tuong, M.D.T.; Yeramian, E.; Schwartz, J.-C. Actions of betahistine at histamine receptors in the brain. Eur. J. Pharmacol. 1985, 111, 73–84. [Google Scholar] [CrossRef]
- Duflo, S.G.D.; Gestreau, C.; Lacour, M. Fos expression in the rat brain after exposure to gravito-inertial force changes. Brain Res. 2000, 861, 333–344. [Google Scholar] [CrossRef]
- Kirtane, M.V.; Bhandari, A.; Narang, P.; Santani, R. Cinnarizine: A contemporary review. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 1060–1068. [Google Scholar] [CrossRef]
- Emanuel, M.; Will, J. Cinnarizine in the treatment of peripheral vascular disease: Mechanisms related to its clinical action. Proc. R. Soc. Med. 1977, 70, 7–12. [Google Scholar] [CrossRef]
- Gil, A.; Nachum, Z.; Tal, D.; Shupak, A. A comparison of cinnarizine and transdermal scopolamine for the prevention of seasickness in naval crew: A double-blind, randomized, crossover study. Clin. Neuropharmacol. 2012, 35, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, S.; Pathak, K. Recent advances in delivery systems and therapeutics of cinnarizine: A poorly water soluble drug with absorption window in stomach. J. Drug Deliv. 2014, 2014, 479246. [Google Scholar] [CrossRef]
- Arab, S.F.; Düwel, P.; Jüngling, E.; Westhofen, M.; Lückhoff, A. Inhibition of voltage-gated calcium currents in type II vestibular hair cells by cinnarizine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Plescia, F.; Salvago, P.; Dispenza, F.; Messina, G.; Cannizzaro, E.; Martines, F. Efficacy and pharmacological appropriateness of cinnarizine and dimenhydrinate in the treatment of vertigo and related symptoms. Int. J. Environ. Res. Public Health 2021, 18, 4787. [Google Scholar] [CrossRef] [PubMed]
- Haress, N.G. Cinnarizine: Comprehensive Profile. Profiles Drug Subst. Excip. Relat. Methodol. 2015, 40, 1–41. [Google Scholar]
- Yao, X.; Gao, S.; Yan, N. Structural basis for pore blockade of human voltage-gated calcium channel Cav1. 3 by motion sickness drug cinnarizine. Cell Res. 2022, 32, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Doweck, I.; Gordon, C.R.; Spitzer, O.; Melamed, Y.; Shupak, A. Effect of cinnarizine in the prevention of seasickness. Aviat. Space Environ. Med. 1994, 65, 606–609. [Google Scholar]
- Powell-Dunford, N.; Bushby, A. Management of sea sickness in susceptible flight crews. Mil. Med. 2017, 182, e1846–e1850. [Google Scholar] [CrossRef]
- Weerts, A.; Pattyn, N.; Van de Heyning, P.; Wuyts, F. Evaluation of the effects of anti-motion sickness drugs on subjective sleepiness and cognitive performance of healthy males. J. Psychopharmacol. 2014, 28, 655–664. [Google Scholar] [CrossRef]
- Patel, P.N.; Ambizas, E.M. Meclizine: Safety and Efficacy in the Treatment and Prevention of Motion Sickness. Clin. Med. Insights Ther. 2011, 3, CMT.S6237. [Google Scholar] [CrossRef]
- Dobie, T.G.; Dobie, T.G. Pharmacological treatment of motion sickness. Motion Sick. A Motion Adapt. Syndr. 2019, 65, 183–217. [Google Scholar]
- Yoshikawa, T.; Nakamura, T.; Shibakusa, T.; Sugita, M.; Naganuma, F.; Iida, T.; Miura, Y.; Mohsen, A.; Harada, R.; Yanai, K. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J. Nutr. 2014, 144, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, N. Expression of histidine decarboxylase and its roles in inflammation. Int. J. Mol. Sci. 2019, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Takehiko, W.; Atsushi, Y.; Kazutaka, M.; Hiroshi, W. Pharmacology of α fluoromethylhistidine, a specific inhibitor of histidine decarboxylase. Trends Pharmacol. Sci. 1990, 11, 363–367. [Google Scholar] [CrossRef]
- Bender, A.; Scheiber, J.; Glick, M.; Davies, J.W.; Azzaoui, K.; Hamon, J.; Urban, L.; Whitebread, S.; Jenkins, J.L. Analysis of pharmacology data and the prediction of adverse drug reactions and off-target effects from chemical structure. ChemMedChem Chem. Enabling Drug Discov. 2007, 2, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, L.M. Motion sickness may be caused by a neurohumoral action of acetylcholine. Med. Hypotheses 2009, 73, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Golding, J.F.; Wesnes, K.A.; Leaker, B.R. The effects of the selective muscarinic M3 receptor antagonist darifenacin, and of hyoscine (scopolamine), on motion sickness, skin conductance & cognitive function. Br. J. Clin. Pharmacol. 2018, 84, 1535–1543. [Google Scholar] [PubMed]
- Spinks, A.; Wasiak, J. Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Syst. Rev. 2011, 2011, CD002851. [Google Scholar] [CrossRef]
- Golding, J.; Stott, J. Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. Br. J. Clin. Pharmacol. 1997, 43, 633–637. [Google Scholar] [CrossRef]
- Flicker, C.; Ferris, S.H.; Serby, M. Hypersensitivity to scopolamine in the elderly. Psychopharmacology 1992, 107, 437–441. [Google Scholar] [CrossRef]
- Mollayeva, T.; Shapiro, C.M. Medication Effects. In Encyclopedia of Sleep; Kushida, C.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 330–337. [Google Scholar] [CrossRef]
- Sherman, C.R. Motion Sickness: Review of Causes and Preventive Strategies. J. Travel Med. 2002, 9, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Khilnani, A.K.; Thaddanee, R.; Khilnani, G. Anti vertigo Drugs-Revisited. NJIRM 2013, 4, 118–128. [Google Scholar]
- Zhang, L.L.; Wang, J.Q.; Qi, R.R.; Pan, L.L.; Li, M.; Cai, Y.L. Motion Sickness: Current Knowledge and Recent Advance. CNS Neurosci. Ther. 2016, 22, 15–24. [Google Scholar] [CrossRef]
- Wood, C.D.; Stewart, J.J.; Wood, M.J.; Struve, F.A.; Straumanis, J.J.; Mims, M.E.; Patrick, G.Y. Habituation and motion sickness. J. Clin. Pharmacol. 1994, 34, 628–634. [Google Scholar]
- Koranteng, R.D.; Swindle, E.J.; Davis, B.J.; Dearman, R.J.; Kimber, I.; Flanagan, B.F.; Coleman, J.W. Differential regulation of mast cell cytokines by both dexamethasone and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580. Clin. Exp. Immunol. 2004, 137, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Brunton, L.L. Goodman & Gilman’s The Pharmacological Basis of Therapeutics; Mc Graw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Tsurufuji, S.; Sugio, K.; Takemasa, F. The role of glucocorticoid receptor and gene expression in the anti-inflammatory action of dexamethasone. Nature 1979, 280, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Koyama, T. Effects of acute and chronic administration of high-dose corticosterone and dexamethasone on regional brain dopamine and serotonin metabolism in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1996, 20, 147–156. [Google Scholar] [CrossRef]
- Kohl, R.L.; Lewis, M.R. Mechanisms underlying the antimotion sickness effects of psychostimulants. Aviat. Space Environ. Med. 1987, 58, 1215–1218. [Google Scholar]
- Imming, P.; Sinning, C.; Meyer, A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006, 5, 821–834. [Google Scholar] [CrossRef]
- Renaud, J.-P.; Chung, C.-w.; Danielson, U.H.; Egner, U.; Hennig, M.; Hubbard, R.E.; Nar, H. Biophysics in drug discovery: Impact, challenges and opportunities. Nat. Rev. Drug Discov. 2016, 15, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Jarisch, R.; Weyer, D.; Ehlert, E.; Koch, C.; Pinkowski, E.; Jung, P.; Kähler, W.; Girgensohn, R.; Kowalski, J.; Weisser, B. Impact of oral vitamin C on histamine levels and seasickness. J. Vestib. Res. 2014, 24, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.M.R.; Schieber, A. Bioactive compounds in mango (Mangifera indica L.). In Bioactive Foods in Promoting Health; Elsevier Publisher: Oxford, UK, 2010; pp. 507–523. [Google Scholar]
- Masibo, M.; He, Q. Mango bioactive compounds and related nutraceutical properties—A review. Food Rev. Int. 2009, 25, 346–370. [Google Scholar] [CrossRef]
- Kohl, R.L.; Lacey, C.L.; Homick, J.L. An appraisal of the value of vitamin B12 in the prevention of motion sickness. Acta Astronaut. 1983, 10, 219–224. [Google Scholar] [CrossRef]
- Uijtdehaage, S.H.; Stern, R.M.; Koch, K.L. Effects of eating on vection-induced motion sickness, cardiac vagal tone, and gastric myoelectric activity. Psychophysiology 1992, 29, 193–201. [Google Scholar] [CrossRef]
- Williamson, M.; Levine, M.; Stern, R. The effect of meals of varying nutritional composition on subjective and physiological markers of nausea in response to optokinetic motion. Digestion 2005, 72, 254–260. [Google Scholar] [CrossRef]
- Mühlbacher, D.; Tomzig, M.; Reinmüller, K.; Rittger, L. Methodological considerations concerning motion sickness investigations during automated driving. Information 2020, 11, 265. [Google Scholar] [CrossRef]
- Feinle, C.; Grundy, D.; Read, N.W. Fat increases vection-induced nausea independent of changes in gastric emptying. Physiol. Behav. 1995, 58, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Horowitz, M.; Feinle-Bisset, C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: Implications for the pathophysiology of obesity. Am. J. Clin. Nutr. 2007, 86, 531–541. [Google Scholar] [CrossRef]
- Stewart, J.J.; Wood, M.J.; Wood, C.D.; Mims, M.E. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology 1991, 42, 111–120. [Google Scholar] [CrossRef]
- Wood, M.; Wood, C.; Manno, J.; Manno, B.; Redetzki, H. Nuclear medicine evaluation of motion sickness and medications on gastric emptying time. Aviat. Space Environ. Med. 1987, 58, 1112–1114. [Google Scholar] [PubMed]
- Stewart, J.J.; Wood, M.J.; Wood, C.D.; Mims, M.E. Effects of motion sickness and antimotion sickness drugs on gastric function. J. Clin. Pharmacol. 1994, 34, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, G.W.; Lin, H.C. Ileal brake: Neuropeptidergic control of intestinal transit. Curr. Gastroenterol. Rep. 2006, 8, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Mera, Y.; Ishii, Y.; Tadaki, H.; Tomimoto, D.; Kuroki, Y.; Kawai, T.; Ohta, T.; Kakutani, M. JTT-130, a novel intestine-specific inhibitor of microsomal triglyceride transfer protein, suppresses food intake and gastric emptying with the elevation of plasma peptide YY and glucagon-like peptide-1 in a dietary fat-dependent manner. J. Pharmacol. Exp. Ther. 2011, 336, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Williamson, M.J.; Muth, E.R.; Stern, R.M. The effect of liquid carbohydrate and protein meais on gastric tachyarrhythmia and susceptibility to vection-induced motion sickness. Gastroenterology 2001, 5, A716–A717. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Y.; Chen, S.; Li, F.; Chen, X.; Liu, Y. Integrated metabolomics and network pharmacology approach to exploring the potential mechanism of tianxiang capsule for treating motion sickness. J. Ethnopharmacol. 2021, 275, 114107. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.-C.; Sun, W.M.; Chen, Y.-H.; Kim, H.; Hasler, W.; Owyang, C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G481–G489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Zhang, M.; Fu, X. Three statistical experimental designs for enhancing yield of active compounds from herbal medicines and anti-motion sickness bioactivity. Pharmacogn. Mag. 2015, 11, 435. [Google Scholar]
- Weimer, K.; Schulte, J.; Maichle, A.; Muth, E.R.; Scisco, J.L.; Horing, B.; Enck, P.; Klosterhalfen, S. Effects of ginger and expectations on symptoms of nausea in a balanced placebo design. PLoS ONE 2012, 7, e49031. [Google Scholar] [CrossRef]
- Grøntved, A.; Brask, T.; Kambskard, J.; Hentzer, E. Ginger root against seasickness: A conctrolled trial on the open sea. Acta Oto-Laryngol. 1988, 105, 45–49. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Zaghlool, S.S.; Shehata, B.A.; Abo-Seif, A.A.; Abd El-Latif, H.A. Protective effects of ginger and marshmallow extracts on indomethacin-induced peptic ulcer in rats. J. Nat. Sci. Biol. Med. 2015, 6, 421. [Google Scholar] [CrossRef] [PubMed]
- Ilaiyaraja, N.; Singsit, D.; Patil, M.M.; Priyadharshini, S.; Rashmi, V.; Khanum, F. Motion sickness-relieving effects of Tamzin, a herbal formulation: In vitro and in vivo studies. Food Biosci. 2020, 35, 100595. [Google Scholar] [CrossRef]
- Zhong, W.; Zhu, J.; Yi, J.; Zhao, C.; Shi, Y.; Kang, Q.; Huang, J.; Hao, L.; Lu, J. Biochemical analysis reveals the systematic response of motion sickness mice to ginger (Zingiber officinale) extract’s amelioration effect. J. Ethnopharmacol. 2022, 290, 115077. [Google Scholar] [CrossRef]
- Maheswari, D.U.; Anand, T.; Padma, A.; Ilaiyaraja, N.; Khanum, F. Evaluation of the effect of herbal extracts and their bioactive compounds against motion sickness by regulating neurotransmitter levels in vitro and in vivo. S. Afr. J. Bot. 2020, 130, 130–140. [Google Scholar] [CrossRef]
- Heimes, K.; Hauk, F.; Verspohl, E.J. Mode of action of peppermint oil and (-)-menthol with respect to 5-HT3 receptor subtypes: Binding studies, cation uptake by receptor channels and contraction of isolated rat ileum. Phytother. Res. 2011, 25, 702–708. [Google Scholar] [CrossRef]
- Masruroh, N.; Safitri, Y.I.; Laili, U.; Andriani, R.A.D.; Abidah, S.N. The Effectiveness Of Giving Ginger And Mint Leaves To The Incidence Of Emesis Gravidarum. Int. J. Psychosoc. Rehabil. 2020, 24, 1003–1010. [Google Scholar]
- Oz, M.; El Nebrisi, E.G.; Yang, K.-H.S.; Howarth, F.C.; Al Kury, L.T. Cellular and molecular targets of menthol actions. Front. Pharmacol. 2017, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, E. Herbs for Motion Sickness. Altern. Complement. Ther. 2016, 22, 74–78. [Google Scholar] [CrossRef]
- Takumida, M.; Ishibashi, T.; Hamamoto, T.; Hirakawa, K.; Anniko, M. Expression of transient receptor potential channel melastin (TRPM) 1–8 and TRPA1 (ankyrin) in mouse inner ear. Acta Oto-Laryngol. 2009, 129, 1050–1060. [Google Scholar] [CrossRef]
- Deshetty, U.M.; Tamatam, A.; Bhattacharjee, M.; Perumal, E.; Natarajan, G.; Khanum, F. Ameliorative effect of hesperidin against motion sickness by modulating histamine and histamine H1 receptor expression. Neurochem. Res. 2020, 45, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Shahbaz, O.; Teskey, G.; Beever, A.; Kachour, N.; Venketaraman, V.; Darmani, N.A. Mechanisms of nausea and vomiting: Current knowledge and recent advances in intracellular emetic signaling systems. Int. J. Mol. Sci. 2021, 22, 5797. [Google Scholar] [CrossRef] [PubMed]
- Deshetty, U.M.; Tamatam, A.; Patil, M.M. Menthol, a bioactive constituent of Mentha, attenuates motion sickness in mice model: Involvement of dopaminergic system. J. Food Biochem. 2021, 45, e13863. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.P.; de Campos Rodrigues, C.; Cardoso, C.A.F.; Cytrynbaum, N.; Kaufman, R.; Rzetelna, H.; Goldwasser, G.; Santos, A.; Oliveira, L.; Geller, M. Clinical Evaluation of the Use of Ginger Extract in the Preventive Management of Motion Sickness. Curr. Ther. Res. 2020, 92, 100591. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Wood, M.J.; Wood, C.D. Electrogastrograms during motion sickness in fasted and fed subjects. Aviat. Space Environ. Med. 1989, 60, 214–217. [Google Scholar] [PubMed]
- Hu, S.; Grant, W.F.; Stern, R.M.; Koch, K.L. Motion sickness severity and physiological correlates during repeated exposures to a rotating optokinetic drum. Aviat. Space Environ. Med. 1991, 62, 308–314. [Google Scholar]
- Robb, S.L. Music assisted progressive muscle relaxation, progressive muscle relaxation, music listening, and silence: A comparison of relaxation techniques. J. Music Ther. 2000, 37, 2–21. [Google Scholar] [CrossRef]
- Sang, F.D.Y.P.; Billar, J.P.; Golding, J.F.; Gresty, M.A. Behavioral methods of alleviating motion sickness: Effectiveness of controlled breathing and a music audiotape. J. Travel Med. 2003, 10, 108–111. [Google Scholar] [CrossRef]
- Keshavarz, B.; Hecht, H. Pleasant music as a countermeasure against visually induced motion sickness. Appl. Ergon. 2014, 45, 521–527. [Google Scholar] [CrossRef]
- Peck, K.; Russo, F.; Campos, J.L.; Keshavarz, B. Examining potential effects of arousal, valence, and likability of music on visually induced motion sickness. Exp. Brain Res. 2020, 238, 2347–2358. [Google Scholar] [CrossRef]
- Jerath, R.; Edry, J.W.; Barnes, V.A.; Jerath, V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 2006, 67, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.N.; Porta, C.; Casucci, G.; Casiraghi, N.; Maffeis, M.; Rossi, M.; Bernardi, L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 2005, 46, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.M.; Gevirtz, R. Heart rate variability biofeedback: How and why does it work? Front. Psychol. 2014, 5, 756. [Google Scholar] [PubMed]
- Choukèr, A.; Kaufmann, I.; Kreth, S.; Hauer, D.; Feuerecker, M.; Thieme, D.; Vogeser, M.; Thiel, M.; Schelling, G. Motion sickness, stress and the endocannabinoid system. PLoS ONE 2010, 5, e10752. [Google Scholar] [CrossRef]
- Martarelli, D.; Cocchioni, M.; Scuri, S.; Pompei, P. Diaphragmatic breathing reduces exercise-induced oxidative stress. Evid.-Based Complement. Altern. Med. 2011, 2011, 932430. [Google Scholar] [CrossRef]
- Paillard, A.; Lamôré, M.; Etard, O.; Millot, J.-L.; Jacquot, L.; Denise, P.; Quarck, G. Is there a relationship between odors and motion sickness? Neurosci. Lett. 2014, 566, 326–330. [Google Scholar]
- Keshavarz, B.; Stelzmann, D.; Paillard, A.; Hecht, H. Visually induced motion sickness can be alleviated by pleasant odors. Exp. Brain Res. 2015, 233, 1353–1364. [Google Scholar] [CrossRef]
- Sharma, K. Prevalence and correlates of susceptibility to motion sickness. Acta Genet. Med. Gemellol. Twin Res. 1997, 46, 105–121. [Google Scholar] [CrossRef]
- Tadese, Z.; Teshome, B.; Mengistu, E. Factors affecting car sickness of passengers traveled by vehicles in North Shewa Zone, Oromia, Ethiopia. J. Environ. Public Health 2022, 2022, 6642603. [Google Scholar] [CrossRef]
- Belani, K.; Sessler, D.I.; Sessler, A.M.; Schroeder, M.; McGuire, J.; Merrifield, B.; Washington, D.E.; Moayeri, A. Leg heat content continues to decrease during the core temperature plateau in humans anesthetized with isoflurane. J. Am. Soc. Anesthesiol. 1993, 78, 856–863. [Google Scholar]
- Saunders, A.; Dugas, J.; Tucker, R.; Lambert, M.; Noakes, T. The effects of different air velocities on heat storage and body temperature in humans cycling in a hot, humid environment. Acta Physiol. Scand. 2005, 183, 241–255. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, S.; Bos, J.E.; Keshavarz, B. The efficacy of airflow and seat vibration on reducing visually induced motion sickness. Exp. Brain Res. 2017, 235, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Acromite, M.T.; Cowings, P.; Toscano, W.; Davis, C.; Porter, H.O. NASA-Navy telemedicine: Autogenic feedback training exercises for motion sickness. In Proceedings of the 81st Annual Scientific Meeting Aerospace Medical Association, Phoenix, AZ, USA, 9–13 May 2010. [Google Scholar]
- Walton, N.; Spencer, T.; Cowings, P.; Toscano, W.B. Autogenic Feedback Training Exercise: Controlling Physiological Responses to Mitigate Motion Sickness; NASA Technical Reports Server (NTRS): Atlanta, GA, USA, 2018.
- Cowings, P.S.; Toscano, W.B.; Reschke, M.F.; Gebreyesus, F.; Rocha, C. Autogenic-Feedback Training Exercise (AFTE) Mitigates the Effects of Spatial Disorientation to Simulated Orion Spacecraft Re-Entry: Individual Differences; NASA Langley Research Center: Hampton, VA, USA, 2017.
- Cowings, P.S. Autogenic-feedback training: A treatment for motion and space sickness. In Motion and Space Sickness; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 353–372. [Google Scholar]
- Golding, J.F.; Paillard, A.C.; Normand, H.; Besnard, S.; Denise, P. Prevalence, predictors, and prevention of motion sickness in zero-G parabolic flights. Aerosp. Med. Hum. Perform. 2017, 88, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mittelstaedt, J.M. Individual predictors of the susceptibility for motion-related sickness: A systematic review. J. Vestib. Res. 2020, 30, 165–193. [Google Scholar] [CrossRef]
- Palmisano, S.; Constable, R. Reductions in sickness with repeated exposure to HMD-based virtual reality appear to be game-specific. Virtual Real. 2022, 26, 1373–1389. [Google Scholar] [CrossRef]
- Hill, K.J.; Howarth, P.A. Habituation to the side effects of immersion in a virtual environment. Displays 2000, 21, 25–30. [Google Scholar] [CrossRef]
- Huang, Y.-D.; Xia, S.-W.; Dai, P.; Han, D.-Y. Role of AQP1 in inner ear in motion sickness. Physiol. Behav. 2011, 104, 749–753. [Google Scholar] [CrossRef]
- Benson, A.J. Motion sickness. In Aviation Medicine; Ernsting, J., King, P., Eds.; Butterworths: London, UK, 1988; pp. 318–338. [Google Scholar]
- Sang, F.Y.P.; Billar, J.; Gresty, M.A.; Golding, J.F. Effect of a novel motion desensitization training regime and controlled breathing on habituation to motion sickness. Percept. Mot. Ski. 2005, 101, 244–256. [Google Scholar] [CrossRef]
- Cowings, P.S.; Toscano, W.B. Autogenic-Feedback Training (AFT) as a Preventive Method for Space Motion Sickness: Background and Experimental Design; Ames Research Center: Moffett Reid, CA, USA, 1993.
- Gahlinger, P.M. Motion sickness: How to help your patients avoid travel travail. Postgrad. Med. 1999, 106, 177–184. [Google Scholar] [CrossRef]
- Leung, A.K.; Hon, K.L. Motion sickness: An overview. Drugs Context 2019, 8, 2019-9-4. [Google Scholar] [CrossRef]
- Koca, C.F.; Bayinir, T. Review of pathophysiology, epidemiology, diagnosis and treatment methods in motion sickness; a special issue. J. Turgut Ozal Med. Cent. 2017, 24, 365–367. [Google Scholar] [CrossRef]
- Mowrey, D.; Clayson, D. Motion sickness, ginger, and psychophysics. Lancet 1982, 319, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Chapes, S.K.; Simske, S.J.; Sonnenfeld, G.; Miller, E.S.; Zimmerman, R.J. Effects of spaceflight and PEG-IL-2 on rat physiological and immunological responses. J. Appl. Physiol. (1985) 1999, 86, 2065–2076. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Rioux, L.-E. Food matrix impact on macronutrients nutritional properties. Food Hydrocoll. 2011, 25, 1915–1924. [Google Scholar] [CrossRef]
- Alessandrini, R.; He, F.J.; Ma, Y.; Scrutinio, V.; Wald, D.S.; MacGregor, G.A. Potential impact of gradual reduction of fat content in manufactured and out-of-home food on obesity in the United Kingdom: A modeling study. Am. J. Clin. Nutr. 2021, 113, 1312–1321. [Google Scholar] [CrossRef]
- Redfield, M.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Mahoney, D.W.; Bailey, K.R.; Burnett, J.C. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002, 40, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.C.; Van Zandt, E.C. A meta-analysis of the virtual reality problem: Unequal effects of virtual reality sickness across individual differences. Virtual Real. 2021, 25, 1221–1246. [Google Scholar] [CrossRef]
- Takeda, N.; Hasegawa, S.; Morita, M.; Matsunaga, T. Pica in rats is analogous to emesis: An animal model in emesis research. Pharmacol. Biochem. Behav. 1993, 45, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Sanger, G.J.; Andrews, P.L. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front. Pharmacol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Lin, C.-T.; Chuang, C.-H.; Wang, Y.-K.; Tsai, S.-F.; Chiu, T.-C.; Ko, L.-W. Neurocognitive characteristics of the driver: A review on drowsiness, distraction, navigation, and motion sickness. J. Neurosci. Neuroeng. 2012, 1, 61–81. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lai, P.-C.; Ko, L.-W.; Chuang, C.-H.; Kuo, B.-C.; Lin, C.-T. An EEG-based classification system of Passenger’s motion sickness level by using feature extraction/selection technologies. In Proceedings of the 2010 International Joint Conference on Neural Networks (IJCNN), Barcelona, Spain, 18–23 July 2010; pp. 1–6. [Google Scholar]
- Padmanaban, N.; Ruban, T.; Sitzmann, V.; Norcia, A.M.; Wetzstein, G. Towards a machine-learning approach for sickness prediction in 360 stereoscopic videos. IEEE Trans. Vis. Comput. Graph. 2018, 24, 1594–1603. [Google Scholar] [CrossRef]
- Hell, S.; Argyriou, V. Machine learning architectures to predict motion sickness using a virtual reality rollercoaster simulation tool. In Proceedings of the 2018 IEEE International Conference on Artificial Intelligence and Virtual Reality (AIVR), Taichung, Taiwan, 10–12 December 2018; pp. 153–156. [Google Scholar]
- Lee, T.M.; Yoon, J.-C.; Lee, I.-K. Motion sickness prediction in stereoscopic videos using 3d convolutional neural networks. IEEE Trans. Vis. Comput. Graph. 2019, 25, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Yoo, S.; Jang, Y. Motion sickness measurement and analysis in virtual reality using deep neural networks algorithm. J. Korea Comput. Graph. Soc. 2019, 25, 23–32. [Google Scholar] [CrossRef]
- Martin, N.; Mathieu, N.; Pallamin, N.; Ragot, M.; Diverrez, J.-M. Virtual reality sickness detection: An approach based on physiological signals and machine learning. In Proceedings of the 2020 IEEE International Symposium on Mixed and Augmented Reality (ISMAR), Virtual, 9–11 November 2020; pp. 387–399. [Google Scholar]
- Keshavarz, B.; Peck, K.; Rezaei, S.; Taati, B. Detecting and predicting visually induced motion sickness with physiological measures in combination with machine learning techniques. Int. J. Psychophysiol. 2022, 176, 14–26. [Google Scholar] [CrossRef] [PubMed]

| Name | Protection Index (%) | Mechanism | Used Dosage | Side Effects | References |
|---|---|---|---|---|---|
| dimenhydrinate | 72.91 | Histamine H1 receptors antagonist | 25 mg to 50 mg 32 (mg/kg) | Headache, drowsiness, blurred vision, eye irritation, drowsy, dizziness, concentration difficulty, fatigue, euphoria, and hallucinations | [30,33,34,35,36] |
| betahistine | weak effect | H3 presynaptic antagonist and a partial H1 postsynaptic agonist | 8 mg–32 mg | Nausea and vomiting, gastric upset and decreased appetite | [28,32,37,38] |
| α-fluoromethylhistidine (α-FMH), | Not mentioned | Histidine decarboxylase inhibitor | 100–200 mg/kg | Sedation effect | [39,40] |
| cinnarizine | Not mentioned | Histamine H1 antagonist | 50 mg | Drowsiness, dry mouth, sedation and, Parkinson’s disease | [30,41,42,43] |
| promethazine | 78 64.33 | Histamine H1 receptors antagonist | 25 to 50 mg | Drowsiness—akathisia—Restlessness dry mouth and extrasystoles | [32,35,44,45,46] |
| cyclizine | 71.24 | Histamine H1 receptors antagonist | 50 mg 3 times a day | Dry mouth, blurred vision, and drowsiness | [33,47,48,49] |
| meclizine | 67.98 | Histamine H1 receptors antagonist. | 20 mg/kg | Drowsiness, dry mouth, and constipation | [33,35,50,51] |
| scopolamine | 78 62.96 | Acetylcholine antagonist | 0.6–1.2 mg for adults 0.25 mg for children | Dry mouth and drowsiness Reduced memoryblurred vision, headache, nausea, abdominal pain, dizziness, sweating, tachycardia, urinary retention, and acute angle-closure glaucoma. | [31,33,35,52,53,54,55,56] |
| dextroamphetamine | 64 | Not well-described | 5 to 10 mg in combination with other antihistamines | Dizziness, dry mouth, blurred vision, anxiety, drowsiness, and the risk of drug dependence | [29,33,57,58] |
| dexamethasone | Not mentioned | Increased endocannabinoids | 0.05 mg/kg | Delayed wound healing, high blood pressure, hyperglycemia muscular weakness, and gastrointestinal disorders | [59,60,61] |
| Food Type | Number of Participants Age and Sex | Study Design | Used Dosage | Apparatus | Mechanism | Effect on MS | References |
|---|---|---|---|---|---|---|---|
| Ginger | N = 64: 32 females, 32 males 20–38 years | balanced placebo design’ | 1000 mg | rotating vection drum | not identified | unchanged | [142] |
| N = 184: 18–65 years | open single-arm study | 160 mg | trip for 15 min | not identified | unchanged | [157] | |
| N = 18: 8 males and 10 females; 18–40 years | double-blind, randomized, placebo-controlled study | 1000 mg 2000 mg | circular vection | prevent the release of vasopressin | reduced nausea | [140] | |
| N = 80: 16–19 years | double-blind randomized design | 1000 mg | full-rigged training-ship | Not identified | unchanged | [143] | |
| Tamazin | N = 36 mice | randomized trial | 400 mg/kg.bw 800 mg/kg.bw 1200 mg/kg.bw | self-manufactured rotation device | reduced histamine level | reduced | [146] |
| Tianxiang | N = 60: male rats | randomized trial | 0.91 g/kg, 1.82 g/kg and 3.64 g/kg per day, | biaxial rotating acceleration stimulator | reduced histamine and acetylcholine | reduced | [139] |
| Menthol | N = 36, female mice | randomized trial | 50 µg/mL | custom-designed centrifuge machine | reduced dopamine | reduced | [156] |
| Hesperidin | N = 36 female mice | randomized trial | 80 mg/kg bw | custom-designed centrifuge machine | reduced histamine and GABA | reduced | [154]. |
| Protein | N = 18: 15 femalesand 3 males; 18 to 20 years | repeated measure design | 53%protein + 12% carbohydrate and 35% fat | rotating optokinetic drum | Increased parasympathetic tone. | reduced | [4] |
| N = 57: 49 males and 8 femals; 18 to 36 years | descriptive correlational study | Not mentioned | real flight | Not mentioned | increased | [5] | |
| Carbohydrate | N = 108: 68 females and 40 males; 18–23 years | A double blind, independent subject design with modified random assignment | 49.2 g carbohydrate, 4.8 g fat, 12 g protein, | rotating optokinetic drum | Not mentioned | not effective reduced. | [4,5] [128] |
| Fat | N = 12: 12 males; 22 to 36 years | Randomized trial | -high-fat meal: 30 g water +30 g margarine. -low fat meal: 50 g beef (1.5 g carbohydrate, 3.6 g protein) + 150 g water, | Rotating vection drum | Not mentioned | increased | [130] |
| Vitamin C | N = 70: 20 females +50 males; 19 to 60 years | Double-blind placebo-controlled crossover study | 500 mg | Inflatable life raft exposed to 1-m-high waves. | decreased histamine level | reduced | [123] |
| Vitamin B12 | N = not mentioned (18 years or older) | Not mentioned | 1000 μg, | Rotator chair assembly | Not mentioned | not effective | [126] |
| Sodium | N = 57 (49 males and 8 females; 18–34 years) | Descriptive correlational study | Not identified | Real flight | Not mentioned | increased | [5] |
| Thiamine | N = 57: 49 males and 8 females; 18–34 years | Descriptive correlational study | Not identified | Real flight | Not mentioned | increased | [5] |
| Calcium | N = 57: 49 males and 8 females; 18–34 years | Descriptive, correlational study | Not identified | Real flight | Not mentioned | increased | [5] |
| Yogurt | N = 7 (age and gender have not mentioned) | Not mentioned | Not identified | Coriolis stimulation (rotating chair) | Not mentioned | increased | [158] |
| Mango | Not mentioned | Observation report | 2–3 mangos | Sailing trips | Not mentioned | decrease | [123]. |
| Food composition | N = 40: 21 males and 19 females; 19.3 mean years | Repeated-measure, counterbalanced crossover design. | 10% protein, 30% fat, and 60% carbohydrates | Rotating drum | increased parasympathetic tone | decreased | [127] |
| Study | Year | Environment | Participants | ML or DL | UQ |
|---|---|---|---|---|---|
| Yu et al. [200] | 2010 | Realistic driving environment | N/A | ML | No |
| Padmanaban et al. [201] | 2018 | 3D videos | N/A | ML | No |
| Hell and Argyriou [202] | 2018 | Rollercoaster simulation tool | 23 subjects | DL | No |
| Li et al. [202] | 2019 | Car driving video | 20 subjects | ML | No |
| Lee et al. [203] | 2019 | 360° stereoscopic video | 19 videos | DL | No |
| Jeong et al. [204] | 2019 | 360° video | N/A | DL | No |
| Martin et al. [205] | 2022 | Video game sessions | 103 subjects | ML | No |
| Keshavarz et al. [206] | 2022 | Video of a bicycle ride | 43 subjects | ML | No |
| Tan et al. [67] | 2022 | On-road driving scenario | 12 subjects | ML | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimzadeh, G.; Tay, A.; Travica, N.; Lacy, K.; Mohamed, S.; Nahavandi, D.; Pławiak, P.; Qazani, M.C.; Asadi, H. Nutritional and Behavioral Countermeasures as Medication Approaches to Relieve Motion Sickness: A Comprehensive Review. Nutrients 2023, 15, 1320. https://doi.org/10.3390/nu15061320
Rahimzadeh G, Tay A, Travica N, Lacy K, Mohamed S, Nahavandi D, Pławiak P, Qazani MC, Asadi H. Nutritional and Behavioral Countermeasures as Medication Approaches to Relieve Motion Sickness: A Comprehensive Review. Nutrients. 2023; 15(6):1320. https://doi.org/10.3390/nu15061320
Chicago/Turabian StyleRahimzadeh, Ghazal, Abdullatif Tay, Nikolaj Travica, Kathleen Lacy, Shady Mohamed, Darius Nahavandi, Paweł Pławiak, Mohammadreza Chalak Qazani, and Houshyar Asadi. 2023. "Nutritional and Behavioral Countermeasures as Medication Approaches to Relieve Motion Sickness: A Comprehensive Review" Nutrients 15, no. 6: 1320. https://doi.org/10.3390/nu15061320
APA StyleRahimzadeh, G., Tay, A., Travica, N., Lacy, K., Mohamed, S., Nahavandi, D., Pławiak, P., Qazani, M. C., & Asadi, H. (2023). Nutritional and Behavioral Countermeasures as Medication Approaches to Relieve Motion Sickness: A Comprehensive Review. Nutrients, 15(6), 1320. https://doi.org/10.3390/nu15061320









