Effects of a Synbiotic on Plasma Immune Activity Markers and Short-Chain Fatty Acids in Children and Adults with ADHD—A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Interventions
2.3. Analysis of Plasma Immune Activity Markers
2.4. Analysis of Plasma Short-Chain Fatty Acids (SCFAs)
2.5. Cell Culture
2.6. Statistical Analysis
3. Results
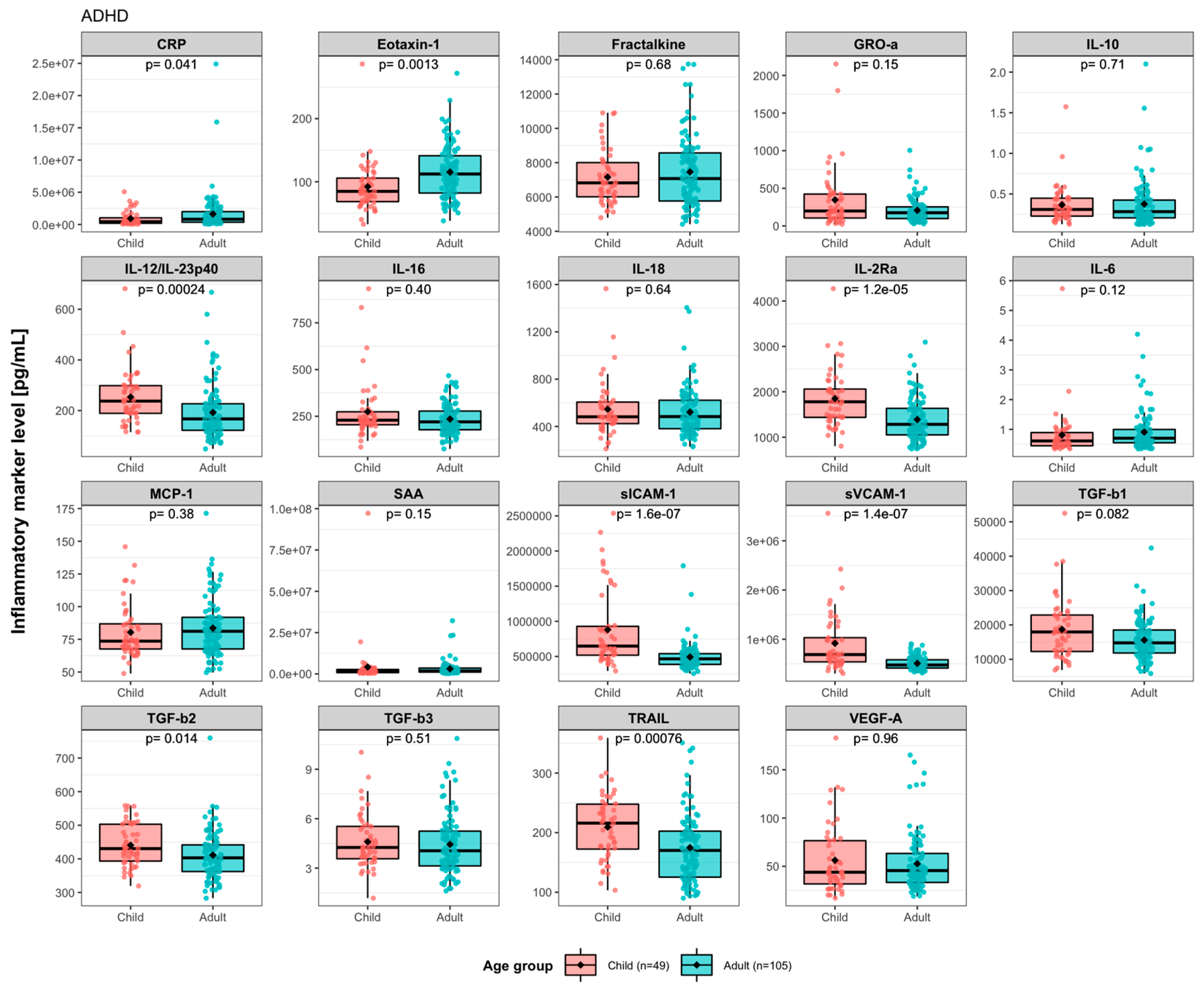
3.1. Baseline Levels of Immune Activity Markers in ADHD Patients
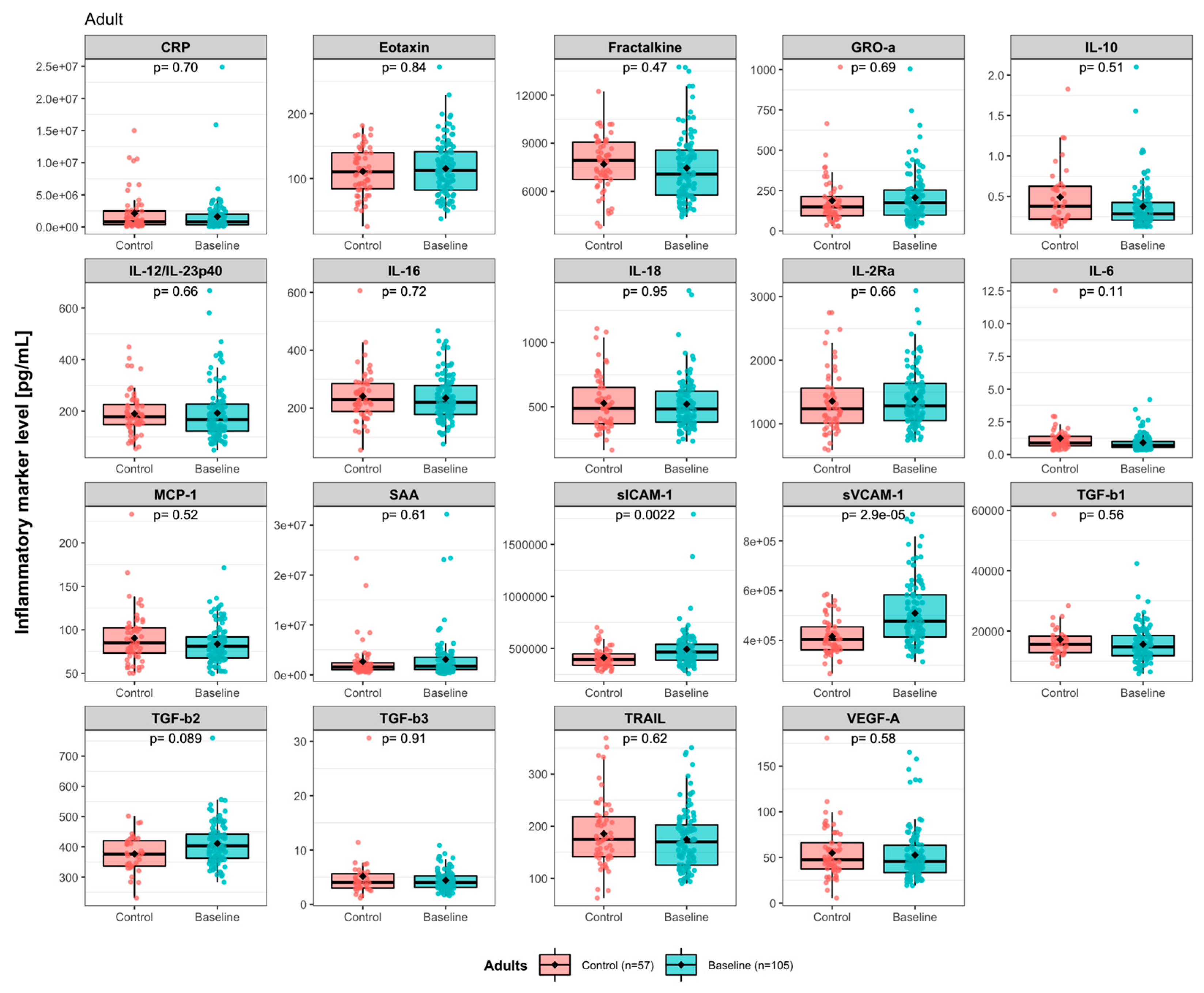
3.2. Effects of Synbiotic 2000 on Immune Activity Markers
3.3. Baseline Levels of Short-Chain Fatty Acids (SCFAs) in ADHD Patients
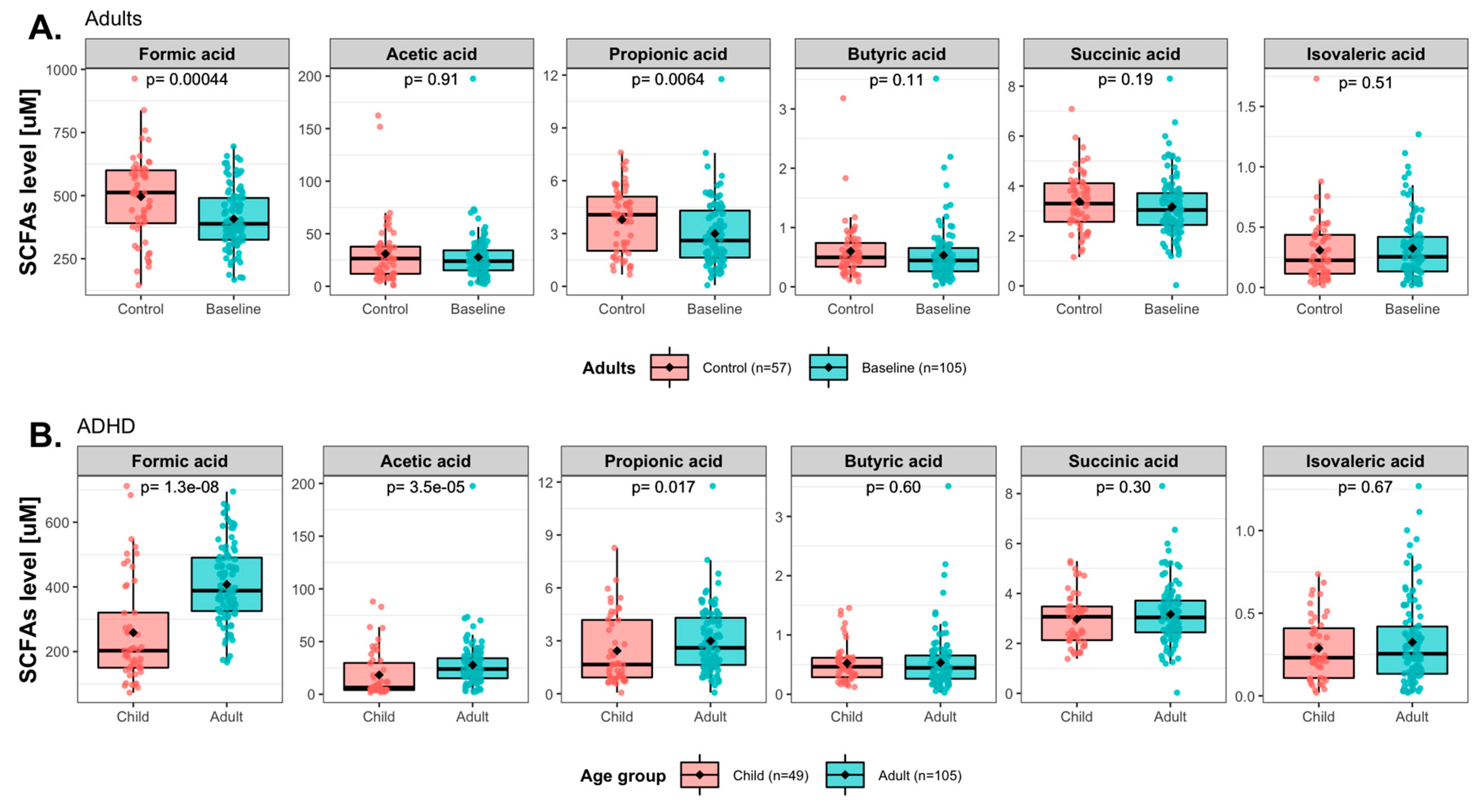
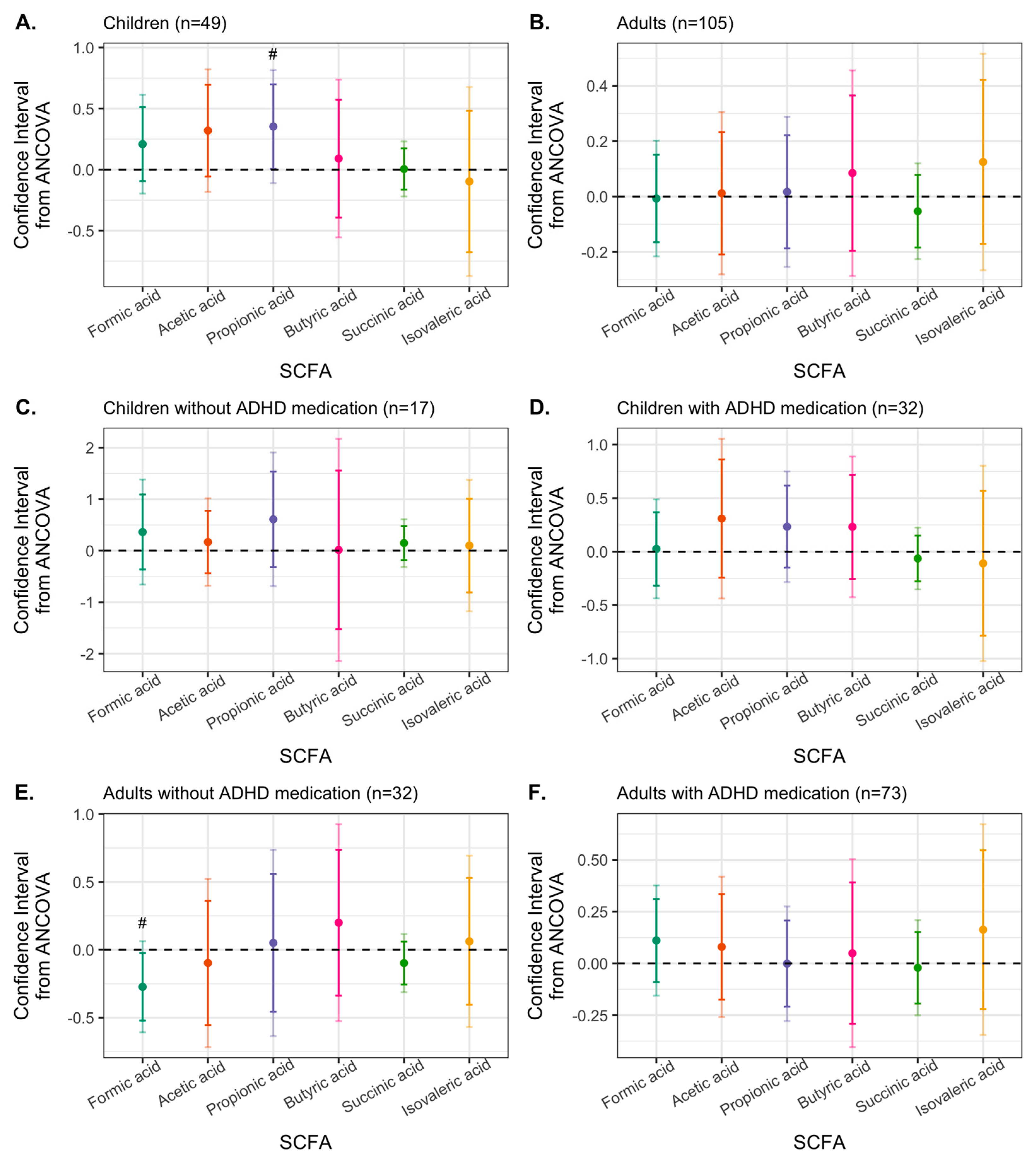
3.4. Effects of Synbiotic 2000 on SCFAs
3.5. Associations between Immune Activity Markers and SCFAs
3.6. Effects of SCFAs on ICAM-1 Expression in Human Aortic Vascular Smooth Muscle Cells In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magnin, E.; Maurs, C. Attention-deficit/hyperactivity disorder during adulthood. Rev. Neurol. 2017, 173, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Banaschewski, T.; Becker, K.; Döpfner, M.; Holtmann, M.; Rösler, M.; Romanos, M. Attention-deficit/hyperactivity disorder. Dtsch. Arztebl. Int. 2017, 114, 149–159. [Google Scholar] [CrossRef]
- Leffa, D.T.; Torres, I.L.; Rohde, L.A. A Review on the role of inflammation in attention-deficit/hyperactivity disorder. Neuroimmunomodulation 2018, 25, 328–333. [Google Scholar] [CrossRef]
- Leffa, D.T.; Caye, A.; Santos, I.; Matijasevich, A.; Menezes, A.; Wehrmeister, F.C.; Oliveira, I.; Vitola, E.; Bau, C.H.D.; Grevet, E.H.; et al. Attention-deficit/hyperactivity disorder has a state-dependent association with asthma: The role of systemic inflammation in a population-based birth cohort followed from childhood to adulthood. Brain Behav. Immun. 2021, 97, 239–249. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T. More than a gut feeling: The microbiota regulates neurodevelopment and behavior. Neuropsychopharmacology 2014, 40, 241–242. [Google Scholar] [CrossRef]
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- MacFabe, D.F. Enteric short-chain fatty acids: Microbial messengers of metabolism, mitochondria, and mind: Implications in autism spectrum disorders. Microb. Ecol. Heal. Dis. 2015, 26, 28177. [Google Scholar] [CrossRef]
- Mora, S.; Martín-González, E.; Prados-Pardo, A.; Moreno, J.; López, M.J.; Pilar-Cuellar, F.; Castro, E.; Díaz, Á.; Flores, P.; Moreno, M. Increased vulnerability to impulsive behavior after streptococcal antigen exposure and antibiotic treatment in rats. Brain Behav. Immun. 2020, 89, 675–688. [Google Scholar] [CrossRef]
- Otten, K.; Keller, L.; Puiu, A.A.; Herpertz-Dahlmann, B.; Seitz, J.; Kohn, N.; Edgar, J.C.; Wagels, L.; Konrad, K. Pre- and postnatal antibiotic exposure and risk of developing attention deficit hyperactivity disorder—A systematic review and meta-analysis combining evidence from human and animal studies. Neurosci. Biobehav. Rev. 2022, 140, 104776. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Zhou, Y.-Y.; Zhou, G.-L.; Li, Y.-C.; Yuan, J.; Li, X.-H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Richarte, V.; Rosales, K.; Corrales, M.; Bellina, M.; Fadeuilhe, C.; Calvo, E.; Ibanez, P.; Sanchez-Mora, C.; Ribases, M.; Ramos-Quiroga, J.A. The gut-brain axis in attention deficit hyperactivity disorder: The role of the microbiota. Rev. Neurol. 2018, 66, S109–S114. [Google Scholar] [PubMed]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Bloemendaal, M.; Aarts, E.; et al. Investigating the gut microbiota composition of individuals with attention-deficit/hyperactivity disorder and association with symptoms. Microorganisms 2020, 8, 406. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current evidence on the role of the gut microbiome in ADHD pathophysiology and therapeutic implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef]
- Sukmajaya, A.C.; Lusida, M.I.; Setiawati, Y. Systematic review of gut microbiota and attention-deficit hyperactivity disorder (ADHD). Ann. Gen. Psychiatry 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Richarte, V.; Sánchez-Mora, C.; Corrales, M.; Fadeuilhe, C.; Vilar-Ribó, L.; Arribas, L.; Garcia, E.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Soler-Artigas, M.; et al. Gut microbiota signature in treatment-naïve attention-deficit/hyperactivity disorder. Transl. Psychiatry 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, C.-Y.; Chou, W.-J.; Lee, M.-J.; Chou, M.-C.; Kuo, H.-C.; Yeh, Y.-M.; Lee, S.-Y.; Huang, L.-H.; Li, S.-C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2019, 29, 287–297. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Dam, S.A.; Wiesmann, M.; Naaijen, J.; van Bodegom, M.; Belzer, C.; Dederen, P.J.; Verweij, V.; Franke, B.; Kozicz, T.; et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019, 177, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2019, 25, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Lavebratt, C.; Yang, L.L.; Giacobini, M.; Forsell, Y.; Schalling, M.; Partonen, T.; Gissler, M. Early exposure to antibiotic drugs and risk for psychiatric disorders: A population-based study. Transl. Psychiatry 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kumperscak, H.G.; Gricar, A.; Ülen, I.; Micetic-Turk, D. A pilot randomized control trial with the probiotic strain lactobacillus rhamnosus GG (LGG) in ADHD: Children and adolescents report better health-related quality of life. Front. Psychiatry 2020, 11, 181. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: A randomized, double-blind, placebo-controlled trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Skott, E.; Yang, L.L.; Stiernborg, M.; Söderström, Å.; Rȕegg, J.; Schalling, M.; Forsell, Y.; Giacobini, M.; Lavebratt, C. Effects of a synbiotic on symptoms, and daily functioning in attention deficit hyperactivity disorder—A double-blind randomized controlled trial. Brain Behav. Immun. 2020, 89, 9–19. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2017, 58, 1243–1249. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol. Neurobiol. 2016, 54, 4432–4451. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, M.; Nankova, B.B.; Shah, P.; Patel, P.; Mally, P.V.; Mishra, R.; La Gamma, E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol. Brain Res. 2005, 142, 28–38. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Eilers, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020, 180, 1067–1080. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Frye, R.E.; Melnyk, S.; MacFabe, D.F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2019, 154, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemes, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts imbalance imbalances the brain: A Review of gut microbiota association with neurological and psychiatric disorders. Front. Med. 2022, 9. [Google Scholar] [CrossRef]
- Ratsika, A.; Pereira, J.S.C.; Lynch, C.M.; Clarke, G.; Cryan, J.F. Microbiota-immune-brain interactions: A lifespan perspective. Curr. Opin. Neurobiol. 2023, 78, 102652. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first? Ann. New York Acad. Sci. 2018, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2014, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2014, 2, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Goldstein, B.I. Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef]
- Anand, D.; Colpo, G.D.; Zeni, G.; Zeni, C.P.; Teixeira, A.L. Attention-deficit/hyperactivity disorder and inflammation: What does current knowledge tell us? A systematic review. Front. Psychiatry 2017, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Wójta-Kempa, M.; Samochowiec, J.; Schiweck, C.; Aichholzer, M.; Reif, A.; Samochowiec, A.; Stańczykiewicz, B. Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2022, 118, 110581. [Google Scholar] [CrossRef]
- Bedrossian, N.; Haidar, M.; Fares, J.; Kobeissy, F.H.; Fares, Y. Inflammation and Elevation of interleukin-12p40 in patients with schizophrenia. Front. Mol. Neurosci. 2016, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. The role of intercellular adhesion molecule-1 in the pathogenesis of psychiatric disorders. Front. Pharmacol. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Alaşehirli, B.; Oguz, E.; Gokcen, C.; Erbagcı, A.B.; Orkmez, M.; Demiryurek, A.T. Relationship between soluble intercellular adhesion molecules and attention-deficit/hyperactivity disorder. Int. J. Psychiatry Med. 2015, 50, 238–247. [Google Scholar] [CrossRef]
- Meixensberger, S.; Kuzior, H.; Fiebich, B.; Süß, P.; Runge, K.; Berger, B.; Nickel, K.; Denzel, D.; Schiele, M.; Michel, M.; et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics 2021, 11, 1134. [Google Scholar] [CrossRef]
- Thomas, A.J.; Ferrier, I.N.; Kalaria, R.N.; Davis, S.; O’Brien, J.T. Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulate cortex in major depression in the elderly. Br. J. Psychiatry 2002, 181, 129–134. [Google Scholar] [CrossRef]
- Thomas, A.J.; Davis, S.; Ferrier, I.; Kalaria, R.N.; O’Brien, J.T. Elevation of cell adhesion molecule immunoreactivity in the anterior cingulate cortex in bipolar disorder. Biol. Psychiatry 2004, 55, 652–655. [Google Scholar] [CrossRef]
- Carias, E.; Hamilton, J.; Robison, L.S.; Delis, F.; Eiden, R.; Quattrin, T.; Hadjiargyrou, M.; Komatsu, D.; Thanos, P.K. Chronic oral methylphenidate treatment increases microglial activation in rats. J. Neural Transm. 2018, 125, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Santos, V.; Cardoso, F.L.; Magalhães, A.; Ferreira-Teixeira, M.; Leitão, R.A.; Gomes, C.; Rito, M.; Barbosa, M.; Fontes-Ribeiro, C.A.; Silva, A.P. Effect of chronic methylphenidate treatment on hippocampal neurovascular unit and memory performance in late adolescent rats. Eur. Neuropsychopharmacol. 2018, 29, 195–210. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Dal-Pont, G.C.; Tonin, P.T.; Varela, R.B.; Ferreira, C.L.; Gava, F.F.; Andersen, M.L.; Soares, J.C.; Quevedo, J. Coadministration of lithium and celecoxib attenuates the behavioral alterations and inflammatory processes induced by amphetamine in an animal model of mania. Pharmacol. Biochem. Behav. 2019, 183, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Kuhn, D.M. Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. Experiment 2005, 313, 870–876. [Google Scholar] [CrossRef]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Söderström, A.; Giacobini, M.; Lavebratt, C. Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Eur. Neuropsychopharmacol. 2020, 41, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, Z.; Sun, J.; Garcia-Argibay, M.; Du Rietz, E.; Dobrosavljevic, M.; Brikell, I.; Jernberg, T.; Solmi, M.; Cortese, S.; et al. Attention-deficit/hyperactivity disorder as a risk factor for cardiovascular diseases: A nationwide population-based cohort study. World Psychiatry 2022, 21, 452–459. [Google Scholar] [CrossRef]
- Schmidt, C.; Hulthe, J.; Fagerberg, B. Baseline ICAM-1 and VCAM-1 are Increased in initially healthy middle-aged men who develop cardiovascular disease during 6.6 years of follow-up. Angiology 2008, 60, 108–114. [Google Scholar] [CrossRef]
- Plaudis, H.; Pupelis, G.; Zeiza, K.; Boka, V. Early low volume oral synbiotic/prebiotic supplemented enteral stimulation of the gut in patients with severe acute pancreatitis: A prospective feasibility study. Acta Chir. Belg. 2012, 112, 131–138. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Bengmark, S.; Kanellakopoulou, K.; Kotzampassi, K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J. Trauma Inj. Infect. Crit. Care 2009, 67, 815–821. [Google Scholar] [CrossRef]
- Vidot, H.; Cvejic, E.; Finegan, L.J.; Shores, E.A.; Bowen, D.G.; Strasser, S.I.; McCaughan, G.W.; Carey, S.; Allman-Farinelli, M.; Shackel, N.A. Supplementation with synbiotics and/or branched chain amino acids in hepatic encephalopathy: A pilot randomised placebo-controlled clinical study. Nutrients 2019, 11, 1810. [Google Scholar] [CrossRef]
- Spindler-Vesel, A.; Bengmark, S.; Vovk, I.; Cerovic, O.; Kompan, L. Synbiotics, Prebiotics, glutamine, or peptide in early enteral nutrition: A randomized study in trauma patients. J. Parenter. Enter. Nutr. 2007, 31, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, P.; Heikkinen, A.; Harrer, A.; Pilz, G.; Kunz, A.; Golaszewski, S.M.; Reuss, R.; Oschmann, P.; Kraus, J. Circadian rhythmicity of inflammatory serum parameters: A neglected issue in the search of biomarkers in multiple sclerosis. J. Neurol. 2012, 260, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Upham, J.W.; Lee, P.T.; Holt, B.J.; Heaton, T.; Prescott, S.L.; Sharp, M.J.; Sly, P.D.; Holt, P. Development of interleukin-12-producing capacity throughout childhood. Infect. Immun. 2002, 70, 6583–6588. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Anuurad, E.; Zhang, W.; Kim, K.; Berglund, L. Diverging trajectory patterns of systemic versus vascular inflammation over age in healthy Caucasians and African Americans. Atherosclerosis 2015, 239, 509–515. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2018, 45, 1–8. [Google Scholar] [CrossRef]
- McKeown, C.; Hisle-Gorman, E.; Eide, M.; Gorman, G.H.; Nylund, C.M. Association of Constipation and fecal incontinence with attention-deficit/hyperactivity disorder. Pediatrics 2013, 132, e1210–e1215. [Google Scholar] [CrossRef]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Thomas, S.; Baumgart, D.C. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology 2011, 20, 1–18. [Google Scholar] [CrossRef]
- Jones, S.C.; Banks, R.E.; Haidar, A.; Gearing, A.J.; Hemingway, I.K.; Ibbotson, S.H.; Dixon, M.F.; Axon, A.T. Adhesion molecules in inflammatory bowel disease. Gut 1995, 36, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Quintero, M.A.; Jain, A.; Czul, F.; Barkin, J.S.; Abreu, M.T. Serum amyloid a as a surrogate marker for mucosal and histologic inflammation in patients with Crohn’s disease. Inflamm. Bowel Dis. 2017, 23, 158–164. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, L.; Gargari, B.P.; Jafarabadi, M.A.; Alipour, B. Effects of probiotic yogurt on glycemic indexes and endothelial dysfunction markers in patients with metabolic syndrome. Nutrition 2019, 62, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Mohammadshahi, M.; Shayanpour, S.; Haghighizadeh, M.H. Effect of synbiotic and probiotic supplementation on serum levels of endothelial cell adhesion molecules in hemodialysis patients: A randomized control study. Probiotics Antimicrob. Proteins 2018, 11, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Yang, M.-C.; Wu, Y.-Y.; Chen, P.-H.; Hsu, C.-M.; Chen, L.-W. Lactobacillus plantarum reverse diabetes-induced Fmo3 and ICAM expression in mice through enteric dysbiosis-related c-Jun NH2-terminal kinase pathways. PLoS ONE 2018, 13, e0196511. [Google Scholar] [CrossRef]
- Cabrera, S.M.; Coren, A.T.; Pant, T.; Ciecko, A.E.; Jia, S.; Roethle, M.F.; Simpson, P.M.; Atkinson, S.N.; Salzman, N.H.; Chen, Y.-G.; et al. Probiotic normalization of systemic inflammation in siblings of type 1 diabetes patients: An open-label pilot study. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.; Mitrea, L.; Vodnar, D. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.A.E.; Vanhoutvin, S.A.L.W.; Troost, F.J.; Rijkers, G.; De Bruïne, A.; Bast, A.; Venema, K.; Brummer, R.-J.M. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kołodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-κB and PPARα. J. Nutr. Biochem. 2004, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Naruszewicz, M. Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J. Physiol. Pharmacol. 2009, 60, 123–131. [Google Scholar] [PubMed]
- Yoshida, A.; Takahashi, H.K.; Iwagaki, H.; Yoshino, T.; Morichika, T.; Yokoyama, M.; Itoh, H.; Mori, S.; Akagi, T.; Nishibori, M.; et al. Essential role of ICAM-1/LFA-1 interaction in synergistic effect of IL-18 and IL-12 on IFN-γ production in human PBMC. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002, 365, 181–186. [Google Scholar] [CrossRef]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Gillberg, T.; Landberg, R.; Giacobini, M.; Lavebratt, C. Lower plasma concentrations of short-chain fatty acids (SCFAs) in patients with ADHD. J. Psychiatr. Res. 2022, 156, 36–43. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021, 184, 5916–5931. [Google Scholar] [CrossRef] [PubMed]



| Children (n = 49) | Adults (n = 105) | ||||
|---|---|---|---|---|---|
| Placebo (n = 21) | Synbiotic 2000 (n = 28) | Placebo (n = 54) | Synbiotic 2000 (n = 51) | ||
| Median (IQR)/ N (%) | Median (IQR)/ N (%) | Median (IQR)/ N (%) | Median (IQR)/ N (%) | ||
| Age [years] | 13 (8–18) | 14 (8–18) | 36 (19–51) | 37 (19–55) | |
| Body mass index [kg/m2] | 22.8 (17.0–38.3) | 24.3 (19.3–40.4) | |||
| Sex | Female | 4 (19) | 9 (32) | 38 (70) | 36 (71) |
| Male | 17 (81) | 19 (68) | 16 (30) | 15 (29) | |
| Delivery route | Vaginal | 18 (86) | 21 (75) | 48 (89) | 43 (84) |
| C-section | 3 (14) | 4 (14) | 5 (9) | 7 (14) | |
| Acute C-section | 0 (0) | 1 (4) | 1 (2) | 1 (2) | |
| Unknown | 0 (0) | 2 (7) | 0 (0) | 0 (0) | |
| Breastfed | <3 months | 2 (10) | 5 (18) | 7 (13) | 9 (18) |
| 3–6 months | 0 (0) | 4 (14) | 12 (22) | 10 (20) | |
| >6 months | 18 (86) | 17 (61) | 29 (54) | 22 (43) | |
| None | 0 (0) | 0 (0) | 1 (2) | 1 (2) | |
| Unknown | 1 (5) | 2 (7) | 5 (9) | 9 (18) | |
| Antibiotic drugs a | >3 times | 0 (0) | 1 (4) | 3 (6) | 1 (2) |
| 1–3 times | 5 (24) | 8 (29) | 15 (28) | 11 (22) | |
| None | 16 (76) | 18 (64) | 34 (63) | 36 (71) | |
| Unknown | 0 (0) | 1 (4) | 2 (4) | 3 (6) | |
| Melatonin medication | Yes † | 14 (67) | 9 (32) | 12 (22) | 37 (73) |
| No † | 7 (33) | 19 (68) | 42 (78) | 14 (27) | |
| ADHD medication b | Yes † | 14 (67) | 18 (64) | 36 (67) | 37 (73) |
| No † | 7 (33) | 10 (36) | 18 (33) | 14 (27) | |
| Other prescribed drugs for adults c | Yes † | 0 (0) | 0 (0) | 24 (44) | 30 (59) |
| No † | 21 (100) | 28 (100) | 30 (56) | 21 (41) | |
| Dietary supplements d | Yes | 13 (62) | 15 (54) | 39 (72) | 37 (73) |
| No | 8 (38) | 13 (46) | 15 (28) | 14 (26) | |
| ICD-10 code e | F90.0 | 6 (29) | 4 (14) | 9 (17) | 10 (20) |
| F90.0B | 12 (57) | 18 (64) | 28 (52) | 31 (61) | |
| F90.0C | 2 (10) | 5 (18) | 13 (24) | 7 (14) | |
| F90.0X | 0 (0) | 0 (0) | 2 (4) | 2 (4) | |
| F90.1 | 1 (5) | 0 (0) | 0 (0) | 0 (0) | |
| F90.8 | 0 (0) | 0 (0) | 1 (2) | 0 (0) | |
| F98.8 | 0 (0) | 1 (4) | 1 (2) | 1 (2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.L.; Stiernborg, M.; Skott, E.; Xu, J.; Wu, Y.; Landberg, R.; Arefin, S.; Kublickiene, K.; Millischer, V.; Nilsson, I.A.K.; et al. Effects of a Synbiotic on Plasma Immune Activity Markers and Short-Chain Fatty Acids in Children and Adults with ADHD—A Randomized Controlled Trial. Nutrients 2023, 15, 1293. https://doi.org/10.3390/nu15051293
Yang LL, Stiernborg M, Skott E, Xu J, Wu Y, Landberg R, Arefin S, Kublickiene K, Millischer V, Nilsson IAK, et al. Effects of a Synbiotic on Plasma Immune Activity Markers and Short-Chain Fatty Acids in Children and Adults with ADHD—A Randomized Controlled Trial. Nutrients. 2023; 15(5):1293. https://doi.org/10.3390/nu15051293
Chicago/Turabian StyleYang, Liu L., Miranda Stiernborg, Elin Skott, Jingjing Xu, Yujiao Wu, Rikard Landberg, Samsul Arefin, Karolina Kublickiene, Vincent Millischer, Ida A. K. Nilsson, and et al. 2023. "Effects of a Synbiotic on Plasma Immune Activity Markers and Short-Chain Fatty Acids in Children and Adults with ADHD—A Randomized Controlled Trial" Nutrients 15, no. 5: 1293. https://doi.org/10.3390/nu15051293
APA StyleYang, L. L., Stiernborg, M., Skott, E., Xu, J., Wu, Y., Landberg, R., Arefin, S., Kublickiene, K., Millischer, V., Nilsson, I. A. K., Schalling, M., Giacobini, M., & Lavebratt, C. (2023). Effects of a Synbiotic on Plasma Immune Activity Markers and Short-Chain Fatty Acids in Children and Adults with ADHD—A Randomized Controlled Trial. Nutrients, 15(5), 1293. https://doi.org/10.3390/nu15051293







