Noninvasive Ventilation and Rapid Enteral Feeding Advances in Preterm Infants—2-Year Follow-Up of the STENA-Cohort
Abstract
1. Introduction
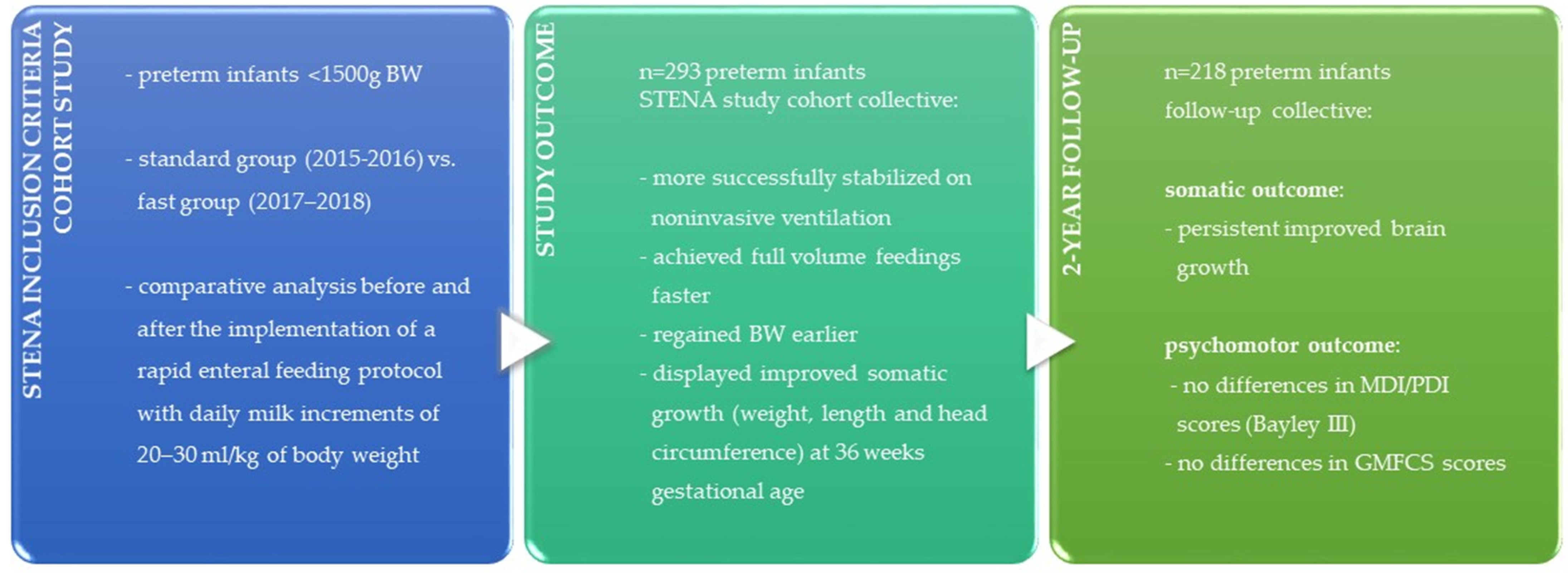
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Demographics
3.2. Somatic and Psychomotor Outcome with 2 Years Corrected Age
4. Discussion
4.1. Main Results
4.2. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cormack, B.E.; Harding, J.E.; Miller, S.P.; Bloomfield, F.H. The Influence of Early Nutrition on Brain Growth and Neurodevelopment in Extremely Preterm Babies: A Narrative Review. Nutrients 2019, 11, 2029. [Google Scholar] [CrossRef]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.R.; Pohlandt, F.; Bode, H.; Mihatsch, W.A.; Sander, S.; Kron, M.; Steinmacher, J. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009, 123, e101-9. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H. Relationships between Neonatal Nutrition and Growth to 36 Weeks’ Corrected Age in ELBW Babies-Secondary Cohort Analysis from the Provide Trial. Nutrients 2020, 12, 760. [Google Scholar] [CrossRef]
- Harding, J.E.; Cormack, B.E.; Alexander, T.; Alsweiler, J.M.; Bloomfield, F.H. Advances in nutrition of the newborn infant. Lancet 2017, 389, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Ehrenkranz, R.A. Neurodevelopmental outcomes and nutritional strategies in very low birth weight infants. Semin. Fetal Neonatal Med. 2017, 22, 42–48. [Google Scholar] [CrossRef]
- Malikiwi, A.I.; Lee, Y.-M.; Davies-Tuck, M.; Wong, F.Y. Postnatal nutritional deficit is an independent predictor of bronchopulmonary dysplasia among extremely premature infants born at or less than 28 weeks gestation. Early Hum. Dev. 2019, 131, 29–35. [Google Scholar] [CrossRef]
- Thiess, T.; Lauer, T.; Woesler, A.; Neusius, J.; Stehle, S.; Zimmer, K.-P.; Eckert, G.P.; Ehrhardt, H. Correlation of Early Nutritional Supply and Development of Bronchopulmonary Dysplasia in Preterm Infants < 1000 g. Front. Pediatr. 2021, 9, 741365. [Google Scholar] [CrossRef]
- Wemhöner, A.; Ortner, D.; Tschirch, E.; Strasak, A.; Rüdiger, M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm. Med. 2011, 11, 7. [Google Scholar] [CrossRef]
- Milanesi, B.G.; Lima, P.A.; Villela, L.D.; Martins, A.S.; Gomes-Junior, S.C.S.; Moreira, M.E.L.; Méio, Maria Dalva Barbosa Baker. Assessment of early nutritional intake in preterm infants with bronchopulmonary dysplasia: A cohort study. Eur. J. Pediatr. 2021, 180, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Hortensius, L.M.; Janson, E.; van Beek, P.E.; Groenendaal, F.; Claessens, N.H.P.; de Veye, S.; Henriette, F.N.; Eijsermans, M.J.C.; Koopman-Esseboom, C.; Dudink, J.; et al. Nutritional Intake, White Matter Integrity, and Neurodevelopment in Extremely Preterm Born Infants. Nutrients 2021, 13, 3409. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F256–F261. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F128. [Google Scholar] [CrossRef]
- Lapidaire, W.; Lucas, A.; Clayden, J.D.; Clark, C.; Fewtrell, M.S. Human milk feeding and cognitive outcome in preterm infants: The role of infection and NEC reduction. Pediatr. Res. 2022, 91, 1207–1214. [Google Scholar] [CrossRef]
- Karatza, A.A.; Gkentzi, D.; Varvarigou, A. Nutrition of Infants with Bronchopulmonary Dysplasia before and after Discharge from the Neonatal Intensive Care Unit. Nutrients 2022, 14, 3311. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Ramel, S.E.; Georgieff, M.K. Preterm nutrition and the brain. World Rev. Nutr. Diet. 2014, 110, 190–200. [Google Scholar] [CrossRef]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Embleton, N.D.; Moltu, S.J.; Lapillonne, A.; van den Akker, C.H.; Chris, H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef]
- Liu, K.; Abudusalamu, A.; Yang, J.; Su, Y. Effectiveness of early enteral feeding on health outcomes in preterm infants: An overview of systematic reviews. Eur. J. Clin. Nutr. 2022, 1–9. [Google Scholar] [CrossRef]
- Walsh, V.; Brown, J.V.E.; Copperthwaite, B.R.; Oddie, S.J.; McGuire, W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 12, CD013542. [Google Scholar] [CrossRef]
- Pike, K.; Brocklehurst, P.; Jones, D.; Kenyon, S.; Salt, A.; Taylor, D.; Marlow, N. Outcomes at 7 years for babies who developed neonatal necrotising enterocolitis: The ORACLE Children Study. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F318. [Google Scholar] [CrossRef]
- Rees, C.M.; Pierro, A.; Eaton, S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F193. [Google Scholar] [CrossRef]
- Oddie, S.J.; Young, L.; McGuire, W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2021, 8, CD001241. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.; Abbott, J.; Berrington, J.; Bosiak, B.; Bowler, U.; Boyle, E.; Embleton, N.; Hewer, O.; Johnson, S.; Juszczak, E.; et al. Controlled Trial of Two Incremental Milk-Feeding Rates in Preterm Infants. N. Engl. J. Med. 2019, 381, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Fogel, A.; Lauria, M.E.; Ferguson, K.; Smith, E.R. Fast Feed Advancement for Preterm and Low Birth Weight Infants: A Systematic Review and Meta-analysis. Pediatrics 2022, 150. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Thanigainathan, S.; Ninan, B. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2019, 7, CD012937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Meena, J.; Mittal, P.; Shankar, J.; Kumar, P.; Shenoi, A. Routine prefeed gastric aspiration in preterm infants: A systematic review and meta-analysis. Eur. J. Pediatr. 2021, 180, 2367–2377. [Google Scholar] [CrossRef]
- Terek, D.; Celik, M.; Ergin, F.; Erol, E.; Altun Koroglu, O.; Yalaz, M.; Akisu, M.; Kultursay, N. Omitting routine gastric residual checks may help to accelerate enteral feeds and postnatal growth in stable preterm infants. JPEN J. Parenter. Enter. Nutr. 2022, 46, 1198–1202. [Google Scholar] [CrossRef]
- Parker, L.A.; Weaver, M.; Murgas Torrazza, R.J.; Shuster, J.; Li, N.; Krueger, C.; Neu, J. Effect of Gastric Residual Evaluation on Enteral Intake in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 534–543. [Google Scholar] [CrossRef]
- Halemani, K.; Issac, A.; Dhiraaj, S.; Mishra, P. Efficacy of body position on gastric residual in preterm infant: A systematic review and meta-analysis. Clin. Exp. Pediatr. 2022. [Google Scholar] [CrossRef]
- Sokou, R.; Grivea, I.N.; Gounari, E.; Panagiotounakou, P.; Baltogianni, M.; Antonogeorgos, G.; Kokori, F.; Konstantinidi, A.; Gounaris, A.K. Gastric Volume Changes in Preterm Neonates during Intermittent and Continuous Feeding-GRV and Feeding Mode in Preterm Neonates. Children 2021, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Holzfurtner, L.; Shahzad, T.; Dong, Y.; Rekers, L.; Selting, A.; Staude, B.; Lauer, T.; Schmidt, A.; Rivetti, S.; Zimmer, K.-P.; et al. When inflammation meets lung development-an update on the pathogenesis of bronchopulmonary dysplasia. Mol. Cell. Pediatr. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Göpel, W.; Kribs, A.; Roll, C.; Wieg, C.; Teig, N.; Hoehn, T.; Welzing, L.; Vochem, M.; Hoppenz, M.; Bührer, C.; et al. Multi-centre randomised trial of invasive and less invasive surfactant delivery methods showed similar spirometry results at 5–9 years of age. Acta Paediatr. 2022, 111, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, V.; Bhandari, V. Non-Invasive Ventilatory Strategies to Decrease Bronchopulmonary Dysplasia-Where Are We in 2021? Children 2021, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Behnke, J.; Lemyre, B.; Czernik, C.; Zimmer, K.-P.; Ehrhardt, H.; Waitz, M. Non-Invasive Ventilation in Neonatology. Dtsch. Arztebl. Int. 2019, 116, 177–183. [Google Scholar] [CrossRef]
- Abiramalatha, T.; Ramaswamy, V.V.; Bandyopadhyay, T.; Somanath, S.H.; Shaik, N.B.; Pullattayil, A.K.; Weiner, G.M. Interventions to Prevent Bronchopulmonary Dysplasia in Preterm Neonates: An Umbrella Review of Systematic Reviews and Meta-analyses. JAMA Pediatr. 2022, 176, 502–516. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019, 266, 121–129. [Google Scholar] [CrossRef]
- Behnke, J.; Dippel, C.M.; Choi, Y.; Rekers, L.; Schmidt, A.; Lauer, T.; Dong, Y.; Behnke, J.; Zimmer, K.-P.; Bellusci, S.; et al. Oxygen Toxicity to the Immature Lung-Part II: The Unmet Clinical Need for Causal Therapy. Int. J. Mol. Sci. 2021, 22, 694. [Google Scholar] [CrossRef]
- Behnke, J.; Estreich, V.; Oehmke, F.; Zimmer, K.-P.; Windhorst, A.; Ehrhardt, H. Compatibility of rapid enteral feeding advances and noninvasive ventilation in preterm infants-An observational study. Pediatr. Pulmonol. 2022, 57, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Rochow, N.; Schneider, K.T.M.; Hagenah, H.-P.; Scholz, R.; Hesse, V.; Wittwer-Backofen, U.; Straube, S.; Olbertz, D. New percentile values for the anthropometric dimensions of singleton neonates: Analysis of perinatal survey data of 2007-2011 from all 16 states of Germany. Z. Geburtshilfe Neonatol. 2014, 218, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; de Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary From the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bayley, N. Bayley-III: Bayley Scales of Infant and Toddler Development; Giunti OS: Florence, Italy, 2009. [Google Scholar]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Bundesausschuss, G. Richtlinie des Gemeinsamen Bundesausschusses über Maßnahmen zur Qualitätssicherung der Versorgung von Früh-und Reifgeborenen gemäß § 136 Absatz 1 Nummer 2 SGB V in Verbindung mit § 92 Abs. 1 Satz 2 Nr. 13 SGB V (Qualitätssicherungs-Richtlinie Früh-und Reifgeborene/QFR-RL). Bundesanzeiger 2020, 2005, 15–684. [Google Scholar]
- Seppänen, A.-V.; Draper, E.S.; Petrou, S.; Barros, H.; Andronis, L.; Kim, S.W.; Maier, R.F.; Pedersen, P.; Gadzinowski, J.; Lebeer, J.; et al. Follow-up after very preterm birth in Europe. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 113–114. [Google Scholar] [CrossRef]
- Selvanathan, T.; Guo, T.; Kwan, E.; Chau, V.; Brant, R.; Synnes, A.R.; Grunau, R.E.; Miller, S.P. Head circumference, total cerebral volume and neurodevelopment in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 181–187. [Google Scholar] [CrossRef]
- Bushman, E.T.; Blanchard, C.; Sinkey, R.G.; Harris, S.; Casey, B.; Tita, A.T.; Ramani, M.; Harper, L.M. Head Circumference within the Normal Range and Neurodevelopmental Outcomes in Preterm Infants. Am. J. Perinatol. 2021, 38, 1459–1464. [Google Scholar] [CrossRef]
- Toppe, F.; Rasche, T.; Weiss, C.; Schock, A.; Felderhoff-Müser, U.; Müller, H. Relationship between early nutrition and deep gray matter and lateral ventricular volumes of preterm infants at term-equivalent age. World J. Pediatr. 2023, 1–9. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chu, C.-H.; Chen, Y.-J.; Chen, R.-B.; Huang, C.-C. Gestational Age-Related Associations between Early-Life Feeding Trajectories and Growth Outcomes at Term Equivalent Age in Very Preterm Infants. Nutrients 2022, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Moddemann, D.; Poets, C.; Rabi, Y.; Solimano, A.; Roberts, R.S. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: A randomized clinical trial. JAMA 2013, 309, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
| 2-Year Follow-Up Standard Group (2015–2016) n = 99 | 2-Year Follow-Up Fast Group (2017–2018) n = 119 | p-Value | |
|---|---|---|---|
| Birth weight, g | 990 (745–1385) | 990 (825–1340) | 0.994 a |
| Gestational age, weeks | 28.86 (26.57–30.93) | 28.71 (26.43–30.43) | 0.839 a |
| z-scores at birth | |||
| weight | −0.62 (−1.18–−0.16) | −0.65 (−1.38–−0.13) | 0.662 a |
| length | −0.61 (−1.09–−0.29) | −0.59 (−1.17–−0.19) | 0.600 a |
| head circumference | −0.72 (−1.20–−0.27) | −0.80 (−1.24–−0.27) | 0.915 a |
| SGA c, n (%) | 24 (24) | 35 (29) | 0.483 b |
| Male sex, n (%) | 41 (41) | 64 (53) | 0.092 b |
| Multiple birth, n (%) | 33 (33) | 46 (39) | 0.501 b |
| Antenatal corticosteroids, n (%) | 94 (95) | 112 (94) | 1.000 b |
| 2-Year Follow-Up n = 218 | Lost to Follow-Up n = 75 | p-Value | |
|---|---|---|---|
| Standard group (2015–2016) | n = 99 | n = 46 | |
| Birth weight, g | 990 (745–1385) | 1175 (910–1395) | 0.089 a |
| Gestational age, weeks | 28.86 (26.57–30.93) | 30.21 (27.86–31.71) | 0.013 a |
| z-Scores at birth | |||
| weight | −0.62 (−1.18–−0.16) | −0.89 (−1.44–−0.03) | 0.376 a |
| length | −0.61 (−1.09–−0.29) | −0.59 (−1.05–−0.03) | 0.833 a |
| head circumference | −0.72 (−1.20–−0.27) | −0.79 (−1.23–−0.38) | 0.441 a |
| SGA c, n (%) | 24 (24) | 17 (37) | 0.166 b |
| Male sex, n (%) | 41 (41) | 27 (59) | 0.078 b |
| Multiple birth, n (%) | 33 (33) | 27 (59) | 0.007 b |
| Antenatal corticosteroids, n (%) | 94 (95) | 40 (87) | 0.075 b |
| Fast group (2017–2018) | n = 119 | n = 29 | |
| Birth weight, g | 990 (825–1.340) | 1.320 (995–1.440) | 0.003 a |
| Gestational age, weeks | 28.71 (26.43–30.43) | 30.43 (28.14–32.86) | 0.001 a |
| z-Scores at birth | |||
| weight | −0.65 (−1.38–−0.13) | −1.07 (−1.65–−0.32) | 0.158 a |
| length | −0.59 (−1.17–−0.19) | −0.66 (−1.38–−0.25) | 0.504 a |
| head circumference | −0.80 (−1.24–−0.27) | −0.90 (−1.19–−0.37) | 0.623 a |
| SGA c, n (%) | 35 (29) | 11 (38) | 0.506 b |
| Male sex, n (%) | 64 (53) | 64 (59) | 0.794 b |
| Multiple birth, n (%) | 46 (39) | 12 (41) | 0.954 b |
| Antenatal corticosteroids, n (%) | 112 (94) | 25 (86) | 0.209 b |
| 2-Year Follow-Up Standard Group (2015–2016) n = 99 | 2-Year Follow-Up Fast Group (2017–2018) n = 119 | p-Value | |
|---|---|---|---|
| z-Scores at 2 years corrected age | |||
| weight | −0.74 (−1.43–−0.05) | −0.53 (−1.24–0.09) | 0.256 a |
| length head circumference | −0.54 (−1.39–0.19) −0.94 (−2.15–−0.10) | −0.45 (−1.31–0.47) −0.55 (−1.38–0.20) | 0.259 a 0.034 a |
| Δz-score (weight 2 years-birth) | 0.07 (−0.79–0.61) | 0.07 (−0.54–0.80) | 0.243 a |
| Δz-score (weight 2 years-36 weeks gestational age) Δz-score (length 2 years-birth) Δz-score (length 2 years-36 weeks gestational age) Δz-score (head 2 years-birth) Δz-score (head 2 years-36 weeks gestational age) | 0.49 (−0.29–1.16) −0.01 (−0.68–0.65) 1.21 (0.25–1.83) −0.40 (−1.15–0.58) 0.43 (−0.80–1.08) | 0.39 (−0.23–0.98) 0.26 (−0.75–0.97) 0.93 (−0.13–1.77) 0.26 (−0.73–1.15) 0.32 (−0.47–1.07) | 0.591 a 0.291 a 0.183 a 0.007 a 0.827 a |
| MDI c | 95 (85–105) | 95 (80–105) | 0.738 a |
| PDI d | 109 (89–125) | 103 (85–119) | 0.122 a |
| GMFCS e | 1 (1-1) | 1 (1-1) | 0.170 a |
| Severe hearing impairment, n (%) | 2 (2) | 5 (4) | 0.638 b |
| Blindness, n (%) | 0 (0) | 1 (1) | 1.000 b |
| 2-Year Follow-Up SGA Subgroup n = 59 | 2-Year Follow-Up NonSGA Subgroup n = 159 | p-Value | |
|---|---|---|---|
| Standard group (2015–2016) z-Scores at 2 years corrected age | n = 24 | n = 75 | |
| weight | −1.18 (−1.91–−0.81) | −0.61 (−1.26–0.05) | 0.012 a |
| length head circumference | −1.54 (−2.17–−0.95) −2.04 (−3.35–−0.80) | −0.43 (−1.10–0.30) −0.74 (−1.61–0.04) | <0.001 a 0.004 a |
| Δz-score (weight 2 years-birth) | 0.48 (0.04–1.10) | −0.14 (0.80–0.42) | 0.008 a |
| Δz-score (weight 2 years-36 weeks gestational age) Δz-score (length 2 years-birth) Δz-score (length 2 years-36 weeks gestational age) Δz-score (head 2 years-birth) Δz-score (head 2 years-36 weeks gestational age) | 0.52 (−0.07–1.62) 0.03 (−0.63–0.57) 1.46 (0.72–2.16) −0.81 (−1.44–0.57) −0.13 (−1.19–0.63) | 0.44 (−0.40–1.10) −0.05 (−0.68–0.66) 1.12 (0.22–1.75) −0.29 (−1.04–0.55) 0.55 (−0.75–1.19) | 0.213 a 0.978 a 0.171 a 0.384 a 0.129 a |
| MD I c | 95 (85–105) | 85 (81–105) | 0.844 a |
| PDI d | 101 (82–122) | 111 (95–126) | 0.247 a |
| GMFCS e | 1 (1-1) | 1 (1-1) | 0.500 a |
| Severe hearing impairment, n (%) | 1 (4) | 1 (1) | 0.950 b |
| Blindness, n (%) | 0 (0) | 0 (0) | - |
| Fast group (2017–2018) z-scores at 2 years corrected age | n = 35 | n = 84 | |
| weight | −1.12 (−1.69–−0.19) | −0.27 (−0.90–0.18) | 0.005 a |
| length head circumference | −0.90 (−1.43–−0.32) −1.12 (−2.14–−0.30) | −0.36 (−1.23–0.57) −0.26 (−1.07–0.25) | 0.030 a 0.012 a |
| Δz-score (weight 2 years-birth) | 0.72 (0.06–1.58) | −0.17 (−0.72–0.60) | <0.001 a |
| Δz-score (weight 2 years-36 weeks gestational age) Δz-score (length 2 years-birth) Δz-score (length 2 years-36 weeks gestational age) Δz-score (head 2 years-birth) Δz-score (head 2 years-36 weeks gestational age) | 0.64 (0.01–1.42) 0.42 (−0.33–1.01) 1.01 (0.20–2.17) 0.15 (−1.20–1.13) 0.37 (−0.33–1.07) | 0.27 (−0.24–0.91) 0.24 (−0.87–0.94) 0.81 (−0.19–1.72) 0.28 (−0.59–1.16) 0.32 (−0.47–1.07) | 0.112 a 0.407 a 0.345 a 0.700 a 0.967 a |
| MDI c | 85 (79–105) | 95 (85–106) | 0.198 a |
| PDI d | 96 (85–116) | 103 (87–120) | 0.310 a |
| GMFCS e | 1 (1-1) | 1 (1-1) | 1.000 a |
| Severe hearing impairment, n (%) | 1 (3) | 4 (5) | 1.000 b |
| Blindness, n (%) | 1 (3) | 0 (0) | 0.650 b |
| 2-Year Follow-Up Standard Group (2015–2016) n = 24 | 2-Year Follow-Up Fast Group (2017–2018) n = 35 | p-Value | |
|---|---|---|---|
| z-Scores at 2 years corrected age | |||
| weight | −1.18 (−1.91–−0.81) | −1.12 (−1.69–−0.19) | 0.511 a |
| length head circumference | −1.54 (−2.17–−0.95) −2.04 (−3.35–−0.80) | −0.90 (−1.43–−0.32) −1.12 (−2.14–−0.30) | 0.079 a 0.114 a |
| Δz-score (weight 2 years-birth) | 0.48 (0.04–1.10) | 0.72 (0.06–1.58) | 0.457 a |
| Δz-score (weight 2 years-36 weeks gestational age) Δz-score (length 2 years-birth) Δz-score (length 2 years-36 weeks gestational age) Δz-score (head 2 years-birth) Δz-score (head 2 years-36 weeks gestational age) | 0.52 (−0.07–1.62) 0.03 (−0.63–0.57) 1.46 (0.72–2.16) −0.81 (−1.44–0.57) −0.13 (−1.19–0.63) | 0.64 (0.01–1.42) 0.42 (−0.33–1.01) 1.01 (0.20–2.17) 0.15 (−1.20–1.13) 0.37 (−0.33–1.07) | 0.955 a 0.315 a 0.302 a 0.213 a 0.265 a |
| MDI c | 95 (85–105) | 85 (79–105) | 0.224 a |
| PDI d | 101 (82–122) | 96 (85–116) | 0.747 a |
| GMFCS e | 1 (1-1) | 1 (1-1) | 0.377 a |
| Severe hearing impairment, n (%) | 1 (4) | 1 (3) | 1.000 b |
| Blindness, n (%) | 0 (0) | 1 (3) | 1.000 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behnke, J.; Estreich, V.; Oehmke, F.; Neubauer, B.A.; Windhorst, A.; Ehrhardt, H. Noninvasive Ventilation and Rapid Enteral Feeding Advances in Preterm Infants—2-Year Follow-Up of the STENA-Cohort. Nutrients 2023, 15, 1292. https://doi.org/10.3390/nu15051292
Behnke J, Estreich V, Oehmke F, Neubauer BA, Windhorst A, Ehrhardt H. Noninvasive Ventilation and Rapid Enteral Feeding Advances in Preterm Infants—2-Year Follow-Up of the STENA-Cohort. Nutrients. 2023; 15(5):1292. https://doi.org/10.3390/nu15051292
Chicago/Turabian StyleBehnke, Judith, Vanessa Estreich, Frank Oehmke, Bernd Axel Neubauer, Anita Windhorst, and Harald Ehrhardt. 2023. "Noninvasive Ventilation and Rapid Enteral Feeding Advances in Preterm Infants—2-Year Follow-Up of the STENA-Cohort" Nutrients 15, no. 5: 1292. https://doi.org/10.3390/nu15051292
APA StyleBehnke, J., Estreich, V., Oehmke, F., Neubauer, B. A., Windhorst, A., & Ehrhardt, H. (2023). Noninvasive Ventilation and Rapid Enteral Feeding Advances in Preterm Infants—2-Year Follow-Up of the STENA-Cohort. Nutrients, 15(5), 1292. https://doi.org/10.3390/nu15051292






