Abstract
Type 2 diabetes mellitus (T2DM) is a major cause of morbidity and mortality, and it is a major risk factor for the early onset of cardiovascular diseases (CVDs). More than genetics, food, physical activity, walkability, and air pollution are lifestyle factors, which have the greatest impact on T2DM. Certain diets have been shown to be associated with lower T2DM and cardiovascular risk. Diminishing added sugar and processed fats and increasing antioxidant-rich vegetable and fruit intake has often been highlighted, as in the Mediterranean diet. However, less is known about the interest of proteins in low-fat dairy and whey in particular, which have great potential to improve T2DM and could be used safely as a part of a multi-target strategy. This review discusses all the biochemical and clinical aspects of the benefits of high-quality whey, which is now considered a functional food, for prevention and improvement of T2DM and CVDs by insulin- and non-insulin-dependent mechanisms.
1. Introduction
Cardiovascular diseases (CVDs) are the major cause of death globally. Around 17.9 million people died from CVDs in 2019, representing 32% of all deaths [1]. Type 2 diabetes mellitus (T2DM) is a major comorbidity associated with CVDs. Globally, overall CVDs affect around 32.2% of all people with T2DM [2], and CVDs are a major cause of mortality among people with T2DM, accounting for approximately half of all deaths.
Global diabetes is a pandemic with a prevalence in 20–79-year-olds in 2021 of 10.5% (536.6 million people), which should reach 12.2% (783.2 million) in 2045 [3]. Individuals with T2DM are at high risk for microvascular complications (including retinopathy, nephropathy, and neuropathy) as well as macrovascular issues (cardiovascular comorbidities) caused by hyperglycemia and insulin resistance [4]. Diabetes and hypertension share similar risk factors, such as dyslipidemia, obesity, endothelial dysfunction, and vascular inflammation leading to atherosclerosis.
In general population, diabetes and CVDs are linked to various parameters in particular diet, physical activity, walkability, and air pollution (NO2 and PM 2.5) more than genetics [5,6,7].
Diet is a crucial factor to prevent diabetes and CVDs, and patients with T2DM may benefit from diminishing added sugars and increasing proteins and non-processed as well as vegetable fats [8,9,10]. We know this in particular, as decades of added sugar damage to health have been largely underestimated in comparison with “bad fats”, such as cholesterol or saturated fat, whose harmful effects have been pointed out excessively worldwide. We now know that the Sugar Research Foundation (SRF) since the 1950s has constantly paid millions of U.S. dollars to feed people with false information based on warped or false studies [11]. This has been only recently been clearly denounced according to precise documents from the SFR in the Journal of the American Medical Association (JAMA) [12] and has been reproduced in the New York Times [13]. The real “bad fats” that have a clear negative impact on diabetes and CVDs are essentially processed fats and trans-fats but not necessarily saturated fats [14].
Meta-analyses suggest that certain food interventions and diets such as the Mediterranean diet have a beneficial role on CVD prevention in populations, including individuals with diabetes [15,16]. Red meat and processed meats seem to be associated with T2DM, whereas soy and milk products seem to protect against T2DM. Egg and fish intake do not appear to be significantly associated with a decrease in T2DM risk [17].
Dairy products, in particular low-fat dairy, are negatively associated with T2DM [18]. Milk protein is made primarily of whey and casein proteins, representing approximately 20 and 80% of the total proteins, respectively [19].
Whey, a by-product of cheese and curd manufacturing, was formerly considered a waste product. It is a protein complex derived from milk, which is nowadays presented as a functional food, having a number of health benefits [20]. The biological components of whey include β-lactoglobulin (β-Lg) (45–57%), α-lactalbumin (α-La) (15–25%), immunoglobulins (IGs) (10–15%), glycomacropeptide (up to 15%), serum albumin (SA) (10%), lactoferrin (Lf) (≈1%), and lactoperoxidase (<1%). Interestingly, Hippocrates already praised the health benefits of whey in Ancient Greece, and it was used as a medicine through the Middle Age (for burn soothing, vitality, and to improve various illnesses) [21]. There are diverse types of whey protein products available for nutrition and food supplements: whey protein concentrates (WPCs) (which contain 25%–80% protein), whey protein isolates (WPIs) (containing ≥ 90% protein), whey protein hydrolysates, β–Lg, α-La, and protein-peptone fraction [22].
The goal of this review is to discuss all the biochemical and clinical aspects of the benefits of whey for prevention and improvement of postprandial hyperglycemia, T2DM, and CVDs.
2. Whey and Dairy Product Intake Induce Benefits on T2DM Parameters
In a meta-analysis on cohort studies, Tong et al. reported that T2DM risk could be decreased by 5% with dairy products and 10% for low-fat dairy products with daily intakes [18]. Another meta-analysis of seventeen cohort studies demonstrated significant inverse associations between T2DM risk and intake of total dairy products and low-fat dairy products [23].
2.1. Whey Intake Improved Insulin Secretion and Postprandial Glycemia
Milk-derived whey as well as casein proteins can produce insulin secretion in obese, pre-diabetic, and also type 2 diabetes individuals [24,25,26,27,28,29]. Studies in humans have shown that whey protein decreases postprandial glycemia and could be used in medical/nutritional therapy to regulate blood sugar [30,31]. In diabetic subjects, whey intake has been associated with a reduction of postprandial hyperglycemia [32,33]. Even a small 15 g dose of whey protein consumed shortly before mixed-macronutrient meals stimulates insulin release, improves postprandial glycemia (−13%), and increases satiety in T2DM subjects (p < 0.05) [34].
Various studies have similarly reported positive effects of whey protein on insulin secretion [35]. The intake of 50 g WPI associated with maltodextrin increased insulin production by 96% versus maltodextrin alone in pre-diabetic adults (p < 0.05) and a 21% decrease in postprandial blood glucose after protein meals (p < 0.0001) [36]. Interestingly, for practical nutrition, the addition of whey (27.6 g) to high-glycemic-index meals (such as bread and mashed potatoes and meatballs) increases insulin release (31% for breakfast and 57% for lunch, both p < 0.05) and diminishes postprandial blood glucose (−21%, p < 0.05) excursion in type 2 diabetic subjects [37].
In lean and healthy subjects, whey consumption has also been shown to decrease blood sugar [36,38]. Between whey, tuna, turkey, and egg albumin, the measure of postprandial glucose and insulin concentrations in 22 lean, healthy men provided the best results for whey [24]. Blood glucose was significantly lower for whey meal than for turkey (p < 0.023) and eggs (p < 0.001), indicating a faster glucose uptake in cells, but not with the tuna meal. Blood insulin was also significantly higher for whey compared to tuna, turkey, and eggs (all p < 0.001).
Moreover, whey protein may have beneficial effects on some symptoms of metabolic syndrome and improve cardiovascular risk factors [35,39,40]. Metabolic syndrome is a combination of hyperglycemia, hypertension, excess body fat around the waist, and abnormal cholesterol or triglyceride levels, increasing the risk of diabetes, heart disease, and stroke [41]. Moreover, a meta-analysis of 22 randomized controlled trials (RCTs), using the Cochrane method for the elimination of bias, showed that whey intake decreased insulin significantly in patients with metabolic syndrome (weighted mean difference (WMD): −0.94; 95% CI: −1.68, −0.21) but did not have an effect on fasting plasma glucose levels [42]. Meanwhile subgroup analyses showed a significant reduction in fasting plasma glucose levels and other meta-analyses including in obese participants who had also shown improvement in fasting plasma glucose levels after whey protein intake [43,44].
2.2. Effects on Insulin Resistance and Glycated Hemoglobin
A meta-analysis of 22 randomized controlled trials (RCTs) highlighted a significant decrease in glycated hemoglobin (HbA1c) with whey intake in patients with metabolic syndrome (WMD: −0.15; 95% CI: −0.29, −0.01) and in the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (WMD: −0.20; 95% CI: −0.36, −0.05) [42]. Another meta-analysis of 30 RCTs suggested that dairy intake, in particular, low-fat dairy products, has a positive action on HOMA-IR (mean difference (MD) of −1.21; 95% CI −1.74 to −0.67; p < 0.00001; I2 = 92%) [45].
Dairy protein consumption before a meal decreases food intake and, in association with carbohydrates, decreases glycemia by insulin-dependent as well as insulin-independent mechanisms [46,47].
Interestingly, whey can also be effective for controlling blood sugar parameters and inflammation before surgery. Fasting before surgery, which can be prolonged from 10 up to 16 h, can induce hyperglycemia due to a limitation of insulin action by the effect of counter-regulatory hormone action. Whey protein in a drink associated with carbohydrates has been shown to minimize the postoperative insulin resistance (HOMA-IR) and associated acute inflammation vs. carbohydrates alone (2.75 ± 0.72 vs. 5.74 ± 1.16; p < 0.05) [48].
3. Mechanisms of Whey and Dairy Proteins Associated with Decrease in Postprandial Glycemia
3.1. Activity of Amino Acids on Insulin Secretion
It has been confirmed that the insulinotropic effect of dairy proteins is associated with certain amino acids, in particular the branched-chain amino acids (BCAAs) who seem to be of vital importance, especially leucine, isoleucine, valine, lysine, and threonine, inducing insulin secretion with leucine, reportedly having the greatest effect acutely [49,50,51]. Leucine activates glutamate dehydrogenase activity in β-cells, which leads to an increase in Krebs cycle activity, oxygen consumption by these cells, and then to increased insulin production [49]. Leucine and high protein intake also seem to modulate AMP-activated protein kinase (AMPK) and mTOR and influence hypothalamic neuropeptides, reducing the expression of orexigenic neuropeptides (NPY) and AgRP (Agouti-related peptide) and increasing anorexigenic neuropeptide pro-opiomelanocortin (POMC) [51].
Whey protein is an exceptional source of BCAAs, which are easily and quickly digested, leading to a rapid increase in BCAA leads in the circulation and insulin release, which may improve postprandial hyperglycemia (Figure 1) [42]. Glutamate and alanine can also participate in insulin secretion coupling, not alone but by amplifying the stimulation by glucose [50]. Cysteine could also be implicated in this process [52].
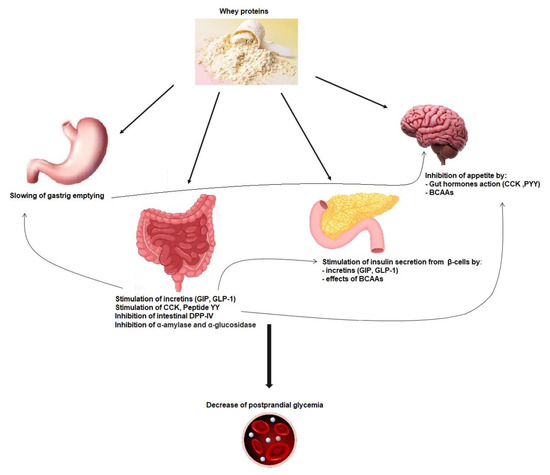
Figure 1.
Mechanisms implicated in whey protein activity on postprandial glycemia reduction. GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagon-like-peptide-1; CCK: cholecystokinin; PYY: peptide YY; DPP-IV: dipeptidyl peptidase-IV; BCAAs: branched-chain amino acids.
3.2. Incretin Secretion and Insulin Secretion
Dairy-protein-derived peptides can also increase the insulin secretion effect through dipeptidyl peptidase-4 (DPP-4) inhibitory activity in the proximal gut, preventing the incretin glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like-peptide-1 (GLP-1) degradation [40] (Figure 1) [53]. Indeed a major part of secreted insulin is a result of the action of both GIP and GLP-1. However, because of the cleaving activity of DPP-4, GIP and GLP-1 have a half-life of only 1–2 min [54]. Thus, inhibiting DPP-IV activity is considered as a way of treatment in T2DM [55]. Incretins also increase the sensitivity of β-cells to glucose, stimulate β-cell proliferation, and protect these cells against apoptosis [56].
When provided shortly prior to a meal, whey dose-dependently reduces (using from 9 to 18 g) the postprandial glycemia (p < 0.0001) and increases GLP-1 levels (p < 0.0001) [57]. Bioactive substances in whey, among which are IGs, Lf, α-La, and glutamine, have been shown to increase incretin hormones and to inhibit dipeptidyl peptidase-IV [31,42,58,59]. Other studies have shown a strong increase in GLP-1 concentrations after a whey drink when compared to a glucose or fructose drink (from 25 to 50 g of whey, p < 0.05) taken before a meal (30 min to 4 h) [60,61].
Some bioactive peptides should be responsible for influencing postprandial incretin responses [62]. Indeed, whey and milk proteins are degraded during low-pH digestion in the stomach and by gastric pepsin and other peptidases. The resulting hydrolyzed proteins pass into the small intestine and are further split by pancreatic proteases into single amino acids and oligopeptides and finally by other enzymes from the brush-border enzymes into dipeptides, tripeptides, and amino acids. Bioactive peptides can contain from two to twenty amino acid residues or more. Some of these peptides have been identified in the gastrointestinal tract as well as in bloodstream after milk intake, but more studies are needed to fully characterize these peptides and their precise role in glycemia management [62].
However, in present hypertriglyceridemia, obesity and high-baseline GLP-1 levels tend to have poorer response to whey proteins [63], and even positive results have been also observed [25]. It could be linked to glucagon-induced increase with whey protein intake [60]. Furthermore, hypertriglyceridemia augments the hyperglycemic effects of glucagon [64].
Whey proteins seem also to inhibit other enzymes such as α-amylase and α-glucosidase [53,65,66].
3.3. Gastric Emptying Effect on Postprandial Glycemia
The actions of whey proteins on gastric emptying, on postprandial glycemia, and on secretion of incretin hormones are linked together. In addition to its impact on insulinotropic effects, GLP-1 induced by whey intake is also able to slow down gastric emptying by relaxing the proximal stomach, reducing antral and duodenal motility, and increasing pyloric tone. This restrains energy intake and can inhibit glucagon secretion, which all together improve postprandial glycaemia (Figure 1) [67]. The function of the gastrointestinal tract is key for glucose homeostasis, especially during the postprandial phase, and slowing gastric emptying can diminish postprandial glycemic excursions in healthy and diabetic subjects (Figure 1) [58,68,69,70]. Other gut hormones, namely, cholecystokinin (CCK) and peptide YY (PYY), can decrease gastric emptying and appetite [71].
The importance of slowing gastric emptying is key in the decrease in postprandial glycemia observed when proteins are added to glucose intake [72]. Likewise, a whey “preload” is able to slow gastric emptying of a following meal in both healthy [73] and T2DM subjects [74]. In diabetic subjects, GLP-1 was higher when whey was ingested (55 g) between −15 min and 90 min before the meal versus during the mean (p < 0.001), even if the incremental area under the curve (iAUC) was not significantly different [74], and both decrease postprandial glucose (363.7 ± 64.5 mmol · min−1 · L−1) and (406.3 ± 85.9 mmol · min−1 · L−1) compared with no whey (734.9 ± 98.9 mmol · min−1 · L−1; p < 0.005).
3.4. Gut Hormones, Amino Acids, and Satiety
Satiety induced by proteins has been demonstrated, in an acute manner, with meals containing from 25 to 90% proteins, leading to a significant decrease in energy intake. It has also been shown with high protein content in ad libitum diets, lasting from a few days up to 6 months [75]. Among the three macronutrients, protein has the greatest satiating action. After protein-containing nutrient intake, signals can be sent to the central nervous system (CNS) via gastric and gut peptide action and via the bloodstream after digestion. Indeed, satiety is induced by various mechanisms, which are both visceral (during digestion) and metabolic (inter-prandial phase) and directed towards the CNS directly at the level of the hypothalamus and indirectly mainly through the vagus nerve [76]. Regarding meal size, the negative feedback control from gastrointestinal signals and bloodstream takes place in the dorsal vagal complex (brainstem) and in the hypothalamus.
The gut peptide hormones upregulated by whey consumption include CCK, PYY, GLP-1, and GPI (Figure 1) [56,58,73]. It has been proposed that high protein meals could induce the greatest production of PYY and the highest satiety feeling in obese as well as normal-weight human subjects [74]. Ghrelin, an orexigenic peptide decreased after consumption of proteins; leptin; and insulin levels are also known to influence satiety [58,60,74].
Although GLP-1 can have an action on peripheral organs through the circulation, it is of note also that GLP-1 can be produced by the pancreas and brain as well [56].
Studies demonstrate that dairy and whey proteins decrease appetite better than other protein sources such as eggs, casein, or soy [73,77,78,79]. In the study from Hall et al., plasma CCK was increased by 60% (iAUC, p < 0.005), GLP-1 by 65% (iAUC, p < 0.05), and GIP by 36% (iAUC, p < 0.01) following a 48 g whey preload when compared with casein, showing the particular potential of whey in this field [73]. Whey, tuna, turkey, and egg albumin meals were compared in terms of appetite measures and energy intake in 22 lean healthy men. The best results were obtained for whey, with a significant reduction of mean energy intake at the ad libitum meal 4 h after (p < 0.001) [24]. Appetite rated by the subjects, postprandial insulin, and energy intake during the meal were strongly related.
4. Other Mechanisms of Whey and Dairy Proteins Associated with the Benefits on T2DM and Cardiovascular Risk
4.1. Effects of Whey Intake on Lipid Profile
Different works have shown that whey consumption ameliorates some biological markers in obesity and T2DM such as fasting lipids.
In a study with whey supplementation in overweight/obese individuals (27 men and women with a mean age of 48 years) for 12 weeks, fasting triglycerides were lowered after 6 weeks (p < 0.025) as well as a total cholesterol and LDL cholesterol after 12 weeks in the whey group (p < 0.001 and 0.003, respectively) [25]. It was also lowered in comparison with a casein group (p < 0.026 and p < 0.045, respectively). In this study, fasting insulin measurements and insulin resistance were also significantly diminished in the whey group versus the control (p < 0.049 and p < 0.034, respectively).
A systematic review and meta-analysis of 22 RCTs in patients with metabolic syndrome, using the Cochrane Collaboration risk of bias tool, showed a significant decrease in the values for triglycerides (WMD: −17.12; 95% CI: −26.52, −7.72), total cholesterol (WMD: −10.88; 95% CI −18.60, −3.17), LDL-cholesterol levels (WMD: −8.47 95% CI: −16.59, −0.36), and total cholesterol/HDL cholesterol ratio (WMD: −0.26; 95% CI: −0.41, −0.10) [42]. Another meta-analysis of 13 trials showed that whey protein supplementation significantly decreased blood triglyceride level by 0.11 mmol/L (95% CI: −0.21, 0 mmol/L), but had no effects on blood total cholesterol (−0.11 mmol/L, 95% CI: −0.27, 0.05 mmol/L), LDL- cholesterol levels (−0.08 mmol/L, 95% CI: −0.23, 0.07 mmol/L), and HDL-cholesterol (0.01 mmol/L, 95% CI: −0.04, 0.05 mmol/L) [80]. Other encouraging results were highlighted in a meta-analysis including nine RCTs, among which, trials on overweight and obese patients and whey intake improved HDL-cholesterol and total cholesterol levels as well as systolic and diastolic blood pressure and fasting plasma glucose (all p < 0.05) [43]. Moreover, in metabolic syndrome, in subjects receiving yogurt enriched with whey (5 g), calcium (500 mg), vitamin D (500 IU), prebiotic fiber (3 g), and probiotic cultures for 10 weeks, a significant reduction of triglyceride levels (p < 0.001) and insulin resistance (HOMA-IR) (p = 0.025), as well as increased HDL-cholesterol levels (p < 0.01), were observed [81].
Altogether, these results indicate that whey intake could improve lipidemia. It has been proposed that protein intake could decrease the production of chylomicrons [40]. The insulinotropic effect of whey proteins may enhance lipoprotein lipase (the enzyme that hydrolyses triglycerides in chylomicrons and very low density lipoprotein (VLDL)) activity and increase the clearance of chylomicrons [29]. Whey proteins can also decrease the intestinal absorption of cholesterol as well as its de novo generation in the liver [25].
4.2. Improvement of Obesity and Weight by Whey Intake
Decreasing weight and obesity can improve diabetes parameters and prevent CVDs. A double-blind RCT on 90 obese subjects for 23 weeks, comparing whey protein to soy (≈56 g/d of protein and 1670 kJ/d) and to an isoenergetic carbohydrate intake, showed significant decreases of body weight and fat mass of 1.8 kg (p < 0.006) and 2.3 kg (p < 0.005), respectively, in comparison with the carbohydrate group [82]. Body weight and composition did not differ significantly between the groups consuming soy and whey or between soy and carbohydrates. No lean mass difference was observed between all groups. Moreover, waist circumference decreased significantly in the whey group versus the two others (p < 0.05). This is supported by a meta-analysis on 30 RCTs, which suggests that low-fat dairy products may have beneficial results on waist circumference in a total of 1348 individuals (MD: −1.09 cm (95% CI 1.68 to −0.58; p < 0.00001; I2 = 94%)) and body weight in 2362 individuals with 0.42 kg less than control (p < 0.00001; I2 = 92%) [45]. In another meta-analysis with nine RCTs included, whey intake led to a significant decrease in body weight (MD = 0.56, 95% CI: 0.30–0.81), lean mass (MD = 0.77, 95% CI: 0.59–0.96), and fat mass (MD = 1.12, 95% CI: 0.77–1.47) [43].
Furthermore, whey has also been shown to decrease fat in the liver of obese patients [83].
People with less protein in their diet have more chances of developing obesity, such as in women and children [84,85,86]. Clinical studies and meta-analyses show that whey decreases fat mass, total weight, and waist circumference when sugar is lowered and replaced by protein in isoenergetic meals in overweight/obese people after a few weeks/months [44,82,87].
Whey proteins and proteins in general also have an interesting metabolic property that can be useful for losing weight and avoiding obesity: digesting and processing proteins requires more energy/calories than lipids and fats for the body [88], called the “thermic effect of food” (TEF). Evidence suggests TEF as a weight-loss tool for research and clinical studies [89]. Indeed, reported TEF for protein is 20–30% of energy content, being 0–3% for fats and 5–10% for carbohydrates [90,91].
Moreover, if a diet for weight loss is only based on dietary energy restriction (500–750 kcal/d energy deficit), the problem is that 25% of the body mass that is lost is muscle/lean mass [86]. Then, high-quality protein is needed to avoid this lean mass loss [84,92]. Whey protein is one of the best quality proteins with regard to its amino acid profile (high essential, branched-chain, and leucine content) and fast digestibility [93]. Whey protein has been found to stimulate muscle protein synthesis with a significantly better yield than casein, soy, and other proteins, and clinical studies have confirmed the interest of whey to avoid losing muscle/lean mass [94,95,96]. Meta-analyses observing shorter-term as well as long-term studies on energy restriction point out that the daily protein intake necessary to avoid lean mass loss would be between 1.2 and 1.5 g protein/kg/d (i.e., ≈89–119 g protein/d for women and 104–138 g protein/d for men) [92,97,98,99]. However, other works suggest that lower quantities (i.e., 0.8–1.2 g protein/kg/d) seem to be enough for the preservation of lean mass during an energy restriction diet [100].
4.3. Effect of Whey Intake on Hypertension
High blood pressure/hypertension is a major global public health issue. Hypertension and diabetes T2DM are interrelated diseases that strongly predispose to CVDs and stroke [101].
Increasingly more clinical studies have shown that milk, fermented milk products, and peptides from whey and casein could bring about significant improvement in blood pressure [102]. Some studies demonstrated that whey intake ameliorates blood pressure in overweight and obese individuals. In a study with whey protein or glucose (27 g) supplementation for 12 weeks (70 subjects), systolic blood pressure was significantly lower with whey after 6 weeks (p < 0.05) as well as diastolic blood pressure (p < 0.05) after 12 weeks [25]. Moreover, an improvement of vascular function was observed as measured by a decreased augmentation index (AI), which is an indirect measure of arterial stiffness, after 12 weeks of whey intake (p < 0.05).
Potent reductions in systolic blood pressure have been observed with several whey peptides containing four or fewer amino acid residues [103]. The short peptides described by FitzGerald et al. are YGLF f(50–53) in α-La, IPA f(78–80) in β-Lg, FP f(221–222) in bovine serum albumin, and GKP f(18–20) in β2-microglobulin, which produced maximum decreases in systolic blood pressure of 23, 31, 27, and 26 mmHg, respectively.
4.4. Importance of Antioxidant and Anti-Inflammatory Potential of Whey Proteins with Regard to T2DM and Cardiovascular Health
Whey protein can exert antioxidative effects, in the first place, as an enhancer of the synthesis of reduced glutathione (GSH) and can also activate the endogenous antioxidative enzyme system. The correlation between low glutathione (GSH) and diabetes has been described. Diabetes has been linked to oxidative damage and decreased GSH content (also increased GSSG/GSH ratio, oxidized glutathione/reduced glutathione) in different tissues [104,105,106]. The decrease in GSH is, in most cases, associated with an increased activity of NF-kB, an inflammation node [107], and GSH is key in lowering oxidative stress and insulin resistance [52,108].
Several studies have highlighted that in obese patients, oxidative stress is also associated with a decrease in GSH levels [109,110] and a decrease in the GSH/GSSG ratio [111]. Furthermore, nutritional stress caused by a diet high in fats and carbohydrates promotes oxidative stress, as evidenced by increased lipid peroxidation products, decreased antioxidant system, and lower GSH levels [112,113].
If physical activity can lead to an improvement of the antioxidant systems and defenses as well as reduced risks of CVDs in overweight populations, the synergy of resistance exercises in association with whey intake has been shown to be more efficient [114].
Antioxidant/anti-inflammatory capacities of whey and dairy have been shown in various conditions with both low- and full-fat dairy products, as well as fermented dairy foods, even if some studies showed no effect [115,116,117,118]. Moreover, a few studies have already shown the interest of whey in other conditions as in gut inflammation and Crohn’s disease [119,120], leaky gut [121,122], or rheumatoid arthritis [123].
5. Discussion on Long-Term Effects of Whey Protein and of Other Natural Compounds on Glycemic Parameters, T2DM, and Cardiovascular Health
Consuming a 15 g premeal of whey protein before each main meal decreases daily hyperglycemia by 8% (p < 0.05), which helps to maintain the body for longer periods (≈2 h/day, p < 0.05) in an euglycemic area, especially for people with T2DM [32]. Although most of the recent evidence in systematic reviews and meta-analysis show beneficial effects of whey protein supplementation on postprandial and short-term glycemic response, as well as blood lipid profile, other long-term clinical data are needed for better understanding the benefits of whey intake on postprandial and baseline glycemia after several weeks/months [42,124,125,126]. Increasingly more studies have investigated the effects of whey and its bioactive peptides and biochemical and biological pathways, especially on longer periods on glucose and lipid metabolism, hypertension, oxidative stress and inflammation, and vascular health [127,128]. Some studies suggest that regular whey intake may positively affect long-term glycemic control [129,130]. Whey may also consolidate arterial walls, which can prevent and improve cardiovascular diseases [25,59]. Altogether, these results indicate a potential for whey in preventing and improving pre-diabetic and diabetic conditions, as well as hypertension and cardiovascular diseases.
Moreover, of importance, the best whey products, WPC and WPI, are concentrates obtained by microfiltration at low temperature (cold-process) and without applying extreme pH (acid or basic) as is it used in the “ion exchange” process, in order to protect the most sensitive amino acids such as sulfur amino acids, tryptophan, and others. Meanwhile, most of the whey encountered on the market are processed with heat and/or with strong acids or bases, both of which can denature the 3D structure of proteins and oxidize some key amino acids, which have strong benefits for our health [131,132,133].
Indeed, whey proteins can be denatured by heat, inducing the stabilization of disulfide bonds, gelation, and possible loss of a part of antioxidant activity [22,132,133]. Heat-treated WPC solutions can generate these disulfide-bonded aggregates between all proteins present in whey: β-Lg, α–La, IgG, SA, and Lf [131]. Heat stability of the major whey protein is relative to globular proteins (β-Lg and α-La), and complete denaturation may occur at 90 °C beyond 10 min [134]. For minor whey proteins, i.e., SA and IGs, denaturation starts at ≈65 °C, whereas for the major whey proteins, denaturation starts only above 70–75 °C [135,136]. Thus, the order of sensitivity to heat-induced denaturation is the following: IGs > SA > β-Lg A > β-Lg B > α-La [135,136,137]. This denaturation can increase the oxidability of various sensitive amino acids. With heat, milk and whey proteins can be altered by Maillard reactions [138]. Among the several types of amino acids, the sulfur amino acids are more prone to oxidation. Methionine, for example, is very easily oxidized, producing methionine sulfoxide as a first step, which has been found in heated milk, whey, and casein products [139,140].
Other natural products and foods than dairy and whey are known to have the potential to prevent or improve T2DM. Studies and meta-analyses suggest that certain food interventions and diets such as the Mediterranean diet in particular, rich in fruits and vegetables, nuts, beans, cereals, and fish rather than red meat, and unsaturated oils and fats such as olive oil, have a beneficial role in CVDs, prevention in populations including people with T2DM [15,16,141]. An amount of 200 g/day of fruit intake seems to be a threshold to reduce the risk of T2DM [142,143], and it is linked with their rich content in dietary polyphenols, which may also decrease T2DM risk and associated complications, although some controversial results have been also published [144,145]. Some alkaloids such as berberine, trigonelline, and capsaicin are also promising compounds that may be useful in the treatment of T2DM through various mechanisms including the inhibition of α-glucosidase and DPP-IV and modulation of oxidative stress [146].
Moreover, natural sugars present in plants such as iminosugars (monosaccharide sugars in which nitrogen replaces the ring oxygen) that can inhibit gut α-glucosidase and reduce carbohydrate breakdown in the upper gastrointestinal tract [147,148,149]. Some medicines have already been developed from this research such as Glyset® and Zavesca®, which are derived from the natural compound 1-deoxynojirimycin [147]. Meanwhile, mainly at the level of the digestive system, undigested saccharides become a food source for microbial fermentation [150].
Since diabetes is a multifactorial condition, it is not surprising that there is still no simple cure or drug for managing blood glucose levels, T2DM, and other cardiovascular comorbidities. The main drugs are biguanides, such as metformin, which reduce gluconeogenesis in the liver; sulfonylureas, which stimulate insulin secretion; thiazolidinediones, which are insulin sensitizers; and various other tracks, among which are aldose reductase and tyrosine phosphatase 1B, free fatty acid receptor 1 (FFAR1), G-protein-coupled receptor (GPCR), peroxisome-proliferator-activated receptor-γ (PPARγ), sodium glucose co-transporter-2 (SGLT2), α-glucosidase, aldose reductase, glycogen phosphorylase (GP) [151]. In the future, efficient strategies against T2DM should focus on multi-target compounds [152]. This is where natural compounds such as whey, which has various action pathways, seem useful and promising and would deserve longer-term studies (months, years) to assess their efficacy on various T2DM and glycemia parameters. The magnitude of hyperglycemia reduction induced by whey has been found to be comparable with some interventions with drugs such as sulfonylureas, and several authors conclude that this should have implications for nutritional strategies against T2DM [74]. Whey could be associated with other natural compounds such as polyphenols, alkaloids, or iminosugars.
Furthermore, metformin and several drugs that have been developed have various side effects (mild and serious) that are also responsible for a lack of adherence and compliance from the subjects [153]. On the contrary, whey is a very safe nutrient/supplement. For nutrition interventions, whey should be used to adjust the protein intake in order to reach the global daily needs between 0.8 and 1.2 g/kg body weight/day, which is necessary for optimization of body functions, but probably not higher, even though there is no evidence that too much protein can damage the kidneys of healthy people [154]. High-protein meals are not advised for subjects with renal issues as they can lead to glomerular hyper-filtration, raise the pressure inside the kidneys, and accelerate chronic kidney diseases [155,156].
Finally, new axes could emerge more strongly in T2DM research, such as the impact of intestinal microbiota on the regulation of insulin content, insulin resistance, and the regulation of blood glucose [157]. Various pathological pathways may be implicated in the process, through gut barrier health, inflammation, levels of incretins, or the production of short-chain fatty acids (SCFAs). Several measures based on intestinal flora, diets, and supplements such as probiotics and others could be used to treat and even prevent T2DM [158]. Various studies have already shown the benefits of whey with strong prebiotic effect on the gut microbiota on normal weight as well as and obese subjects with increase in Bifidobacterium and Lactobacillus groups [159] and on gut inflammation, Crohn’s disease [119,120], and leaky gut improvement [121,122].
Moreover, the impact of whey intake, alone or in association with polyphenol compounds for example, on the improvement of antioxidant/anti-inflammatory status as well as the increase in the time passed in euglycemia state (also associated with less oxidative stress [160]) are areas that deserve to be studied for T2DM management.
Thus, together with healthy diets, without forgetting other key parameters such as physical activity and avoiding too high air pollution [5], high-quality whey can be a valuable tool for managing postprandial hyperglycemia and associated oxidative stress, blood lipid profile, and insulin resistance, and it can globally contribute to prevention and improvement of T2DM and CVDs, even if more long-term studies are needed.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Scientific adviser at Protelicious Ltd.
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.B.; Ravnskjær, L.; Loft, S.; Andersen, K.K.; Bräuner, E.V.; Baastrup, R.; Yao, C.; Ketzel, M.; Becker, T.; Brandt, J.; et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ. Int. 2016, 91, 243–250. [Google Scholar] [CrossRef]
- Thomas, D.E.; Elliott, E.J. The use of low-glycaemic index diets in diabetes control. Br. J. Nutr. 2010, 104, 797–802. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, Z.L.; Wang, P.Y.; Qin, L.Q. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: Meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 781–789. [Google Scholar] [CrossRef]
- Viana, L.V.; Gross, J.L.; Azevedo, M.J. Dietary intervention in patients with gestational diabetes mellitus: A systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care 2014, 37, 3345–3355. [Google Scholar] [CrossRef]
- Hass, H.B. What’s new in sugar research. In Proceedings of the American Society of Sugar Beet Technologists, Denver, CO, USA, 2–5 February 1954. [Google Scholar]
- Kearns, C.E.; Schmidt, L.A.; Glantz, S.A. Sugar Industry and Coronary Heart Disease Research: A Historical Analysis of Internal Industry Documents. JAMA Intern. Med. 2016, 176, 1680–1685. [Google Scholar] [CrossRef]
- O’Connor, A. How the Sugar Industry Shifted Blame to Fat. Available online: http://www.nytimes.com/2016/09/13/well/eat/how-the-sugar-industry-shifted-blame-to-fat.html?_r=0 (accessed on 12 September 2016).
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients 2020, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef]
- Tong, X.; Dong, J.Y.; Wu, Z.W.; Li, W.; Qin, L.Q. Dairy consumption and risk of type 2 diabetes mellitus: A meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. Handbook of Milk Composition; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Walzem, R.L.; Dillard, C.J.; German, J.B. Whey components: Millennia of evolution create functionalities for mammalian nutrition: What we know and what we may be overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef]
- Kosikowski, F.V. Whey and Whey Foods, Cheese and Fermented Milk Foods; Kosikowski, F.V., Ed.; Edwards Brothers: New York, NY, USA, 1982; pp. 446–469. [Google Scholar]
- Jovanović, S.; Barać, M.; Maćej, O. Whey Proteins-Properties and Possibility of Application. Mljekarstvo 2005, 55, 215–233. [Google Scholar]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br. J. Nutr. 2010, 104, 1241–1248. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity 2010, 18, 1354–1359. [Google Scholar] [CrossRef]
- Jonker, J.T.; Wijngaarden, M.A.; Kloek, J.; Groeneveld, Y.; Gerhardt, C.; Brand, R.; Kies, A.K.; Romijn, J.A.; Smit, J.W. Effects of low doses of casein hydrolysate on post-challenge glucose and insulin levels. Eur. J. Intern. Med. 2011, 22, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Gunnerud, U.J.; Ostman, E.M.; Björck, I.M. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur. J. Clin. Nutr. 2013, 67, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Manders, R.J.; Hansen, D.; Zorenc, A.H.; Dendale, P.; Kloek, J.; Saris, W.H.; van Loon, L.J. Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients. J. Med. Food 2014, 17, 758–763. [Google Scholar] [CrossRef]
- Mortensen, L.S.; Holmer-Jensen, J.; Hartvigsen, M.L.; Jensen, V.K.; Astrup, A.; de Vrese, M.; Holst, J.J.; Thomsen, C.; Hermansen, K. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur. J. Clin. Nutr. 2012, 66, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.L.; Ward, L.S.; Bastian, E.D.; Jenkins, A.L.; Campbell, J.; Vuksan, V. A whey protein supplement decreases post-prandial glycemia. Nutr. J. 2009, 8, 47. [Google Scholar] [CrossRef]
- Adams, R.L.; Broughton, K.S. Insulinotropic Effects of Whey: Mechanisms of Action, Recent Clinical Trials, and Clinical Applications. Ann. Nutr. Metab. 2016, 69, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Taylor, G.S.; Brunsgaard, L.H.; Walker, M.; Bowden Davies, K.A.; Stevenson, E.J.; West, D.J. Thrice daily consumption of a novel, premeal shot containing a low dose of whey protein increases time in euglycemia during 7 days of free-living in individuals with type 2 diabetes. BMJ Open Diabetes Res. Care 2022, 10, e002820. [Google Scholar] [CrossRef]
- Comerford, K.B.; Pasin, G. Emerging Evidence for the Importance of Dietary Protein Source on Glucoregulatory Markers and Type 2 Diabetes: Different Effects of Dairy, Meat, Fish, Egg, and Plant Protein Foods. Nutrients 2016, 8, 446. [Google Scholar] [CrossRef]
- King, D.G.; Walker, M.; Campbell, M.D.; Breen, L.; Stevenson, E.J.; West, D.J. A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 550–557. [Google Scholar] [CrossRef]
- Pal, S.; Radavelli-Bagatini, S. The effects of whey protein on cardiometabolic risk factors. Obes. Rev. 2013, 14, 324–343. [Google Scholar] [CrossRef]
- Hoefle, A.S.; Bangert, A.M.; Stamfort, A.; Gedrich, K.; Rist, M.J.; Lee, Y.M.; Skurk, T.; Daniel, H. Metabolic responses of healthy or prediabetic adults to bovine whey protein and sodium caseinate do not differ. J. Nutr. 2015, 145, 467–475. [Google Scholar] [CrossRef]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Björck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [CrossRef]
- Power, O.; Hallihan, A.; Jakeman, P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids 2009, 37, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.T.; Lira, F.S.; Rosa, J.C.; de Oliveira, E.P.; Oyama, L.M.; Santos, R.V.; Pimentel, G.D. Dietary whey protein lessens several risk factors for metabolic diseases: A review. Lipids Health Dis. 2012, 11, 67. [Google Scholar] [CrossRef]
- Bjørnshave, A.; Hermansen, K. Effects of dairy protein and fat on the metabolic syndrome and type 2 diabetes. Rev. Diabet Stud. 2014, 11, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Amirani, E.; Milajerdi, A.; Reiner, Ž.; Mirzaei, H.; Mansournia, M.A.; Asemi, Z. Effects of whey protein on glycemic control and serum lipoproteins in patients with metabolic syndrome and related conditions: A systematic review and meta-analysis of randomized controlled clinical trials. Lipids Health Dis. 2020, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Wirunsawanya, K.; Upala, S.; Jaruvongvanich, V.; Sanguankeo, A. Whey Protein Supplementation Improves Body Composition and Cardiovascular Risk Factors in Overweight and Obese Patients: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018, 37, 60–70. [Google Scholar] [CrossRef]
- Badely, M.; Sepandi, M.; Samadi, M.; Parastouei, K.; Taghdir, M. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta-analysis. Diabetes Metab. Syndr. 2019, 13, 3121–3131. [Google Scholar] [CrossRef]
- Sochol, K.M.; Johns, T.S.; Buttar, R.S.; Randhawa, L.; Sanchez, E.; Gal, M.; Lestrade, K.; Merzkani, M.; Abramowitz, M.K.; Mossavar-Rahmani, Y.; et al. The Effects of Dairy Intake on Insulin Resistance: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 2237. [Google Scholar] [CrossRef]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Jacobs, D.R.; Van Horn, L.; Slattery, M.L.; Kartashov, A.I.; Ludwig, D.S. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA Study. JAMA 2002, 287, 2081–2089. [Google Scholar] [CrossRef]
- Perrone, F.; da-Silva-Filho, A.C.; Adôrno, I.F.; Anabuki, N.T.; Leal, F.S.; Colombo, T.; da Silva, B.D.; Dock-Nascimento, D.B.; Damião, A.; de Aguilar-Nascimento, J.E. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance. A randomized trial. Nutr. J. 2011, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186. [Google Scholar] [CrossRef]
- Potier, M.; Darcel, N.; Tomé, D. Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 54–58. [Google Scholar] [CrossRef]
- Jain, S.K. L-cysteine supplementation as an adjuvant therapy for type-2 diabetes. Can. J. Physiol. Pharmacol. 2012, 90, 1061–1064. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013, 61, 7500–7506. [Google Scholar] [CrossRef]
- Psallas, M.; Manes, C. Incretins in type 2 diabetes mellitus: Cardiovascular and anti-atherogenic effects beyond glucose lowering. Hippokratia 2012, 16, 100–105. [Google Scholar]
- Deacon, C.F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Östman, E.M.; Björck, I.M. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE 2012, 7, e44731. [Google Scholar] [CrossRef]
- Mignone, L.E.; Wu, T.; Horowitz, M.; Rayner, C.K. Whey protein: The “whey” forward for treatment of type 2 diabetes. World J. Diabetes 2015, 6, 1274–1284. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem. 2013, 24, 1–5. [Google Scholar] [CrossRef]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int. J. Obes. 2007, 31, 1696–1703. [Google Scholar] [CrossRef]
- Wu, T.; Little, T.J.; Bound, M.J.; Borg, M.; Zhang, X.; Deacon, C.F.; Horowitz, M.; Jones, K.L.; Rayner, C.K. A Protein Preload Enhances the Glucose-Lowering Efficacy of Vildagliptin in Type 2 Diabetes. Diabetes Care 2016, 39, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.; Drummond, E.; Brennan, L. Bioavailability of milk protein-derived bioactive peptides: A glycaemic management perspective. Nutr. Res. Rev. 2016, 29, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Almario, R.U.; Buchan, W.M.; Rocke, D.M.; Karakas, S.E. Glucose-lowering effect of whey protein depends upon clinical characteristics of patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000420. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Morita, S.; Vranic, M. Resistance to insulin but not to glucagon in lean human hypertriglyceridemics. Diabetes 1980, 29, 899–905. [Google Scholar] [CrossRef]
- Baba, W.N.; Mudgil, P.; Kamal, H.; Kilari, B.P.; Gan, C.Y.; Maqsood, S. Identification and characterization of novel α-amylase and α-glucosidase inhibitory peptides from camel whey proteins. J. Dairy Sci. 2021, 104, 1364–1377. [Google Scholar] [CrossRef]
- Konrad, B.; Anna, D.; Marek, S.; Marta, P.; Aleksandra, Z.; Józefa, C. The Evaluation of Dipeptidyl Peptidase (DPP)-IV, α-Glucosidase and Angiotensin Converting Enzyme (ACE) Inhibitory Activities of Whey Proteins Hydrolyzed with Serine Protease Isolated from Asian Pumpkin (Cucurbita ficifolia). Int. J. Pept. Res. Ther. 2014, 20, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Edelbroek, M.A.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.K.; Samsom, M.; Jones, K.L.; Horowitz, M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001, 24, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Kojecky, V.; Bernatek, J.; Horowitz, M.; Zemek, S.; Bakala, J.; Hep, A. Prevalence and determinants of delayed gastric emptying in hospitalised Type 2 diabetic patients. World J. Gastroenterol. 2008, 14, 1564–1569. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Fraser, R.J.; Bryant, L.K.; Chapman, M.J.; Wishart, J.; Holloway, R.H.; Butler, R.; Horowitz, M. The relationship between gastric emptying, plasma cholecystokinin, and peptide YY in critically ill patients. Crit. Care 2007, 11, R132. [Google Scholar] [CrossRef]
- Karamanlis, A.; Chaikomin, R.; Doran, S.; Bellon, M.; Bartholomeusz, F.D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am. J. Clin. Nutr. 2007, 86, 1364–1368. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tomé, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 2012, 25, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [CrossRef]
- Uhe, A.M.; Collier, G.R.; O’Dea, K. A comparison of the effects of beef, chicken and fish protein on satiety and amino acid profiles in lean male subjects. J. Nutr. 1992, 122, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Tahavorgar, A.; Vafa, M.; Shidfar, F.; Gohari, M.; Heydari, I. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr. Res. 2014, 34, 856–861. [Google Scholar] [CrossRef]
- Zhang, J.W.; Tong, X.; Wan, Z.; Wang, Y.; Qin, L.Q.; Szeto, I.M. Effect of whey protein on blood lipid profiles: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 879–885. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Brett, N.R.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Stote, K.S.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Clevidence, B.A. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J. Nutr. 2011, 141, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Maiolo, E.; Corazza, M.; Van Dijke, E.; Schneiter, P.; Boss, A.; Carrel, G.; Giusti, V.; Lê, K.A.; Quo Chong, D.G.; et al. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin. Nutr. 2011, 30, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef]
- Roberge, J.B.; Van Hulst, A.; Barnett, T.A.; Drapeau, V.; Benedetti, A.; Tremblay, A.; Henderson, M. Lifestyle Habits, Dietary Factors, and the Metabolically Unhealthy Obese Phenotype in Youth. J. Pediatr. 2019, 204, 46–52.e1. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Eneli, I.; Xu, J.; Tindall, A.; Watowicz, R.; Worthington, J.; Tanner, K.; Pratt, K.; Walston, M. Using a Revised Protein-Sparing Modified Fast (rPSMF) for Children and Adolescents with Severe Obesity: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 3061. [Google Scholar] [CrossRef]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Ravn, A.M.; Gregersen, N.T.; Christensen, R.; Rasmussen, L.G.; Hels, O.; Belza, A.; Raben, A.; Larsen, T.M.; Toubro, S.; Astrup, A. Thermic effect of a meal and appetite in adults: An individual participant data meta-analysis of meal-test trials. Food Nutr. Res 2013, 57, 19676. [Google Scholar] [CrossRef]
- Tappy, L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod. Nutr. Dev. 1996, 36, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. S1), A8–A15. [Google Scholar] [CrossRef]
- Smith, G.I.; Commean, P.K.; Reeds, D.N.; Klein, S.; Mittendorfer, B. Effect of Protein Supplementation During Diet-Induced Weight Loss on Muscle Mass and Strength: A Randomized Controlled Study. Obesity 2018, 26, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Nesbit, B.A.; Kiel, J.R.; Sheedy, J.L.; Arterburn, L.M.; Collins, A.E.; Ford, S.A.; Henderson, R.M.; Coleman, C.D.; Beavers, D.P. Effect of an Energy-Restricted, Nutritionally Complete, Higher Protein Meal Plan on Body Composition and Mobility in Older Adults With Obesity: A Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 929–935. [Google Scholar] [CrossRef]
- Merra, G.; Miranda, R.; Barrucco, S.; Gualtieri, P.; Mazza, M.; Moriconi, E.; Marchetti, M.; Chang, T.F.; De Lorenzo, A.; Di Renzo, L. Very-low-calorie ketogenic diet with aminoacid supplement versus very low restricted-calorie diet for preserving muscle mass during weight loss: A pilot double-blind study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2613–2621. [Google Scholar]
- Leidy, H.J.; Carnell, N.S.; Mattes, R.D.; Campbell, W.W. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity 2007, 15, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Evans, E.; Baum, J.I.; Seyler, J.; Erickson, D.J.; Boileau, R.A. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J. Nutr. 2005, 135, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Martens, E.A.; Hochstenbach-Waelen, A.; Lemmens, S.G.; Westerterp-Plantenga, M.S. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J. Nutr. 2013, 143, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Escobar, C. Diabetes and hypertension. What is new. Minerva Cardioangiol. 2009, 57, 705–722. [Google Scholar] [PubMed]
- Huth, P.J.; DiRienzo, D.B.; Miller, G.D. Major scientific advances with dairy foods in nutrition and health. J. Dairy Sci. 2006, 89, 1207–1221. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirokawa, J.; Tagami, S.; Kawakami, Y.; Urata, Y.; Kondo, T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: Regulation of glutathione synthesis and efflux. Diabetologia 1995, 38, 201–210. [Google Scholar] [CrossRef]
- Thornalley, P.J.; McLellan, A.C.; Lo, T.W.; Benn, J.; Sönksen, P.H. Negative association between erythrocyte reduced glutathione concentration and diabetic complications. Clin. Sci. 1996, 91, 575–582. [Google Scholar] [CrossRef]
- Samiec, P.S.; Drews-Botsch, C.; Flagg, E.W.; Kurtz, J.C.; Sternberg, P.; Reed, R.L.; Jones, D.P. Glutathione in human plasma: Decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Arnalich, F.; Hernanz, A.; López-Maderuelo, D.; De la Fuente, M.; Arnalich, F.M.; Andres-Mateos, E.; Fernández-Capitán, C.; Montiel, C. Intracellular glutathione deficiency is associated with enhanced nuclear factor-kappaB activation in older non-insulin dependent diabetic patients. Free Radic. Res. 2001, 35, 873–884. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Micinski, D.; Jain, S.K. Hyperglycemia (high-glucose) decreases L-cysteine and glutathione levels in cultured monocytes and blood of Zucker diabetic rats. Mol. Cell Biochem. 2019, 459, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.A.; Saad, E.A.; Elsharkawy, A.A.; Attia, Z.R. Pro-inflammatory adipocytokines, oxidative stress, insulin, Zn and Cu: Interrelations with obesity in Egyptian non-diabetic obese children and adolescents. Adv. Med. Sci. 2015, 60, 179–185. [Google Scholar] [CrossRef]
- Uzun, H.; Konukoglu, D.; Gelisgen, R.; Zengin, K.; Taskin, M. Plasma protein carbonyl and thiol stress before and after laparoscopic gastric banding in morbidly obese patients. Obes. Surg. 2007, 17, 1367–1373. [Google Scholar] [CrossRef]
- Zamora-Mendoza, R.; Rosas-Vargas, H.; Ramos-Cervantes, M.; Garcia-Zuniga, P.; Perez-Lorenzana, H.; Mendoza-Lorenzo, P.; Perez-Ortiz, A.C.; Estrada-Mena, F.J.; Miliar-Garcia, A.; Lara-Padilla, E.; et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int. J. Obes. 2018, 42, 618–624. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef]
- Andrich, D.E.; Melbouci, L.; Ou, Y.; Auclair, N.; Mercier, J.; Grenier, J.C.; Lira, F.S.; Barreiro, L.B.; Danialou, G.; Comtois, A.S.; et al. A Short-Term High-Fat Diet Alters Glutathione Levels and IL-6 Gene Expression in Oxidative Skeletal Muscles of Young Rats. Front. Physiol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Vatani, D.S.; Golzar., F.A.K. Changes in antioxidant status and cardiovascular risk factors of overweight young men after six weeks supplementation of whey protein isolate and resistance training. Appetite 2012, 59, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Anderson, B.D.; Cifelli, C.J. The Effects of Dairy Product and Dairy Protein Intake on Inflammation: A Systematic Review of the Literature. J. Am. Coll. Nutr. 2021, 40, 571–582. [Google Scholar] [CrossRef]
- Ballard, K.D.; Bruno, R.S.; Seip, R.L.; Quann, E.E.; Volk, B.M.; Freidenreich, D.J.; Kawiecki, D.M.; Kupchak, B.R.; Chung, M.Y.; Kraemer, W.J.; et al. Acute ingestion of a novel whey-derived peptide improves vascular endothelial responses in healthy individuals: A randomized, placebo controlled trial. Nutr. J. 2009, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes Dos Santos, C.; Pinto, P.; Re, R.; Rémond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Feng, Q.; Song, X.; Wang, L.; Sun, L. Whey protein preloading can alleviate stress adaptation disorder and improve hyperglycemia in women with gestational diabetes mellitus. Gynecol. Endocrinol. 2021, 37, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Tanaka, A.; Yamauchi, K.; Iwatsuki, K. Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis. Br. J. Nutr. 2014, 111, 1801–1810. [Google Scholar] [CrossRef]
- Benjamin, J.; Makharia, G.; Ahuja, V.; Anand Rajan, K.D.; Kalaivani, M.; Gupta, S.D.; Joshi, Y.K. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: A randomized controlled trial. Dig. Dis. Sci. 2012, 57, 1000–1012. [Google Scholar] [CrossRef]
- Brimelow, R.E.; West, N.P.; Williams, L.T.; Cripps, A.W.; Cox, A.J. A role for whey-derived lactoferrin and immunoglobulins in the attenuation of obesity-related inflammation and disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 1593–1602. [Google Scholar] [CrossRef]
- Li, Y.; Østergaard, M.V.; Jiang, P.; Chatterton, D.E.; Thymann, T.; Kvistgaard, A.S.; Sangild, P.T. Whey protein processing influences formula-induced gut maturation in preterm pigs. J. Nutr. 2013, 143, 1934–1942. [Google Scholar] [CrossRef]
- Katayama, K.; Matsuno, T.; Waritani, T.; Terato, K.; Shionoya, H. Supplemental treatment of rheumatoid arthritis with natural milk antibodies against enteromicrobes and their toxins: Results of an open-labelled pilot study. Nutr. J. 2011, 10, 2. [Google Scholar] [CrossRef]
- Nouri, M.; Pourghassem Gargari, B.; Tajfar, P.; Tarighat-Esfanjani, A. A systematic review of whey protein supplementation effects on human glycemic control: A mechanistic insight. Diabetes Metab. Syndr. 2022, 16, 102540. [Google Scholar] [CrossRef]
- Oberoi, A.; Giezenaar, C.; Rigda, R.S.; Lange, K.; Horowitz, M.; Jones, K.L.; Chapman, I.; Soenen, S. Comparative Effects of Co-Ingesting Whey Protein and Glucose Alone and Combined on Blood Glucose, Plasma Insulin and Glucagon Concentrations in Younger and Older Men. Nutrients 2022, 14, 3111. [Google Scholar] [CrossRef]
- Giglio, B.M.; Lobo, P.C.B.; Pimentel, G.D. Effects of whey protein supplementation on adiposity, body weight, and glycemic parameters: A synthesis of evidence. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc. Nutr. Soc. 2016, 75, 328–341. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V.; Dhaliwal, S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br. J. Nutr. 2010, 104, 716–723. [Google Scholar] [CrossRef]
- Watson, L.E.; Phillips, L.K.; Wu, T.; Bound, M.J.; Checklin, H.L.; Grivell, J.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. A whey/guar "preload" improves postprandial glycaemia and glycated haemoglobin levels in type 2 diabetes: A 12-week, single-blind, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2019, 21, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jesudason, D.R.; Stevens, J.E.; Keogh, J.B.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Sustained effects of a protein ’preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: A randomized clinical trial. Diabetes Res. Clin. Pract. 2015, 108, e31–e34. [Google Scholar] [CrossRef]
- Havea, P.; Singh, H.; Creamer, L.K.; Campanella, O.H. Electrophoretic characterization of the protein products formed during heat treatment of whey protein concentrate solutions. J. Dairy Sci. 1998, 65, 79–91. [Google Scholar] [CrossRef]
- Havea, P.; Singh, H.; Creamer, L.K. Characterization of heat-induced aggregates of beta-lactoglobulin, alpha-lactalbumin and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res. 2001, 68, 483–497. [Google Scholar] [CrossRef]
- Schokker, E.P.; Singh, H.; Pinder, D.N.; Creamer, L.K. Heat-induced aggregation of β-lactoglobulin AB at pH 2.5 as influenced by ionic strength and protein concentration. J. Agric. Food Chem. 2000, 10, 233–240. [Google Scholar] [CrossRef]
- Edwards, P.J.B.; Jameson, G.B.; Palmano, K.P.; Creamer, L.K. Heat-resistant structural features of bovine β -lactoglobulin A revealed by NMR H/D exchange observations. Int. Dairy J. 2002, 12, 331–344. [Google Scholar] [CrossRef]
- Dannenberg, F.; Kessler, H.G. Reaction kinetics of the denaturation of whey proteins in milk. J. Food Sci. 1988, 53, 258–263. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W.; Pearce, K.N. Kinetics of denaturation and aggregation of whey proteins in skim milk heated in an ultra-high temperature (UHT) pilot plant. Int. Dairy J. 1998, 8, 311–318. [Google Scholar] [CrossRef]
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of heat and high-hydrostatic pressure treatments on the aggregation of whey proteins in whey protein concentrate solutions. Food New Zealand 2004, 4, 29–35. [Google Scholar]
- Meltretter, J.; Wüst, J.; Pischetsrieder, M. Comprehensive analysis of nonenzymatic post-translational β-lactoglobulin modifications in processed milk by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 6971–6981. [Google Scholar] [CrossRef]
- Meltretter, J.; Becker, C.M.; Pischetsrieder, M. Identification and site-specific relative quantification of beta-lactoglobulin modifications in heated milk and dairy products. J. Agric. Food Chem. 2008, 56, 5165–5171. [Google Scholar] [CrossRef]
- Baxter, J.H.; Lai, C.S.; Phillips, R.R.; Dowlati, L.; Chio, J.J.; Luebbers, S.T.; Dimler, S.R.; Johns, P.W. Direct determination of methionine sulfoxide in milk proteins by enzyme hydrolysis/high-performance liquid chromatography. J. Chromatogr. A 2007, 1157, 10–16. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Miao, S.; Huang, Y.; Liu, Z.; Tian, H.; Yin, X.; Tang, W.; Steffen, L.M.; Xi, B. Fruit intake decreases risk of incident type 2 diabetes: An updated meta-analysis. Endocrine 2015, 48, 454–460. [Google Scholar] [CrossRef]
- Xiao, J.B.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Perez de Heredia, F.; Jirillo, E.; Morabito, G.; Marcos, A.; Serafini, M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can. J. Physiol. Pharmacol. 2013, 91, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural Alkaloids and Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew Chem. Int. Ed. Engl. 2023, 62, e202217809. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5, 6 & 6, 6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar] [CrossRef]
- Esposito, A.; D’Alonzo, D.; Fenza, M.; Gregorio, E.; Tamanini, A.; Lippi, G.; Dechecchi, M.C.; Guaragna, A. Synthesis and Therapeutic Applications of Iminosugars in Cystic Fibrosis. Int. J. Mol. Sci. 2020, 21, 3353. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- McGovern, A.; Tippu, Z.; Hinton, W.; Munro, N.; Whyte, M.; de Lusignan, S. Comparison of medication adherence and persistence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2018, 20, 1040–1043. [Google Scholar] [CrossRef]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary protein intake and renal function. Nutr. Metab. 2005, 2, 25. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Appel, L.J.; Anderson, C.A.; Miller, E.R. Effect of a high-protein diet on kidney function in healthy adults: Results from the OmniHeart trial. Am. J. Kidney Dis. 2013, 61, 547–554. [Google Scholar] [CrossRef]
- Friedman, A.N. High-protein diets: Potential effects on the kidney in renal health and disease. Am. J. Kidney Dis. 2004, 44, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharm. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, T.; Wang, W.; Dai, Q.; Ma, X. Will intestinal flora therapy become a new target in type-2 diabetes mellitus? A review based on 13 clinical trials. Nutr. Hosp. 2022, 39, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moya, T.; López-Nicolás, R.; Planes, D.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In vitro modulation of gut microbiota by whey protein to preserve intestinal health. Food Funct. 2017, 8, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).