Dietary-Derived Exosome-like Nanoparticles as Bacterial Modulators: Beyond MicroRNAs
Abstract
1. Introduction
2. Can DENPs Impact Bacteria? A Glance of Scientific Evidence
2.1. Exosomes-like Nanoparticles from Edible Plants
2.1.1. Ginger Exosome-like Nanoparticles
2.1.2. Lemon Exosome-like Nanoparticles
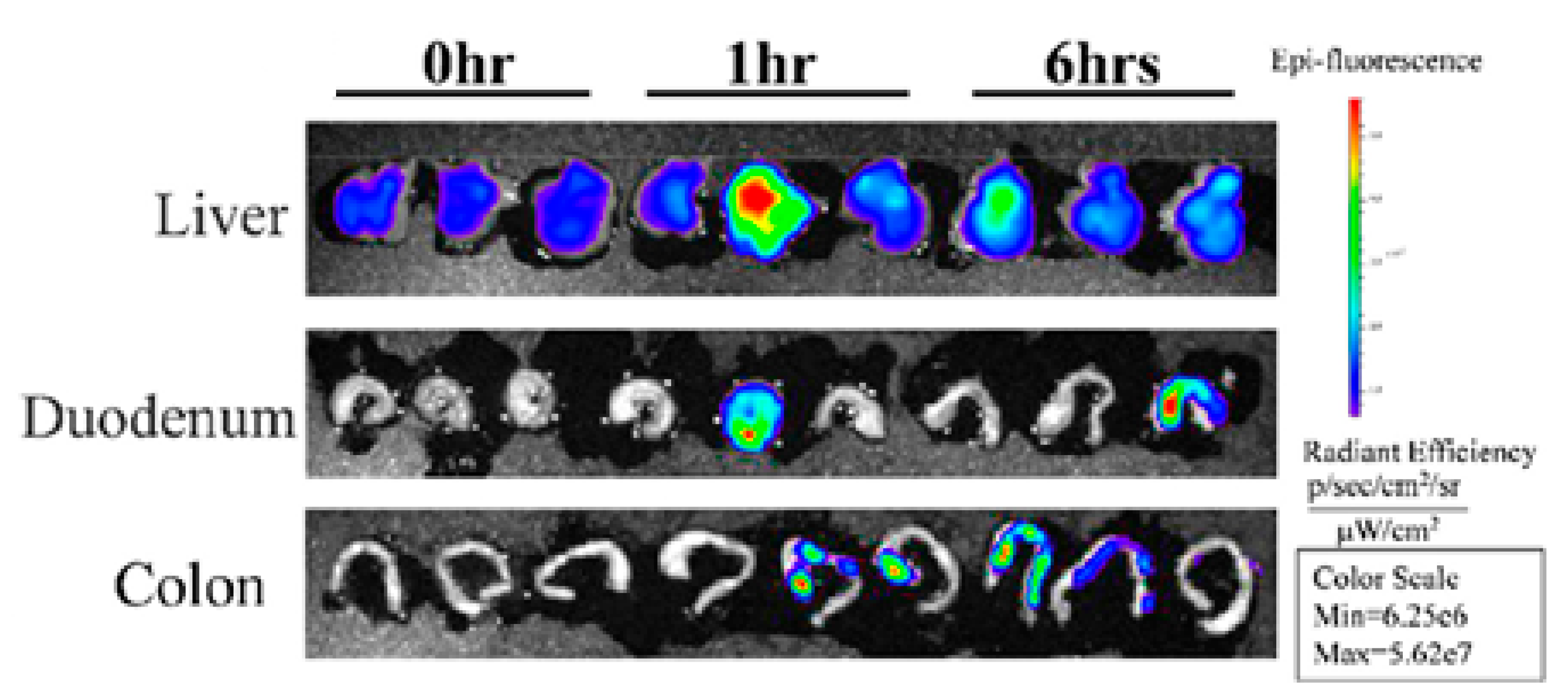
2.1.3. Coconut Exosome-like Nanoparticles
2.1.4. Tartary Buckwheat-Derived Nanovesicles
2.2. Exosomes-like Nanoparticles from Animal-Derived Food
2.2.1. Milk Exosomes
2.2.2. Honey Exosome-like Nanoparticles
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tlaskalova-Hogenova, H.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Vannucci, L.; Tuckova, L.; Rossmann, P.; Hrncir, T.; Kverka, M.; Zakostelska, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.S.; Wessjohann, L.A.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2021, 128, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, L.; Fu, P. Gut microbiota–derived short-chain fatty acids and kidney diseases. Drug Des. Dev. Ther. 2017, 11, 3531–3542. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Wagner, A.E. Impact of Food-Derived Bioactive Compounds on Intestinal Immunity. Biomolecules 2021, 11, 1901–1918. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Rueda-Robles, A.; Rodríguez-Lara, A.; Meyers, M.S.; Sáez-Lara, M.J.; Álvarez-Mercado, A.I. Effect of Probiotics on Host-Microbiota in Bacterial Infections. Pathogens 2022, 11, 986. [Google Scholar] [CrossRef]
- Oliver, S.P. Foodborne Pathogens and Disease Special Issue on the National and International PulseNet Network. Foodborne Pathog. Dis. 2019, 16, 439–440. [Google Scholar] [CrossRef]
- Addis, M.; Sisay, D. A Review on Major Food Borne Bacterial Illnesses. J. Trop. Dis. 2015, 3, 176. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Genet. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Aggarwal, S.; Singh, D.V.; Acharya, N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microb. Cell 2020, 7, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Knox, K.W.; Vesk, M.; Work, E. Relation Between Excreted Lipopolysaccharide Complexes and Surface Structures of a Lysine-Limited Culture of Escherichia coli. J. Bacteriol. 1966, 92, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Lee, J.; Jang, S.C.; Kim, S.R.; Jang, M.H.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Pulmonary Inflammation Induced by Bacteria-Free Outer Membrane Vesicles from Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 2013, 49, 637–645. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, J.-S.; Bin Park, S.; Lee, A.R.; Lee, J.S.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Kim, J.-H.; et al. Significant increase in the secretion of extracellular vesicles and antibiotics resistance from methicillin-resistant Staphylococcus aureus induced by ampicillin stress. Sci. Rep. 2020, 10, 21066. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef]
- Fonseca, S.; Carvalho, A.L.; Miquel-Clopés, A.; Jones, E.J.; Juodeikis, R.; Stentz, R.; Carding, S.R. Extracellular vesicles produced by the human gut commensal bacterium Bacteroides thetaiotaomicron elicit anti-inflammatory responses from innate immune cells. Front. Microbiol. 2022, 13, 1050271. [Google Scholar] [CrossRef]
- Furuyama, N.; Sircili, M.P. Outer Membrane Vesicles (OMVs) Produced by Gram-Negative Bacteria: Structure, Functions, Biogenesis, and Vaccine Application. BioMed Res. Int. 2021, 2021, 1490732. [Google Scholar] [CrossRef]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front. Cell. Infect. Microbiol. 2022, 12, 962216. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, L.P.; Fuhrmann, G. Bacterial extracellular vesicles: Understanding biology promotes applications as nanopharmaceuticals. Adv. Drug Deliv. Rev. 2021, 173, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-J.; Chen, J.-Y.; Tang, C.-H.; Zhao, Q.-Y.; Zhang, J.-M.; Qin, Y.-C. Edible plant extracellular vesicles: An emerging tool for bioactives delivery. Front. Immunol. 2022, 13, 1028418. [Google Scholar] [CrossRef]
- Reif, S.; Atias, A.; Musseri, M.; Koroukhov, N.; Gerstl, R.G. Beneficial Effects of Milk-Derived Extracellular Vesicles on Liver Fibrosis Progression by Inhibiting Hepatic Stellate Cell Activation. Nutrients 2022, 14, 4049. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape exosomelike nanoparticles induce intestinal stem cells and protect mice from dss-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and coli-tis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; López de las Hazas, M.-C.; Margollés, A.; Dávalos, A.; García-Ruiz, A. Eating microRNAs: Pharmacological opportunities for cross-kingdom regulation and implications in host gene and gut microbiota modulation. Br. J. Pharmacol. 2021, 178, 2218–2245. [Google Scholar] [CrossRef]
- Kim, S.Q.; Kim, K.-H. Emergence of Edible Plant-Derived Nanovesicles as Functional Food Components and Nanocarriers for Therapeutics Delivery: Potentials in Human Health and Disease. Cells 2022, 11, 2232. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: Results from the Food4Gut randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Munir, J.; Lee, M.; Ryu, S. Exosomes in Food: Health Benefits and Clinical Relevance in Diseases. Adv. Nutr. Int. Rev. J. 2020, 11, 687–696. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Ma, R.-H.; Ni, Z.-J.; Zhu, Y.-Y.; Thakur, K.; Zhang, F.; Zhang, Y.-Y.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. 2020, 12, 519–542. [Google Scholar] [CrossRef]
- Ramzan, M.; Zeshan, B. Assessment of the Phytochemical Analysis and Antimicrobial Potentials of Zingiber zerumbet. Molecules 2023, 28, 409. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, N.; Kong, L.; Wang, M.; Lei, H. Promoting effects of 6-Gingerol on probiotic adhesion to colonic epithelial cells. Food Sci. Technol. 2021, 41, 678–686. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Mao, Y.; Han, M.; Chen, C.; Wang, X.; Han, J.; Gao, Y.; Wang, S. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale 2021, 13, 20157–20169. [Google Scholar] [CrossRef]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. AAPS PharmSciTech 2021, 22, 206. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Dico, A.L.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Takakura, H.; Nakao, T.; Narita, T.; Horinaka, M.; Nakao-Ise, Y.; Yamamoto, T.; Iizumi, Y.; Watanabe, M.; Sowa, Y.; Oda, K.; et al. Citrus limon L.-Derived Nanovesicles Show an Inhibitory Effect on Cell Growth in p53-Inactivated Colorectal Cancer Cells via the Macropinocytosis Pathway. Biomedicines 2022, 10, 1352. [Google Scholar] [CrossRef]
- Otang, W.; Afolayan, A. Antimicrobial and antioxidant efficacy of Citrus limon L. peel extracts used for skin diseases by Xhosa tribe of Amathole District, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 102, 46–49. [Google Scholar] [CrossRef]
- Hamdan, D.; Ashour, M.L.; Mulyaningsih, S.; El-Shazly, A.; Wink, M. Chemical Composition of the Essential Oils of Variegated Pink-Fleshed Lemon (Citrus x limon L. Burm. f.) and their Anti-Inflammatory and Antimicrobial Activities. Z. Naturforsch. C J. Biosci. 2013, 68, 275–284. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Ouazzou, A.A.; Condón, S.; García-Gonzalo, D.; Pagán, R. Inactivation of Escherichia coli O157:H7 in fruit juices by combined treatments of citrus fruit essential oils and heat. Int. J. Food Microbiol. 2012, 159, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Wang, Y.; Chen, F.; Yu, Z.; Wang, L.; Chen, S.; Guo, M. Effect of citrus lemon oil on growth and adherence of Streptococcus mutans. World J. Microbiol. Biotechnol. 2013, 29, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Mu, J.; Teng, Y.; He, L.; Xu, F.; Zhang, X.; Sundaram, K.; Kumar, A.; Sriwastva, M.K.; Lawrenz, M.B.; et al. Lemon Exosome-like Nanoparticles-Manipulated Probiotics Protect Mice from C. diff Infection. iScience 2020, 23, 101571. [Google Scholar] [CrossRef]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Raimondo, S.; Urzì, O.; Meraviglia, S.; Di Simone, M.; Corsale, A.M.; Ganji, N.R.; Piccionello, A.P.; Polito, G.; Presti, E.L.; Dieli, F.; et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J. Cell. Mol. Med. 2022, 26, 4195–4209. [Google Scholar] [CrossRef]
- Stevens, Y.; Van Rymenant, E.; Grootaert, C.; Van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed]
- Segura-Badilla, O.; Lazcano-Hernández, M.; Kammar-García, A.; Vera-López, O.; Aguilar-Alonso, P.; Ramírez-Calixto, J.; Navarro-Cruz, A.R. Use of coconut water (Cocus nucifera L.) for the development of a symbiotic functional drink. Heliyon 2020, 6, e03653. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, I.; Mulyanto, A.; Khasanah, T.U. The effectiveness of coconut water in inhibiting shigella sp. bacteria from diarrhea. Medisains 2019, 17, 8. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dey, S.; Mandal, M.; Sarkar, S.; Maria-Neto, S.; Franco, O.L. Identification and structural insights of three novel antimicrobial peptides isolated from green coconut water. Peptides 2009, 30, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Z.; Xu, X.; Li, M.; Li, P. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 2018, 272, 372–378. [Google Scholar] [CrossRef]
- van Beek, A.; Sovran, B.; Hugenholtz, F.; Meijer, B.; Hoogerland, J.A.; Mihailova, V.; Van Der Ploeg, C.; Belzer, C.; Boekschoten, M.V.; Hoeijmakers, J.H.J.; et al. Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1−/Δ7 Mice. Front. Immunol. 2016, 7, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 63, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Valido, E.; Stoyanov, J.; Gorreja, F.; Stojic, S.; Niehot, C.; Jong, J.K.-D.; Llanaj, E.; Muka, T.; Glisic, M. Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients 2022, 15, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, M.-L.; Zhu, W.-J.; Cao, Y.-N.; Peng, L.-X.; Yan, Z.-Y.; Zhao, G. In Vitro Effects of Tartary Buckwheat-Derived Nanovesicles on Gut Microbiota. J. Agric. Food Chem. 2022, 70, 2616–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Yan, B.-B.; Xiao, Y.; Zhou, Y.-M.; Liu, T.-Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, Y.; Zhao, Q.; Wang, Y.; Li, C.; Zou, L.; Hu, Y. Effects of Tartary Buckwheat Protein on Gut Microbiome and Plasma Metabolite in Rats with High-Fat Diet. Foods 2021, 10, 2457. [Google Scholar] [CrossRef]
- Cheng, W.; Cai, C.; Kreft, I.; Turnšek, T.L.; Zu, M.; Hu, Y.; Zhou, M.; Liao, Z. Tartary Buckwheat Flavonoids Improve Colon Lesions and Modulate Gut Microbiota Composition in Diabetic Mice. Evid.-Based Complement. Altern. Med. 2022, 2022, 4524444. [Google Scholar] [CrossRef]
- He, X.; Li, W.; Chen, Y.; Lei, L.; Li, F.; Zhao, J.; Zeng, K.; Ming, J. Dietary fiber of Tartary buckwheat bran modified by steam explosion alleviates hyperglycemia and modulates gut microbiota in db/db mice. Food Res. Int. 2022, 157, 111386. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Yan, B.; Zhao, S.; Zhou, X. Regulation of tartary buckwheat-resistant starch on intestinal microflora in mice fed with high-fat diet. Food Sci. Nutr. 2020, 8, 3243–3251. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory Function of Buckwheat-Resistant Starch Supplementation on Lipid Profile and Gut Microbiota in Mice Fed with a High-Fat Diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar] [CrossRef]
- Ong, S.; Blenkiron, C.; Haines, S.; Acevedo-Fani, A.; Leite, J.; Zempleni, J.; Anderson, R.; McCann, M. Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity? Nutrients 2021, 13, 2505. [Google Scholar] [CrossRef]
- Jiang, X.; You, L.; Zhang, Z.; Cui, X.; Zhong, H.; Sun, X.; Ji, C.; Chi, X. Biological Properties of Milk-Derived Extracellular Vesicles and Their Physiological Functions in Infant. Front. Cell Dev. Biol. 2021, 9, 693534. [Google Scholar] [CrossRef]
- García-Martínez, J.; Pérez-Castillo, M.; Salto, R.; López-Pedrosa, J.M.; Rueda, R.; Girón, M.D. Beneficial Effects of Bovine Milk Exosomes in Metabolic Interorgan Cross-Talk. Nutrients 2022, 14, 1442. [Google Scholar] [CrossRef]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Di Wu, D.; Zhou, F.; Mutai, E. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J. Nutr. 2017, 147, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am. J. Physiol. Liver Physiol. 2019, 317, G618–G624. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, e1901251. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef]
- Du, C.; Quan, S.; Nan, X.; Zhao, Y.; Shi, F.; Luo, Q.; Xiong, B. Effects of oral milk extracellular vesicles on the gut microbiome and serum metabolome in mice. Food Funct. 2021, 12, 10938–10949. [Google Scholar] [CrossRef]
- Nader, R.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.; Fajloun, Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef]
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey antibacterial activity: A neglected aspect of honey quality assurance as functional food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Schuh, C.M.A.P.; Aguayo, S.; Zavala, G.; Khoury, M. Exosome-like vesicles in Apis mellifera bee pollen, honey and royal jelly contribute to their antibacterial and pro-regenerative activity. J. Exp. Biol. 2019, 222 Pt 20, jeb.208702. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Sabadini, C.; Alvarez, S.; Barrera, N.P.; Schuh, C.M.; Aguayo, S. Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides against Oral Streptococci. Int. J. Nanomed. 2021, 16, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Li, X.; An, T.T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M.J.; Eudy, J.; et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J. Extracell. Vesicles 2021, 10, e12069. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Aguayo, S.; Ramirez, O.; Vallejos, C.; Ruiz, J.; Morales, B. Extracellular Vesicles derived from Apis mellifera Royal Jelly promote wound healing by modulating inflammation and cellular responses RJEVs modulate inflammation in wound healing. arXiv 2022, arXiv:07.21.501009. [Google Scholar] [CrossRef]

| Reference | Direct | Indirect * | ||||
|---|---|---|---|---|---|---|
| Inhibit | Promoted | No Effect | Inhibit | Promoted | ||
| [48] | Lactobacillus rhamnosus (LGG) | x | - | - | ||
| Lactobacillus reuteri (L. reuteri) | x | - | - | |||
| Lactobacillus murinus (L. murinus) | x | - | - | |||
| Bacillus fragilis (B. fragilis) | x | x | ||||
| Escherichia coli (E. coli) | x | x | ||||
| Ruminococcaceae sp. (TSD-27) | x | - | - | |||
| Listeria monocytogenes (L. monocytogenes) | - | - | - | x | ||
| [49] | Porphyromonas gingivalis (P. gingivalis) | x | - | - | ||
| Fusobacterium nucleatum (F. nucleatum) | x | - | - | |||
| Prevotella intermedia (P. intermedia) | x | - | - | |||
| Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) | x | - | - | |||
| Streptococcus gordonii (S. gordonii) | x | - | - | |||
| Ref. | Sample | Isolation | Animal | EVs | Conclusions |
|---|---|---|---|---|---|
| [86] | Bovine Milk | Differential ultracentrifugation | Female and male C57BL/6 3 weeks | Exosome and RNA-depleted (ERD) VS exosome and RNA-sufficient (ERS) diets | Sex alone does not affect microbial communities. 7 weeks. Two unclassified families from phylum Firmicutes were more abundant in ERD mice than in ERS mice. 15 weeks. one of the two unclassified families from the phylum of Firmicutes and another unclassified family from the phylum Tenericutes were more abundant in ERS mice compared with ERD mice 47 weeks. the family of Verrucomicrobiaceae was more abundant in ERD mice compared with ERS mice, whereas Lachnospiraceae and two unclassified families from phyla Firmicutes and Tenericutes were more abundant in ERSmice than in ERD mice |
| [87] | Raw milk | Chymosin or hydrochloric acid treatment combined with ultracentrifugation/ultrafiltration | Specific-pathogen-free female C57BL/6 3 weeks | Low mEVs VS Middle mEVs VS High mEVs VS control PBS | Increase of Clostridiaceae, Ruminococcaceae Lachnospiraceae with EVs (with an increase of mEVs) and decrease in S24_7. |
| [88] | Raw milk from Holstein cows | Chymosin combined with ultracentrifugation | Specific-pathogen-free male C57BL/6 7–8 weeks | Control group VS DSS group VS DSS + mEVs-Low dose | Genus level: Depletion of Enterorhabdus and unclassified_Bacteroidia in the DSS group, but recovered in the mEVs group Family level: Increased Enterococcaceae and Desulfovibrionales-unclassified Desulfovibrionaceae in the DSS group but unchanged in the mEVs group |
| [89] | Bovine raw milk | Ultracentrifugation | Specific-pathogen-free female and male C57BL/6 6–8 weeks | High EVs VS Middle EVs VS Low EVs VS Control PBS | Female Genus: increase Muribaculum, Turicibacter. Decrease Desulfovibrio, Marvinbryantia. Male Genus: increase Akkermansia. Decrease Desulfovibrio. Phylum: increased Verrucomicrobia and Cyanobacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzaneque-López, M.C.; Sánchez-López, C.M.; Pérez-Bermúdez, P.; Soler, C.; Marcilla, A. Dietary-Derived Exosome-like Nanoparticles as Bacterial Modulators: Beyond MicroRNAs. Nutrients 2023, 15, 1265. https://doi.org/10.3390/nu15051265
Manzaneque-López MC, Sánchez-López CM, Pérez-Bermúdez P, Soler C, Marcilla A. Dietary-Derived Exosome-like Nanoparticles as Bacterial Modulators: Beyond MicroRNAs. Nutrients. 2023; 15(5):1265. https://doi.org/10.3390/nu15051265
Chicago/Turabian StyleManzaneque-López, Mari Cruz, Christian M. Sánchez-López, Pedro Pérez-Bermúdez, Carla Soler, and Antonio Marcilla. 2023. "Dietary-Derived Exosome-like Nanoparticles as Bacterial Modulators: Beyond MicroRNAs" Nutrients 15, no. 5: 1265. https://doi.org/10.3390/nu15051265
APA StyleManzaneque-López, M. C., Sánchez-López, C. M., Pérez-Bermúdez, P., Soler, C., & Marcilla, A. (2023). Dietary-Derived Exosome-like Nanoparticles as Bacterial Modulators: Beyond MicroRNAs. Nutrients, 15(5), 1265. https://doi.org/10.3390/nu15051265






