Increased Plasma L-Arginine Levels and L-Arginine/ADMA Ratios after Twelve Weeks of Omega-3 Fatty Acid Supplementation in Amateur Male Endurance Runners
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Sample Collection
2.4. Fatty Acid Analysis
2.5. Amino Acid Assessment
2.6. Statistical Analysis
3. Results
3.1. Omega-3 Polyunsaturated Fatty Acids in RBCs
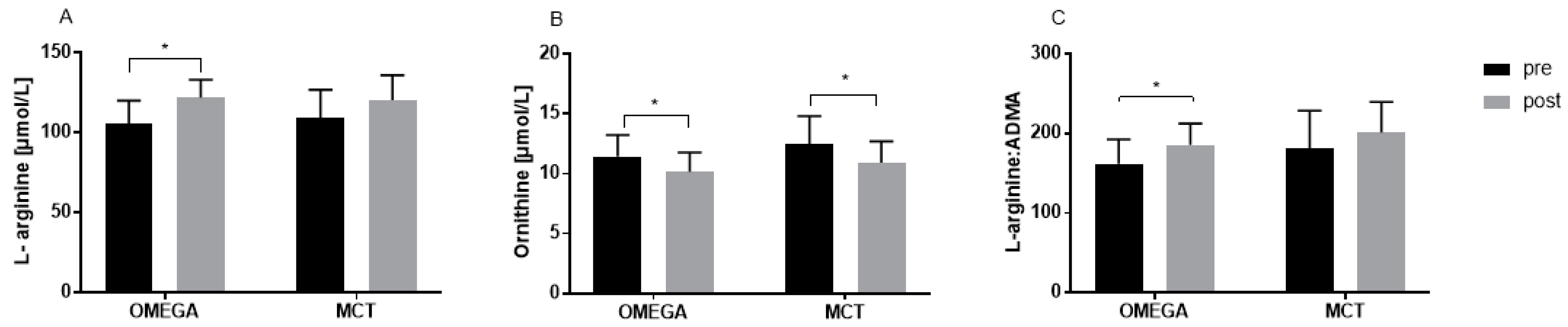
3.2. Plasma L-arginine and Its Metabolites at Resting Conditions
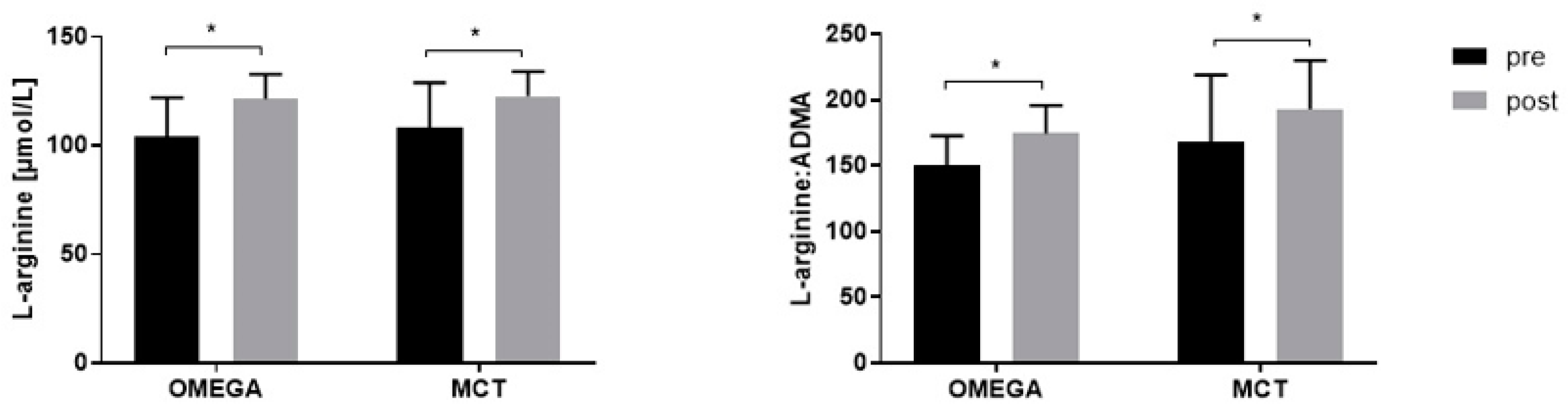
3.3. Plasma L-arginine and Its Metabolites Post-Exercise
3.4. Plasma L-arginine, the L-arg/ADMA Ratio and Running Economy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calder, P.C. Very Long-Chain n-3 Fatty Acids and Human Health: Fact, Fiction and the Future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gong, C.; Jin, K.; Zhou, L.; Xiao, Y.; Ma, L. Omega-3 Fatty Acid Supplementation and Coronary Heart Disease Risks: A Meta-Analysis of Randomized Controlled Clinical Trials. Front. Nutr. 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- AlAmmar, W.A.; Albeesh, F.H.; Ibrahim, L.M.; Algindan, Y.Y.; Yamani, L.Z.; Khattab, R.Y. Effect of Omega-3 Fatty Acids and Fish Oil Supplementation on Multiple Sclerosis: A Systematic Review. Nutr. Neurosci. 2021, 24, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; da Silva, B.G.C.; da Silva, T.G.; Mintem, G.C.; Bielemann, R.M.; Gigante, D.P. Omega-3 Supplementation and Diabetes: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4435–4448. [Google Scholar] [CrossRef]
- López-Seoane, J.; Martinez-Ferran, M.; Romero-Morales, C.; Pareja-Galeano, H. N-3 PUFA as an Ergogenic Supplement Modulating Muscle Hypertrophy and Strength: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Xin, G.; Eshaghi, H. Effect of Omega-3 Fatty Acids Supplementation on Indirect Blood Markers of Exercise-induced Muscle Damage: Systematic Review and Meta-analysis of Randomized Controlled Trials. Food Sci. Nutr. 2021, 9, 6429–6442. [Google Scholar] [CrossRef]
- Tomczyk, M.; Jost, Z.; Chroboczek, M.; Urbański, R.; Calder, P.C.; Fisk, H.L.; Sprengel, M.; Antosiewicz, J. Effects of 12 Weeks of Omega-3 Fatty Acid Supplementation in Long-Distance Runners. Med. Sci. Sports Exerc. 2022. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Kurisu, S.; Yoshimizu, A.; Sasaki, N.; Matsuura, H.; Kajiyama, G.; Oshima, T. Regular Aerobic Exercise Augments Endothelium-Dependent Vascular Relaxation in Normotensive As Well As Hypertensive Subjects. Circulation 1999, 100, 1194–1202. [Google Scholar] [CrossRef]
- Epstein, F.H.; Moncada, S.; Higgs, A. The L-Arginine-Nitric Oxide Pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Álvares, T.S.; Meirelles, C.M.; Bhambhani, Y.N.; Paschoalin, V.M.F.; Gomes, P.S.C. L-Arginine as a Potential Ergogenic Aid in Healthy Subjects. Sports Med. 2011, 41, 233–248. [Google Scholar] [CrossRef]
- Surdacki, A.; Nowicki, M.; Sandmann, J.; Tsikas, D.; Boeger, R.H.; Bode-Boeger, S.M.; Kruszelnicka-Kwiatkowska, O.; Kokot, F.; Dubiel, J.S.; Froelich, J.C. Reduced Urinary Excretion of Nitric Oxide Metabolites and Increased Plasma Levels of Asymmetric Dimethylarginine in Men with Essential Hypertension. J. Cardiovasc. Pharmacol. 1999, 33, 652–658. [Google Scholar] [CrossRef]
- Leone, A.; Moncada, S.; Vallance, P.; Calver, A.; Collier, J. Accumulation of an Endogenous Inhibitor of Nitric Oxide Synthesis in Chronic Renal Failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical Dimethylarginine: A New Combined Parameter for Renal Function and Extent of Coronary Artery Disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Päivä, H.; Kähönen, M.; Lehtimäki, T.; Alfthan, G.; Viikari, J.; Laaksonen, R.; Hutri-Kähönen, N.; Laitinen, T.; Taittonen, L.; Raitakari, O.T.; et al. Levels of Asymmetrical Dimethylarginine Are Predictive of Brachial Artery Flow-Mediated Dilation 6 Years Later. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2010, 212, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, R.N.; Beyer-Westendorf, J.; Bode-Böger, S.M.; Eggebrecht, L.; Konstantinides, S.; Martens-Lobenhoffer, J.; Nagler, M.; Prochaska, J.; Wild, P. Homoarginine and Methylarginines Independently Predict Long-Term Outcome in Patients Presenting with Suspicion of Venous Thromboembolism. Sci. Rep. 2021, 11, 9569. [Google Scholar] [CrossRef]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma Arginine/ADMA Ratio as a Sensitive Risk Marker for Atherosclerosis: Shimane CoHRE Study. Atherosclerosis 2015, 239, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Anderssohn, M.; Rosenberg, M.; Schwedhelm, E.; Zugck, C.; Lutz, M.; Lüneburg, N.; Frey, N.; Böger, R.H. The L-Arginine–Asymmetric Dimethylarginine Ratio Is an Independent Predictor of Mortality in Dilated Cardiomyopathy. J. Card. Fail. 2012, 18, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, C.; Bonnevie-Svendsen, M.; Melsom, H.S.; Melau, J.; Seljeflot, I.; Hisdal, J. Reduced L-Arginine and L-Arginine-ADMA-Ratio, and Increased SDMA after Norseman Xtreme Triathlon. Sports 2021, 9, 120. [Google Scholar] [CrossRef]
- Peoples, G.E.; McLennan, P.L.; Howe, P.R.C.; Groeller, H. Fish Oil Reduces Heart Rate and Oxygen Consumption During Exercise. J. Cardiovasc. Pharmacol. 2008, 52, 540–547. [Google Scholar] [CrossRef]
- Kawabata, F.; Neya, M.; Hamazaki, K.; Watanabe, Y.; Kobayashi, S.; Tsuji, T. Supplementation with Eicosapentaenoic Acid-Rich Fish Oil Improves Exercise Economy and Reduces Perceived Exertion during Submaximal Steady-State Exercise in Normal Healthy Untrained Men. Biosci. Biotechnol. Biochem. 2014, 78, 2081–2088. [Google Scholar] [CrossRef]
- Ritz, P.P.; Rogers, M.B.; Zabinsky, J.S.; Hedrick, V.E.; Rockwell, J.A.; Rimer, E.G.; Kostelnik, S.B.; Hulver, M.W.; Rockwell, M.S. Dietary and Biological Assessment of the Omega-3 Status of Collegiate Athletes: A Cross-Sectional Analysis. PLoS ONE 2020, 15, e0228834. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Kirby, B.S.; Clark, I.E.; Rice, H.M.; Fulkerson, E.; Wylie, L.J.; Wilkerson, D.P.; Vanhatalo, A.; Wilkins, B.W. Physiological Demands of Running at 2-Hour Marathon Race Pace. J. Appl. Physiol. 2021, 130, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The Use of Gas Chromatography to Analyze Compositional Changes of Fatty Acids in Rat Liver Tissue during Pregnancy. J. Vis. Exp. 2014, 85, e51445. [Google Scholar] [CrossRef] [PubMed]
- Carling, R.S.; McDonald, B.A.; Austin, D.; Burden, D.; Correia, J.; Leung, J.; Mayers, B.; John, C. Challenging the Status Quo: A Comparison of Ion Exchange Chromatography with Liquid Chromatography–Mass Spectrometry and Liquid Chromatography–Tandem Mass Spectrometry Methods for the Measurement of Amino Acids in Human Plasma. Ann. Clin. Biochem. Int. J. Lab. Med. 2020, 57, 277–290. [Google Scholar] [CrossRef]
- Harris, W.S.; Rambjør, G.S.; Windsor, S.L.; Diederich, D. N-3 Fatty Acids and Urinary Excretion of Nitric Oxide Metabolites in Humans. Am. J. Clin. Nutr. 1997, 65, 459–464. [Google Scholar] [CrossRef]
- Newens, K.J.; Thompson, A.K.; Jackson, K.G.; Wright, J.; Williams, C.M. DHA-Rich Fish Oil Reverses the Detrimental Effects of Saturated Fatty Acids on Postprandial Vascular Reactivity. Am. J. Clin. Nutr. 2011, 94, 742–748. [Google Scholar] [CrossRef][Green Version]
- Bercea, C.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 Polyunsaturated Fatty Acids and Hypertension: A Review of Vasodilatory Mechanisms of Docosahexaenoic Acid and Eicosapentaenoic Acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Eid, H.M.; Arnesen, H.; Hjerkinn, E.M.; Lyberg, T.; Ellingsen, I.; Seljeflot, I. Effect of Diet and Omega-3 Fatty Acid Intervention on Asymmetric Dimethylarginine. Nutr. Metab. (Lond.) 2006, 3, 4. [Google Scholar] [CrossRef][Green Version]
- Khorrami, E.; Hosseinzadeh-Attar, M.J.; Esmaillzadeh, A.; Alipoor, E.; Hosseini, M.; Emkanjou, Z.; Kolahdouz Mohammadi, R.; Moradmand, S. Effect of Fish Oil on Circulating Asymmetric Dimethylarginine and Adiponectin in Overweight or Obese Patients with Atrial Fibrillation. Food Sci. Nutr. 2020, 8, 2165–2172. [Google Scholar] [CrossRef]
- Żebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gąsior, Z.; Poprzęcki, S. Omega-3 Fatty Acids Supplementation Improves Endothelial Function and Maximal Oxygen Uptake in Endurance-Trained Athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Brandes, R.P.; Phivthong-ngam, L.; Böhme, M.; Nafe, R.; Mügge, A.; Frölich, J.C. Dietary L-Arginine Reduces the Progression of Atherosclerosis in Cholesterol-Fed Rabbits. Circulation 1997, 96, 1282–1290. [Google Scholar] [CrossRef]
- Eid, H. Increased Levels of Asymmetric Dimethylarginine in Populations at Risk for Atherosclerotic Disease. Effects of Pravastatin. Atherosclerosis 2003, 166, 279–284. [Google Scholar] [CrossRef]
- Cuisinier, C.; Ward, R.J.; Francaux, M.; Sturbois, X.; de Witte, P. Changes in Plasma and Urinary Taurine and Amino Acids in Runners Immediately and 24 h after a Marathon. Amino Acids 2001, 20, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Böger, R.H.; Kienke, S.; Junker, W.; Frölich, J.C. Elevatedl-Arginine/Dimethylarginine Ratio Contributes to Enhanced Systemic NO Production by Dietaryl-Arginine in Hypercholesterolemic Rabbits. Biochem. Biophys. Res. Commun. 1996, 219, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; More, R.S.; Mullins, P.A.; Taylor, G.; Petch, M.C.; Schofield, P.M. Aging-Associated Endothelial Dysfunction in Humans Is Reversed by L-Arginine. J. Am. Coll. Cardiol. 1996, 28, 1796–1804. [Google Scholar] [CrossRef][Green Version]
- Lind, L.; Larsson, A.; Teerlink, T. L-Arginine Is Related to Endothelium-Dependent Vasodilation in Resistance and Conduit Arteries in Divergent Ways—The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Atherosclerosis 2009, 203, 544–549. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; DiMenna, F.J.; Blackwell, J.R.; Wallis, G.A.; Jones, A.M. No Effect of Acute L-Arginine Supplementation on O2 Cost or Exercise Tolerance. Eur. J. Appl. Physiol. 2013, 113, 1805–1819. [Google Scholar] [CrossRef]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary Nitrate and Physical Performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-Oil Supplementation Enhances the Effects of Strength Training in Elderly Women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- McGlory, C.; Galloway, S.D.R.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal Changes in Human Skeletal Muscle and Blood Lipid Composition with Fish Oil Supplementation. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 199–206. [Google Scholar] [CrossRef]
- Bergström, J.; Fürst, P.; Hultman, E. Free Amino Acids in Muscle Tissue and Plasma during Exercise in Man. Clin. Physiol. 1985, 5, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.S.; Broberg, S.; Björkman, O.; Wahren, J. Ammonia Metabolism during Exercise in Man. Clin. Physiol. 1985, 5, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.E.; Lambert, M.H.; Montana, V.G.; Parks, D.J.; Blanchard, S.G.; Brown, P.J.; Sternbach, D.D.; Lehmann, J.M.; Wisely, G.B.; Willson, T.M.; et al. Molecular Recognition of Fatty Acids by Peroxisome Proliferator–Activated Receptors. Mol. Cell 1999, 3, 397–403. [Google Scholar] [CrossRef]
- Guelzim, N.; Mariotti, F.; Martin, P.G.P.; Lasserre, F.; Pineau, T.; Hermier, D. A Role for PPARα in the Regulation of Arginine Metabolism and Nitric Oxide Synthesis. Amino Acids 2011, 41, 969–979. [Google Scholar] [CrossRef]
- Wang, C.-P.; Lee, C.-C.; Wu, D.-Y.; Chen, S.; Lee, T.-M. Differential Effects of EPA and DHA on PPARγ-Mediated Sympathetic Innervation in Infarcted Rat Hearts by GPR120-Dependent and -Independent Mechanisms. J. Nutr. Biochem. 2022, 103, 108950. [Google Scholar] [CrossRef]
- Moradi, S.; Alivand, M.; KhajeBishak, Y.; AsghariJafarabadi, M.; Alipour, M.; Chilibeck, P.D.; Alipour, B. The Effect of Omega3 Fatty Acid Supplementation on PPARγ and UCP2 Expressions, Resting Energy Expenditure, and Appetite in Athletes. BMC Sports Sci. Med. Rehabil. 2021, 13, 48. [Google Scholar] [CrossRef]

| Variable | MCT (n = 12) Mean ± SD | OMEGA (n = 14) Mean ± SD | ||
|---|---|---|---|---|
| Age (years) | 37 ± 4 | 37 ± 3 | ||
| Body mass (kg) | 78 ± 8 | 76 ± 11 | ||
| Height (cm) | 180 ± 4 | 181 ± 7 | ||
| VO2peak (mL*kg−1*min−1) | 54.7 ± 7 | 53.6 ± 4 | ||
| RE (mL*kg−1*min−1) | Pre | 47.7 ± 3.3 | Pre | 47.6 ± 1.8 |
| Post | 48.7 ± 2.9 | Post | 46.5 ± 2.4 † | |
| EPA (% of total RBC fatty acids) | Pre | 1.2 ± 0.3 | Pre | 1.1 ± 0.4 |
| Post | 1.2 ± 0.3 | Post | 4.9 ± 1.1 *† | |
| DHA (% of total RBC fatty acids) | Pre | 4.4 ± 1.1 | Pre | 4.7 ± 1.0 |
| Post | 4.5 ± 0.8 | Post | 6.7 ± 0.8 *† | |
| O3I | Pre | 5.6 ± 1.4 | Pre | 5.8 ± 1.3 |
| Post | 5.6 ± 1.1 | Post | 11.6 ± 1.7 *† | |
| Test duration (min: s) | Pre | 1091 ± 144 | Pre | 1111 ± 70 |
| Post | 1137 ± 84 * | Post | 1138 ± 85 | |
| MCT (n = 12) Mean ± SD | OMEGA (n = 14) Mean ± SD | Diff | 95% CI | p | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| L-arginine (µmol/L) | ||||||
| Before | 109.4 ± 17.53 | 105.4 ± 14.67 | −4.003 | −17.4 | 9.394 | 0.744 |
| After | 120.4 ± 15.55 | 122.0 ± 11.12 | 1.621 | −11.78 | 15.02 | 0.952 |
| Change | 11.00 ± 17.21 | 16.63 ± 14.87 | ||||
| p | 0.109 | 0.001 | ||||
| ADMA (µmol/L) | ||||||
| Before | 0.618 ± 0.082 | 0.669 ± 0.147 | 0.051 | −0.059 | 0.161 | 0.496 |
| After | 0.611 ± 0.095 | 0.673 ± 0.139 | 0.062 | −0.482 | 0.172 | 0.360 |
| Change | −0.007 ± 0.086 | 0.004 ± 0.054 | ||||
| p | 0.883 | 0.819 | ||||
| SDMA (µmol/L) | ||||||
| Before | 0.255 ± 0.03 | 0.262 ± 0.036 | 0.007 | −0.025 | 0.04 | 0.851 |
| After | 0.259 ± 0.038 | 0.264 ± 0.038 | 0.004 | −0.028 | 0.037 | 0.940 |
| Change | 0.004 ± 0.031 | 0.001 ± 0.031 | ||||
| p | 0.963 | 0.868 | ||||
| DMA (µmol/L) | ||||||
| Before | 1.334 ± 0.148 | 1.301 ± 0.241 | −0.033 | −0.267 | 0.202 | 0.937 |
| After | 1.361 ± 0.275 | 1.394 ± 0.325 | 0.033 | −0.200 | 0.268 | 0.934 |
| Change | 0.027 ± 0.336 | 0.092 ± 0.314 | ||||
| p | 0.865 | 0.509 | ||||
| L-citrulline (µmol/L) | ||||||
| Before | 33.73 ± 6.184 | 34.97 ± 9.323 | 1.237 | −5.842 | 8.315 | 0.903 |
| After | 35.36 ± 7.092 | 33.8 ± 7.905 | −1.553 | −8.632 | 5.526 | 0.852 |
| Change | 1.626 ± 3.268 | −1.164 ± 3.736 | ||||
| p | 0.113 | 0.265 | ||||
| Ornithine (µmol/L) | ||||||
| Before | 12.49 ± 2.314 | 11.45 ± 1.771 | −1.048 | −2.744 | 0.649 | 0.295 |
| After | 10.91 ± 1.773 | 10.17 ± 1.598 | −0.740 | −2.437 | 0.956 | 0.536 |
| Change | −1.582 ± 1.857 | −1.274 ± 0.991 | ||||
| p | 0.007 | <0.001 | ||||
| L-Arginine:ADMA | ||||||
| Before | 180.9 ± 47.61 | 162.1 ± 30.45 | −18.84 | −51.52 | 13.85 | 0.343 |
| After | 201.5 ± 38.18 | 185.7 ± 26.54 | −15.73 | −48.42 | 16.95 | 0.470 |
| Change | 20.56 ± 41.54 | 23.66 ± 23.48 | ||||
| p | 0.077 | 0.005 | ||||
| MCT (n = 12) Mean ± SD | OMEGA (n = 14) Mean ± SD | Diff | 95% CI | p | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| L-arginine (µmol/L) | ||||||
| Before | 108.1 ± 20.8 | 104.3 ± 17.67 | −3.809 | −18.1 | 10.49 | 0.790 |
| After | 122.7 ± 11.41 | 121.5 ± 11.24 | −1.157 | −15.45 | 13.14 | 0.978 |
| Change | 14.55 ± 17.71 | 17.20 ± 13.75 | ||||
| p | 0.016 | <0.001 | ||||
| ADMA (µmol/L) | ||||||
| Before | 0.663 ± 0.095 | 0.701 ± 0.139 | 0.038 | −0.0611 | 0.137 | 0.615 |
| After | 0.65 ± 0.089 | 0.706 ± 0.102 | 0.056 | −0.043 | 0.155 | 0.361 |
| Change | −0.013 ± 0.078 | 0.004 ± 0.064 | ||||
| p | 0.566 | 0.797 | ||||
| SDMA (µmol/L) | ||||||
| Before | 0.256 ± 0.03 | 0.272 ± 0.045 | 0.016 | −0.019 | 0.051 | 0.489 |
| After | 0.265 ± 0.035 | 0.28 ± 0.039 | 0.015 | −0.02 | 0.05 | 0.545 |
| Change | 0.009 ± 0.034 | 0.008 ± 0.032 | ||||
| p | 0.374 | 0.381 | ||||
| DMA (µmol/L) | ||||||
| Before | 1.505 ± 0.213 | 1.593 ± 0.374 | 0.088 | −0.249 | 0.425 | 0.797 |
| After | 1.628 ± 0.373 | 1.742 ± 0.461 | 0.115 | −0.222 | 0.452 | 0.682 |
| Change | 0.123 ± 0.341 | 0.149 ± 0.462 | ||||
| p | 0.338 | 0.248 | ||||
| L-citrulline (µmol/L) | ||||||
| Before | 34.69 ± 9.013 | 34.65 ± 11.18 | −0.046 | −8.486 | 8.394 | >0.999 |
| After | 36.98 ± 7.893 | 34.17 ± 8.511 | −2.813 | −11.25 | 5.627 | 0.693 |
| Change | 2.288 ± 3.382 | −0.479 ± 4.157 | ||||
| p | 0.052 | 0.952 | ||||
| Ornithine (µmol/L) | ||||||
| Before | 13.18 ± 2.459 | 12.25 ± 1.754 | −0.932 | −2.564 | 0.07 | 0.35 |
| After | 11.66 ± 1.38 | 11.78 ± 1.456 | 0.117 | −1.516 | 1.75 | 0.983 |
| Change | −1.52 ± 2.546 | −0.471 ± 1.497 | ||||
| p | 0.063 | 0.26 | ||||
| L-Arginine:ADMA | ||||||
| Before | 167.5 ± 51.38 | 150.8 ± 22.14 | −16.78 | −47.92 | 14.35 | 0.391 |
| After | 192.9 ± 37.03 | 174.6 ± 21.33 | −18.33 | −49.47 | 12.8 | 0.328 |
| Change | 25.35 ± 42.21 | 23.8 ± 17.42 | ||||
| p | 0.021 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jost, Z.; Tomczyk, M.; Chroboczek, M.; Calder, P.C.; Fisk, H.L.; Przewłócka, K.; Antosiewicz, J. Increased Plasma L-Arginine Levels and L-Arginine/ADMA Ratios after Twelve Weeks of Omega-3 Fatty Acid Supplementation in Amateur Male Endurance Runners. Nutrients 2022, 14, 4749. https://doi.org/10.3390/nu14224749
Jost Z, Tomczyk M, Chroboczek M, Calder PC, Fisk HL, Przewłócka K, Antosiewicz J. Increased Plasma L-Arginine Levels and L-Arginine/ADMA Ratios after Twelve Weeks of Omega-3 Fatty Acid Supplementation in Amateur Male Endurance Runners. Nutrients. 2022; 14(22):4749. https://doi.org/10.3390/nu14224749
Chicago/Turabian StyleJost, Zbigniew, Maja Tomczyk, Maciej Chroboczek, Philip C. Calder, Helena L. Fisk, Katarzyna Przewłócka, and Jędrzej Antosiewicz. 2022. "Increased Plasma L-Arginine Levels and L-Arginine/ADMA Ratios after Twelve Weeks of Omega-3 Fatty Acid Supplementation in Amateur Male Endurance Runners" Nutrients 14, no. 22: 4749. https://doi.org/10.3390/nu14224749
APA StyleJost, Z., Tomczyk, M., Chroboczek, M., Calder, P. C., Fisk, H. L., Przewłócka, K., & Antosiewicz, J. (2022). Increased Plasma L-Arginine Levels and L-Arginine/ADMA Ratios after Twelve Weeks of Omega-3 Fatty Acid Supplementation in Amateur Male Endurance Runners. Nutrients, 14(22), 4749. https://doi.org/10.3390/nu14224749





