Caffeine and the Risk of Diabetic Retinopathy in Type 2 Diabetes Mellitus: Findings from Clinical and Experimental Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Study Design and Subjects
2.2. Clinical and Socio-Demographic Characteristics
2.3. Caffeine Intake
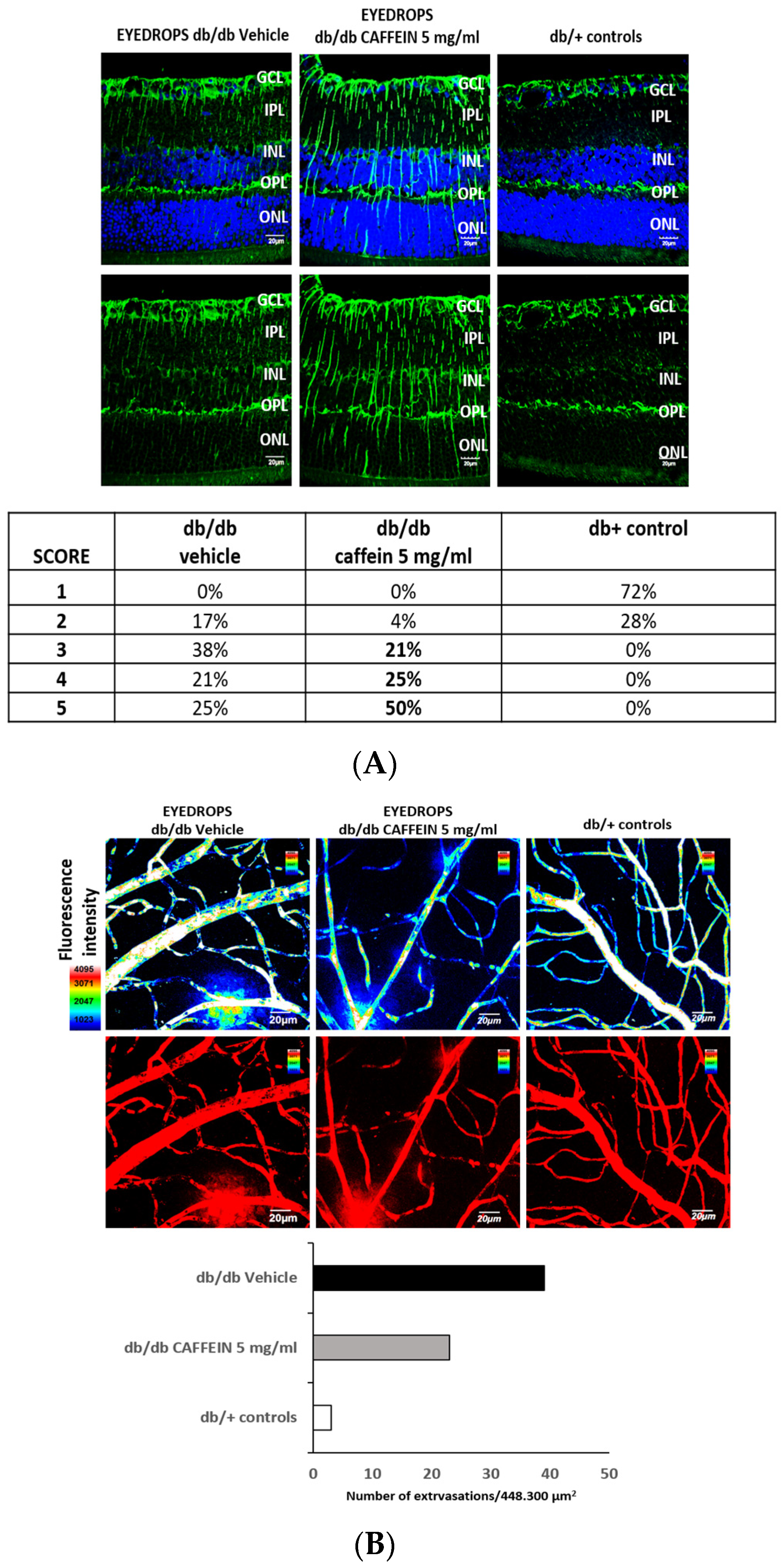
2.4. Experimental Study
2.5. Assessment of Neurovascular Damage
2.6. Statistical Analysis
3. Results
3.1. Clinical Study
Caffeine Intake and Diabetic Retinopathy
3.2. Experimental Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S185–S194. [Google Scholar] [CrossRef]
- Rodriguez-Poncelas, A.; Miravet-Jiménez, S.; Casellas, A.; Barrot De, J.F.; Puente, L.; Franch-Nadal, J.; López-Simarro, F.; Mata-Cases, M.; Mundet-Tudurí, X. Prevalence of diabetic retinopathy in individuals with type 2 diabetes who had recorded diabetic retinopathy from retinal photographs in Catalonia (Spain). Br. J. Ophthalmol. 2015, 99, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Savani, S.; Battram, D.S.; Mclaren, D.H.; Sathasivam, P.; Graham, T.E. Human Nutrition and Metabolism Caffeine Ingestion Before an Oral Glucose Tolerance Test Impairs Blood Glucose Management in Men with Type 2 Diabetes 1,2. J. Nutr. 2004, 134, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Boia, R.; Ambrosio, A.F.; Santiago, A.R. Therapeutic Opportunities for Caffeine and A2A Receptor Antagonists in Retinal Diseases. Ophthalmic Res. 2016, 55, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Hu, F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA 2005, 294, 97–104. [Google Scholar] [CrossRef]
- Dewar, L.; Heuberger, R. The effect of acute caffeine intake on insulin sensitivity and glycemic control in people with diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S631–S635. [Google Scholar] [CrossRef]
- Chieng, D.; Canovas, R.; Segan, L.; Sugumar, H.; Voskoboinik, A.; Prabhu, S.; Ling, L.H.; Lee, G.; Morton, J.B.; Kaye, D.M.; et al. The impact of coffee subtypes on incident cardiovascular disease, arrhythmias, and mortality: Long-term outcomes from the UK Biobank. Eur. J. Prev. Cardiol. 2022, 29, 2240–2249. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Shah, J.; Cheong, Z.Y.; Tan, B.; Wong, D.; Liu, X.; Chua, J. Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients 2022, 14, 5021. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, J.I.; Kwon, S.O.; Hwang, D.D.J. Coffee consumption and diabetic retinopathy in adults with diabetes mellitus. Sci. Rep. 2022, 12, 3547. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Aissopou, E.K.; Katsana, K.; Moiragia, M.; Tentolouris, N.; Sfikakis, P.P.; Protogerou, A.D. Retinal microcirculation in association with caffeinated and alcoholic drinks in subjects at increased cardiovascular risk. Microcirculation 2016, 23, 591–596. [Google Scholar] [CrossRef]
- Neelam, K.; Li, X.; Wong, W.; Shyong, E.; Lee, J.; Van Dam, R.M.; Wong, T. Is Coffee Consumption associated with Age- related Macular Degeneration and Diabetic Retinopathy? All Res. J. Biol. 2014, 5, 7–13. [Google Scholar]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Caffeine Prevents Blood Retinal Barrier Damage in a Model, In Vitro, of Diabetic Macular Edema. J. Cell. Biochem. 2017, 118, 2371–2379. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Alcubierre, N.; Rubinat, E.; Traveset, A.; Martinez-Alonso, M.; Hernandez, M.; Jurjo, C.; Mauricio, D. A prospective cross-sectional study on quality of life and treatment satisfaction in type 2 diabetic patients with retinopathy without other major late diabetic complications. Health Qual. Life Outcomes 2014, 12, 131. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Bernstein, M.S.; Morabia, A.; Sloutskis, D. Definition and prevalence of sedentarism in an urban population. Am. J. Public Health 1999, 89, 862–867. [Google Scholar] [CrossRef]
- Vioque, J.; Navarrete-Muñoz, E.-M.; Gimenez-Monzó, D.; García-de-la-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef]
- US Department of Agriculture Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Available online: https://www.ars.usda.gov/ (accessed on 1 March 2022).
- Palma, I.; Farran, P.; Cervera, P. Tablas de composición de alimentos por medidas caseras de consumo habitual en España; Mc Graw Hill Interamericana, CESNID: Barcelona, Spain, 2008. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. Glial and endothelial blood-retinal barrier responses to amyloid-β in the neural retina of the rat. Clin. Ophthalmol. 2008, 2, 801. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Okuno, T.; Sugiyama, T.; Tominaga, M.; Kojima, S.; Ikeda, T. Effects of caffeine on microcirculation of the human ocular fundus. Jpn. J. Ophthalmol. 2002, 46, 170–176. [Google Scholar] [CrossRef]
- Terai, N.; Spoerl, E.; Pillunat, L.E.; Stodtmeister, R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Ophthalmol. 2012, 90, e524–e528. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H. Bin Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, D.; Sun, H.-P.; Yan, N.; Xu, Y.; Pan, C.-W. Regular Chinese Green Tea Consumption Is Protective for Diabetic Retinopathy: A Clinic-Based Case-Control Study. J Diabetes Res. 2015, 2015, 231570. [Google Scholar] [CrossRef]
- Pereira-Figueiredo, D.; Nascimento, A.A.; Cunha-Rodrigues, M.C.; Brito, R.; Calaza, K.C. Caffeine and Its Neuroprotective Role in Ischemic Events: A Mechanism Dependent on Adenosine Receptors. Cell. Mol. Neurobiol. 2021, 42, 1693–1725. [Google Scholar] [CrossRef]
- Jang, H.; Ahn, H.R.; Jo, H.; Kim, K.A.; Lee, E.H.; Lee, K.W.; Jung, S.H.; Lee, C.Y. Chlorogenic acid and coffee prevent hypoxia-induced retinal degeneration. J. Agric. Food Chem. 2014, 62, 182–191. [Google Scholar] [CrossRef]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef] [PubMed]
- Vioque, J.; Gonzalez, L. Validez de la evaluación de la ingesta dietética. In Nutrición y salud pública. Métodos, bases científicas y aplicaciones; Serra Majem, L., Aranceta Bartrina, J., Eds.; Masson-Elsevier: Barcelona, Spain, 2006; pp. 199–210. ISBN 84-458-1528-8. [Google Scholar]
| Characteristics | No DR (n = 147) | DR (n = 144) | p |
|---|---|---|---|
| Age (years) | 57.9 (10.3) | 60.4 (8.8) | 0.024 |
| Sex (women) | 71 (48.3%) | 72 (50.0%) | 0.863 |
| Educational level | 0.003 | ||
| No university | 130 (88.4%) | 141 (97.9%) | |
| Graduate or higher | 17 (11.6%) | 3 (2.1%) | |
| Tobacco exposure | 81 (55.1%) | 71 (49.3%) | 0.350 |
| Physical activity | 0.855 | ||
| Sedentary | 75 (51.0%) | 76 (52.8%) | |
| Active | 72 (49.0%) | 68 (47.2%) | |
| BMI (Kg/m2) | 31.3 (5.1) | 31.9 (5.6) | 0.358 |
| Waist (cm) | 104.0 (12.1) | 107.0 (11.1) | 0.034 |
| SBP (mmHg) | 134.0 (15.5) | 145.0 (19.9) | <0.001 |
| DBP (mmHg) | 76.6 (10.5) | 77.0 (11.1) | 0.723 |
| Hypertension | 73 (49.7%) | 96 (66.7%) | 0.005 |
| Dyslipidemia | 64 (43.5%) | 74 (51.4%) | 0.221 |
| Diabetes duration (years) | 7.07 (5.5) | 14.0 (9.9) | <0.001 |
| Diabetes treatment | <0.001 | ||
| Insulin | 4 (2.7%) | 17 (11.8%) | |
| OAD | 94 (63.9%) | 64 (44.4%) | |
| OAD + insulin | 13 (8.8%) | 60 (41.7%) | |
| Diet | 36 (24.5%) | 3 (2.1%) | |
| HbA1c (%) | 7.3 (1.2) | 8.2 (1.4) | <0.001 |
| HbA1c (mmol/mol) | 55.9 (12.7) | 66.6 (15.6) | <0.001 |
| Total cholesterol (mg/dL) | 186.0 (36.7) | 185 (36.2) | 0.790 |
| HDL-c (mg/dL) | 48.5 (10.8) | 51.8 (14.8) | 0.031 |
| LDL-c (mg/dL) | 111.0 (30.8) | 106.0 (30.3) | 0.177 |
| Triglycerides (mg/dL) | 138.0 (82.1) | 141.0 (121.0) | 0.827 |
| Variables | No DR (n = 147) | DR (n = 144) | p.overall 1 | OR (95% CI) | p 2 |
|---|---|---|---|---|---|
| Energy intake (kcal/day) | 2200.0 (582.0) | 2090.0 (584.0) | 0.122 | - | - |
| Caffeine (g/day) | 1.9 (2.3) | 1.6 (2.0) | 0.217 | 0.93 (0.84–1.04) | 0.218 |
| Caffeine (g/day) | 0.045 | ||||
| Q1 (0.00–0.10) | 29 (19.7%) | 44 (30.6%) | Ref. | Ref. | |
| Q2 (0.11–1.08) | 43 (29.3%) | 30 (20.8%) | 0.46 (0.24–0.90) | 0.022 | |
| Q3 (1.09–3.10) | 33 (22.4%) | 40 (27.8%) | 0.80 (0.41–1.55) | 0.509 | |
| Q4 (3.11–9.55) | 42 (28.6%) | 30 (20.8%) | 0.47 (0.24–0.92) | 0.027 | |
| Coffee and tea (g/day) | 3.6 (3.3) | 3.0 (2.9) | 0.105 | 0.94 (0.87–1.01) | 0.110 |
| Coffee and tea (g/day) | 0.376 | ||||
| Q1 (0.00–0.95) | 30 (20.4%) | 42 (29.2%) | Ref. | Ref. | |
| Q2 (0.96–2.09) | 38 (25.9%) | 35 (24.3%) | 0.66 (0.34–1.27) | 0.216 | |
| Q3 (2.10–5.02) | 40 (27.2%) | 33 (22.9%) | 0.59 (0.30–1.14) | 0.118 | |
| Q4 (5.03–26.06) | 39 (26.5%) | 34 (23.6%) | 0.63 (0.32–1.21) | 0.162 |
| Diabetic Retinopathy | ||||
|---|---|---|---|---|
| Variables | OR (95% CI) 1 | p | OR (95% CI) 2 | p |
| (Intercept) | 0.00 (0.00–0.10) | 0.001 | 0.00 (0.00–0.12) | 0.002 |
| Caffeine (g/day) | 0.93 (0.81–1.05) | 0.241 | - | - |
| Coffee and tea (g/day) | - | - | 0.91 (0.82–1.01) | 0.087 |
| Age (years) | 1.00 (0.97–1.03) | 0.833 | 1.00 (0.96–1.03) | 0.779 |
| Sex (women) | 0.58 (0.28–1.17) | 0.133 | 0.55 (0.27–1.11) | 0.098 |
| Educational level (no university) | 2.93 (0.84–13.77) | 0.121 | 2.79 (0.80–13.13) | 0.139 |
| Physical activity (active) | 0.91 (0.52–1.59) | 0.744 | 0.90 (0.51–1.56) | 0.702 |
| Hypertension | 1.91 (1.04–3.52) | 0.037 | 1.87 (1.02–3.46) | 0.043 |
| Dyslipidemia | 1.03 (0.59–1.79) | 0.922 | 1.05 (0.60–1.84) | 0.855 |
| Diabetes duration (years) | 1.10 (1.06–1.15) | <0.001 | 1.10 (1.06–1.16) | <0.001 |
| BMI (Kg/m2) | 1.02 (0.96–1.08) | 0.584 | 1.02 (0.96–1.08) | 0.583 |
| HbA1c (%) | 1.56 (1.25–1.97) | <0.001 | 1.57 (1.26–1.99) | <0.001 |
| Tobacco exposure | 0.96 (0.49–1.90) | 0.917 | 1.02 (0.51–2.03) | 0.957 |
| Diabetic Retinopathy | ||||
|---|---|---|---|---|
| Variables | OR (95% CI) 1 | p | OR (95% CI) 2 | p |
| (Intercept) | 0.01 (0.00–0.19) | 0.004 | 0.00 (0.00–0.12) | 0.002 |
| Caffeine (g/day) | ||||
| Q1 (0.00–0.10) | Ref. | Ref. | - | - |
| Q2 (0.11–1.08) | 0.35 (0.16–0.78) | 0.011 | - | - |
| Q3 (1.09–3.10) | 0.88 (0.40–1.91) | 0.741 | - | - |
| Q4 (3.11–9.55] | 0.35 (0.16–0.77) | 0.010 | - | - |
| Coffee and tea (g/day) | ||||
| Q1 (0.00–0.95) | - | - | Ref. | Ref. |
| Q2 (0.96–2.09) | - | - | 0.56 (0.25–1.22) | 0.145 |
| Q3 (2.10–5.02) | - | - | 0.62 (0.28–1.34) | 0.225 |
| Q4 (5.03–26.06) | - | - | 0.53 (0.24–1.18) | 0.123 |
| Age (years) | 0.99 (0.96–1.02) | 0.585 | 1.00 (0.97–1.03) | 0.988 |
| Sex (women) | 0.55 (0.26–1.13) | 0.110 | 0.56 (0.27–1.13) | 0.108 |
| Educational level (no university) | 2.94 (0.80–14.41) | 0.132 | 3.05 (0.87–14.45) | 0.109 |
| Physical activity (active) | 0.87 (0.49–1.53) | 0.629 | 0.92 (0.52–1.61) | 0.763 |
| Hypertension | 2.16 (1.17–4.05) | 0.014 | 1.87 (1.02–3.47) | 0.043 |
| Dyslipidemia | 0.93 (0.53–1.65) | 0.815 | 1.03 (0.59–1.80) | 0.915 |
| Diabetes duration (years) | 1.11 (1.06–1.16) | <0.001 | 1.10 (1.06–1.16) | <0.001 |
| BMI (Kg/m2) | 1.01 (0.96–1.07) | 0.655 | 1.01 (0.96–1.08) | 0.617 |
| HbA1c (%) | 1.60 (1.28–2.03) | <0.001 | 1.55 (1.24–1.97) | <0.001 |
| Tobacco exposure | 0.98 (0.49–1.98) | 0.963 | 0.99 (0.50–1.97) | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcubierre, N.; Granado-Casas, M.; Bogdanov, P.; Hernández, C.; Ramos, H.; Castelblanco, E.; Real, J.; Rubinat-Arnaldo, E.; Traveset, A.; Hernández, M.; et al. Caffeine and the Risk of Diabetic Retinopathy in Type 2 Diabetes Mellitus: Findings from Clinical and Experimental Studies. Nutrients 2023, 15, 1169. https://doi.org/10.3390/nu15051169
Alcubierre N, Granado-Casas M, Bogdanov P, Hernández C, Ramos H, Castelblanco E, Real J, Rubinat-Arnaldo E, Traveset A, Hernández M, et al. Caffeine and the Risk of Diabetic Retinopathy in Type 2 Diabetes Mellitus: Findings from Clinical and Experimental Studies. Nutrients. 2023; 15(5):1169. https://doi.org/10.3390/nu15051169
Chicago/Turabian StyleAlcubierre, Nuria, Minerva Granado-Casas, Patricia Bogdanov, Cristina Hernández, Hugo Ramos, Esmeralda Castelblanco, Jordi Real, Esther Rubinat-Arnaldo, Alicia Traveset, Marta Hernández, and et al. 2023. "Caffeine and the Risk of Diabetic Retinopathy in Type 2 Diabetes Mellitus: Findings from Clinical and Experimental Studies" Nutrients 15, no. 5: 1169. https://doi.org/10.3390/nu15051169
APA StyleAlcubierre, N., Granado-Casas, M., Bogdanov, P., Hernández, C., Ramos, H., Castelblanco, E., Real, J., Rubinat-Arnaldo, E., Traveset, A., Hernández, M., Jurjo, C., Vioque, J., Navarrete-Muñoz, E. M., Simó, R., & Mauricio, D. (2023). Caffeine and the Risk of Diabetic Retinopathy in Type 2 Diabetes Mellitus: Findings from Clinical and Experimental Studies. Nutrients, 15(5), 1169. https://doi.org/10.3390/nu15051169










