Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature
Highlights
- A diet rich in fruits, vegetables, dietary fibers, and fish and following a Mediterranean diet is associated with a lower risk of diabetic retinopathy (DR).
- Conversely, high intakes of diet soda, caloric intake, rice, and choline are linked to an increased risk of DR.
- These findings highlight the importance of incorporating nutritional counseling into diabetes management to potentially reduce the risk of DR.
- Further research is needed to confirm these associations in diverse diabetic populations and to develop more specific clinical guidelines.
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Inclusion Criteria
- Participants—Studies including human subjects with type1, type 2 diabetic mellitus, or both.
- Study design—It included prospective, case–control, cross-sectional, and randomized controlled trials (RCTs).
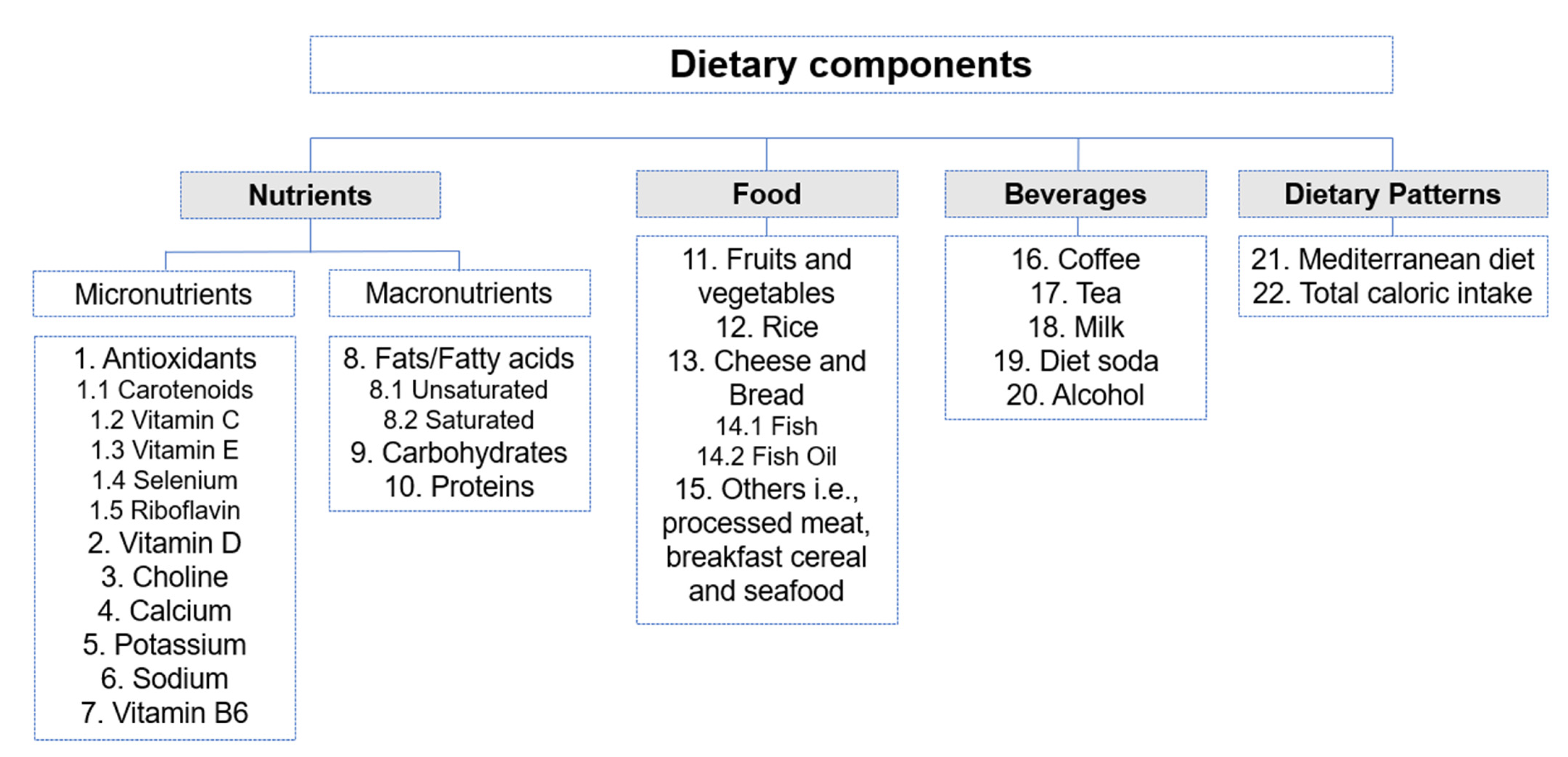
- Interventions or exposure—Studies that evaluated dietary intake using tools such as validated food frequency questionnaires, 24 h dietary recall, dietary history, or general interviewer-administered questionnaires. Dietary intake components included specific food, beverages, micronutrients, macronutrients, and dietary patterns (Figure 3).
- Outcomes—It included prevalence, incidence, or progression of DR with or without DME. Studies that assessed DR outcomes by fundus photography, fundus examination using a direct or indirect ophthalmoscope, and fundus fluorescein angiography were accepted. Different scales for grading the severity of DR such as the Early Treatment Diabetic Retinopathy Study (ETDRS) and the International Classification system of DR were also accepted. The ETDRS is based on seven field stereophotographs, classifying DR from level 10 (absence of retinopathy) to level 85 (vitreous hemorrhage or retinal detachment involving macula). Conversely, the International Classification System grade cases into the categories of: no apparent retinopathy, mild, moderate, and severe non-proliferative retinopathy and final-stage proliferative diabetic retinopathy [30].
2.4. Exclusion Criteria
- Animal studies, in vivo/in vitro studies and reviews.
- Studies that included the non-diabetic, pre-diabetic, or impaired glucose intolerance participants, or patients with special types of diabetes such as gestational diabetes.
- Studies with insufficient data, such as lack of exposure/outcome definitions or absence of statistical analysis which did not enable us to make conclusions.
- Studies that measured only biomarkers in serum, blood, or urine with no relation to dietary intake.
- Studies including intake in the form of supplements containing multiple different types of nutrients.
- Studies describing outcomes using abnormal retinal changes, microvascular complications, or visual acuity but not defined in the form of DR severity.
2.5. Data Extraction
2.6. Study Quality Evaluation
- Selection of participants (maximum of 4 stars).
- Comparability (maximum of 2 stars).
- Exposure (for prospective or cross-sectional designs) or outcome (for case–control designs) (maximum of 3 stars).
3. Results
3.1. Description of Studies
3.2. Measurement of Exposures and Outcomes
3.3. Methodological Quality
3.4. Relationship between Intake of Micronutrients to Diabetic Retinopathy
3.4.1. Antioxidants
Carotenoids
Vitamin C
Vitamin E
Selenium
Riboflavin
3.4.2. Vitamin D
3.4.3. Choline
3.4.4. Calcium
3.4.5. Potassium
3.4.6. Sodium
3.4.7. Vitamin B6
3.5. Relationship between Intake of Macronutrients to Diabetic Retinopathy
3.5.1. Fats/Fatty acids
Monounsaturated Fatty Acids (MUFA)
Polyunsaturated Fatty Acids (PUFA)
3.5.2. Carbohydrates
3.5.3. Proteins
3.6. Relationship between Food Intake to Diabetic Retinopathy
3.6.1. Fruits, Vegetables and Dietary Fiber
3.6.2. Rice
3.6.3. Cheese and Wholemeal Bread
3.6.4. Fish
Fish oil
3.6.5. Other Types of Food
3.7. Relationship between Beverage Intake to Diabetic Retinopathy
3.7.1. Coffee
3.7.2. Tea
3.7.3. Milk
3.7.4. Diet Soda
3.7.5. Alcohol
3.8. Relationship between Broader Dietary Patterns to Diabetic Retinopathy
3.8.1. Mediterranean Dietary Pattern
3.8.2. Total Caloric Intake
4. Discussion
4.1. Protective Associations between Dietary Intake and Diabetic Retinopathy
4.2. Adverse Associations between Dietary Intake and Diabetic Retinopathy
4.3. No Significant Association between Dietary Intake and Diabetic Retinopathy
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blindness, G.B.D.; Vision Impairment, C.; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Lim, C.X.Y.; Wong, T.Y.; Sabanayagam, C. Diabetic Retinopathy in the Asia-Pacific. Asia Pac. J. Ophthalmol. 2018, 7, 3–16. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.Y.; Klein, B.E.K.; et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, E.K.; Pesudovs, K.; Rees, G.; Dirani, M.; Kawasaki, R.; Wong, T.Y.; Lamoureux, E.L. The impact of diabetic retinopathy: Understanding the patient’s perspective. Br. J. Ophthalmol. 2011, 95, 774–782. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. The Effect of Diet and Lifestyle on the Course of Diabetic Retinopathy-A Review of the Literature. Nutrients 2022, 14, 1252. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Guilbert, E.; Perry, R.; Whitmarsh, A.; Sauchelli, S. Short-term effectiveness of nutrition therapy to treat type 2 diabetes in low-income and middle-income countries: Systematic review and meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e056108. [Google Scholar] [CrossRef]
- Lima, V.C.; Cavalieri, G.C.; Lima, M.C.; Nazario, N.O.; Lima, G.C. Risk factors for diabetic retinopathy: A case–control study. Int. J. Retin. Vitr. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.M.; Castañer, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals with Type 2 Diabetes: Prospective Investigation from the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Chia, A.; Chong, M.F.-F.; Man, R.; Tan, G.S.; Lamoureux, E.L.; Wong, T.Y.; Schmetterer, L. The Relationship of Dietary Fish Intake and Diabetic Retinopathy in Asian Patients with Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4284. [Google Scholar]
- Hamad, S.; Zafar, T.A.; Sidhu, J. Parboiled rice metabolism differs in healthy and diabetic individuals with similar improvement in glycemic response. Nutrition 2018, 47, 43–49. [Google Scholar] [CrossRef]
- Wong, M.Y.Z.; Man, R.E.K.; Fenwick, E.K.; Gupta, P.; Li, L.J.; van Dam, R.M.; Chong, M.F.; Lamoureux, E.L. Dietary intake and diabetic retinopathy: A systematic review. PLoS ONE 2018, 13, e0186582. [Google Scholar] [CrossRef]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef] [PubMed]
- She, C.; Shang, F.; Cui, M.; Yang, X.; Liu, N. Association between dietary antioxidants and risk for diabetic retinopathy in a Chinese population. Eye 2021, 35, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, C.; Aida, R.; Kamada, C.; Fujihara, K.; Tanaka, S.; Tanaka, S.; Araki, A.; Yoshimura, Y.; Moriya, T.; Akanuma, Y.; et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: Analysis of data from the Japan Diabetes Complications Study (JDCS). Eur. J. Nutr. 2020, 59, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ren, C.; Zhang, W.; Liu, G.; Lu, P. Association between Dietary Choline Intake and Diabetic Retinopathy: National Health and Nutrition Examination Survey 2005–2008. Curr. Eye Res. 2022, 47, 269–276. [Google Scholar] [CrossRef]
- Kadri, R.; Vishwanath, P.; Parameshwar, D.; Hegde, S.; Kudva, A.A. Dietary associations with diabetic retinopathy-A cohort study. Indian J. Ophthalmol. 2021, 69, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Han, X.; Wu, C.; Keel, S.; Shang, X.; Zhang, L.; He, M. Does daily dietary intake affect diabetic retinopathy progression? 10-year results from the 45 and Up Study. Br. J. Ophthalmol. 2020, 104, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Alali, N.; Albalawi, H.; ALselaimy, R. Diet Sugar-Free Carbonated Soda Beverage, Non-Caloric Flavors Consumption, and Diabetic Retinopathy: Any Linkage. Diabetes Metab. Syndr. Obes. 2021, 14, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, E.K.; Gan, A.T.; Man, R.E.; Sabanayagam, C.; Gupta, P.; Khoo, K.; Aravindhan, A.; Wong, T.Y.; Lamoureux, E.L. Diet soft drink is associated with increased odds of proliferative diabetic retinopathy. Clin. Exp. Ophthalmol. 2018, 46, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bi, M.; Jin, X.; Zhu, M.; Wang, G.; Zhao, P.; Qin, X.; Xu, X.; Sun, X.; Ji, N.; et al. Long-Term Tea Consumption Is Associated with Reduced Risk of Diabetic Retinopathy: A Cross-Sectional Survey among Elderly Chinese from Rural Communities. J. Diabetes Res. 2020, 2020, 1860452. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, F.; Firouzabadi, F.D.; Moosaie, F.; Shadnoush, M.; Poopak, A.; Kermanchi, J.; Abhari, S.M.F.; Forouzanfar, R.; Mansournia, M.A.; Khosravi, A.; et al. Effects of a Mediterranean diet on the development of diabetic complications: A longitudinal study from the nationwide diabetes report of the National Program for Prevention and Control of Diabetes (NPPCD 2016–2020). Maturitas 2021, 153, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, J.I.; Kwon, S.O.; Hwang, D.D. Coffee consumption and diabetic retinopathy in adults with diabetes mellitus. Sci. Rep. 2022, 12, 3547. [Google Scholar] [CrossRef]
- Agrawal, R.; Gupta, P.; Tan, K.A.; Cheung, C.M.; Wong, T.Y.; Cheng, C.Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wu, L.; Fernandez-Loaiza, P.; Sauma, J.; Hernandez-Bogantes, E.; Masis, M. Classification of diabetic retinopathy and diabetic macular edema. World J. Diabetes 2013, 4, 290–294. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, 1–173. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://web.archive.org/web/20210716121605id_/http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 6 October 2022).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, Y.J. Association between Dietary Calcium and Potassium and Diabetic Retinopathy: A Cross-Sectional Retrospective Study. Nutrients 2022, 14, 1086. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Bell, R.A.; Reboussin, B.A.; Rushing, J.; Marshall, J.A.; Hamman, R.F. Antioxidant nutrient intake and diabetic retinopathy. Ophthalmology 1998, 105, 2264–2270. [Google Scholar] [CrossRef]
- Shalini, T.; Jose, S.S.; Prasanthi, P.S.; Balakrishna, N.; Viswanath, K.; Reddy, G.B. Carotenoid status in type 2 diabetes patients with and without retinopathy. Food Funct. 2021, 12, 4402–4410. [Google Scholar] [CrossRef]
- Horikawa, C.; Aida, R.; Tanaka, S.; Kamada, C.; Tanaka, S.; Yoshimura, Y.; Kodera, R.; Fujihara, K.; Kawasaki, R.; Moriya, T.; et al. Sodium Intake and Incidence of Diabetes Complications in Elderly Patients with Type 2 Diabetes-Analysis of Data from the Japanese Elderly Diabetes Intervention Study (J-EDIT). Nutrients 2021, 13, 689. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Zhang, J.; Kuang, X.; Liu, C.; Deng, Q.; Li, D. Relationship between retinol and risk of diabetic retinopathy: A case-control study. Asia Pac. J. Clin. Nutr. 2019, 28, 607–613. [Google Scholar] [CrossRef]
- Chua, J.; Chia, A.R.; Chee, M.L.; Man, R.E.K.; Tan, G.S.W.; Lamoureux, E.L.; Wong, T.Y.; Chong, M.F.; Schmetterer, L. The relationship of dietary fish intake to diabetic retinopathy and retinal vascular caliber in patients with type 2 diabetes. Sci. Rep. 2018, 8, 730. [Google Scholar] [CrossRef]
- Granado-Casas, M.; Ramírez-Morros, A.; Martín, M.; Real, J.; Alonso, N.; Valldeperas, X.; Traveset, A.; Rubinat, E.; Alcubierre, N.; Hernández, M.; et al. Type 1 Diabetic Subjects with Diabetic Retinopathy Show an Unfavorable Pattern of Fat Intake. Nutrients 2018, 10, 1184. [Google Scholar] [CrossRef]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Matsunaga, S.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; et al. Is the Proportion of Carbohydrate Intake Associated with the Incidence of Diabetes Complications?—An Analysis of the Japan Diabetes Complications Study. Nutrients 2017, 9, 113. [Google Scholar] [CrossRef]
- Alcubierre, N.; Navarrete-Muñoz, E.M.; Rubinat, E.; Falguera, M.; Valls, J.; Traveset, A.; Vilanova, M.B.; Marsal, J.R.; Hernandez, M.; Granado-Casas, M.; et al. Association of low oleic acid intake with diabetic retinopathy in type 2 diabetic patients: A case-control study. Nutr. Metab. 2016, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Sahli, M.W.; Nie, J.; LaMonte, M.J.; Lutsey, P.L.; Klein, B.E.; Mares, J.A.; Meyers, K.J.; Andrews, C.A.; Klein, R. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc. Diabetol. 2016, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Sahli, M.W.; Mares, J.A.; Meyers, K.J.; Klein, R.; Brady, W.E.; Klein, B.E.; Ochs-Balcom, H.M.; Donahue, R.P.; Millen, A.E. Dietary Intake of Lutein and Diabetic Retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic Epidemiol. 2016, 23, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alcubierre, N.; Valls, J.; Rubinat, E.; Cao, G.; Esquerda, A.; Traveset, A.; Granado-Casas, M.; Jurjo, C.; Mauricio, D. Vitamin D Deficiency Is Associated with the Presence and Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 374178. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kawasaki, R.; Rogers, S.; Man, R.E.; Itakura, K.; Xie, J.; Flood, V.; Tsubota, K.; Lamoureux, E.; Wang, J.J. The Associations of Dietary Intake of Polyunsaturated Fatty Acids with Diabetic Retinopathy in Well-Controlled Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7473–7479. [Google Scholar] [CrossRef]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; Ohashi, Y.; et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: Analysis of the Japan Diabetes Complications Study (JDCS). J. Clin. Endocrinol. Metab. 2014, 99, 3635–3643. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Tanaka, S.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akanuma, Y.; et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef]
- Roy, M.S.; Janal, M.N. High caloric and sodium intakes as risk factors for progression of retinopathy in type 1 diabetes mellitus. Arch. Ophthalmol. 2010, 128, 33–39. [Google Scholar] [CrossRef]
- Millen, A.E.; Klein, R.; Folsom, A.R.; Stevens, J.; Palta, M.; Mares, J.A. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2004, 79, 865–873. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.; Son, J.I.; Rhee, S.Y.; Kim, D.Y.; Chon, S.; Lim, H.; Woo, J.T. Dietary glutamic acid and aspartic acid as biomarkers for predicting diabetic retinopathy. Sci. Rep. 2021, 11, 7244. [Google Scholar] [CrossRef]
- Roy, M.S.; Stables, G.; Collier, B.; Roy, A.; Bou, E. Nutritional factors in diabetics with and without retinopathy. Am. J. Clin. Nutr. 1989, 50, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Cundiff, D.K.; Nigg, C.R. Diet and diabetic retinopathy: Insights from the Diabetes Control and Complications Trial (DCCT). MedGenMed. 2005, 7, 3. [Google Scholar]
- Engelen, L.; Soedamah-Muthu, S.S.; Geleijnse, J.M.; Toeller, M.; Chaturvedi, N.; Fuller, J.H.; Schalkwijk, C.G.; Stehouwer, C.D. Higher dietary salt intake is associated with microalbuminuria, but not with retinopathy in individuals with type 1 diabetes: The EURODIAB Prospective Complications Study. Diabetologia 2014, 57, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Houtsmuller, A.J.; Zahn, K.J.; Henkes, H.E. Unsaturated fats and progression of diabetic retinopathy. Doc. Ophthalmol. 1980, 48, 363–371. [Google Scholar] [CrossRef]
- Howard-Williams, J.; Patel, P.; Jelfs, R.; Carter, R.D.; Awdry, P.; Bron, A.; Mann, J.I.; Hockaday, T.D. Polyunsaturated fatty acids and diabetic retinopathy. Br. J. Ophthalmol. 1985, 69, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Raman, R.; Kulothungan, V.; Sharma, T. Influence of dietary-fibre intake on diabetes and diabetic retinopathy: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (report 26). Clin. Exp. Ophthalmol. 2012, 40, 288–294. [Google Scholar] [CrossRef]
- Alsbirk, K.E.; Seland, J.H.; Assmus, J. Diabetic retinopathy and visual impairment in a Norwegian diabetic coast population with a high dietary intake of fish oils. Obs. Study. Acta Ophthalmol. 2022, 100, e532–e538. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Li, X.; Wong, W.-L.; Tai, S.E.; Lee, J.; van Dam, R.M.; Wong, T.-Y. Is Coffee Consumption associated with Age-related Macular Degeneration and Diabetic Retinopathy? All Results J. Biol. 2014, 5, 7–13. [Google Scholar]
- Ma, Q.; Chen, D.; Sun, H.P.; Yan, N.; Xu, Y.; Pan, C.W. Regular Chinese Green Tea Consumption is Protective for Diabetic Retinopathy: A Clinic-Based Case-Control Study. J. Diabetes Res. 2015, 2015, 231570. [Google Scholar] [CrossRef]
- Gupta, P.; Fenwick, E.K.; Sabanayagam, C.; Gan, A.T.L.; Tham, Y.C.; Thakur, S.; Man, R.E.K.; Mitchell, P.; Wong, T.Y.; Cheng, C.Y.; et al. Association of alcohol intake with incidence and progression of diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 538–542. [Google Scholar] [CrossRef]
- Fenwick, E.K.; Xie, J.; Man, R.E.; Lim, L.L.; Flood, V.M.; Finger, R.P.; Wong, T.Y.; Lamoureux, E.L. Moderate consumption of white and fortified wine is associated with reduced odds of diabetic retinopathy. J. Diabetes Complicat. 2015, 29, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.; Kruidhof, J.S.; Grobbee, D.E.; Chaturvedi, N.; Fuller, J.H.; Soedamah-Muthu, S.S. Alcohol consumption and risk of microvascular complications in type 1 diabetes patients: The EURODIAB Prospective Complications Study. Diabetologia 2008, 51, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Harjutsalo, V.; Feodoroff, M.; Forsblom, C.; Groop, P.H.; FinnDiane Study Group. Patients with Type 1 diabetes consuming alcoholic spirits have an increased risk of microvascular complications. Diabet. Med. 2014, 31, 156–164. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E.K. Alcohol Consumption and the Prevalence of Diabetic Retinopathy. Ophthalmology 1992, 99, 926–932. [Google Scholar] [CrossRef]
- Thapa, R.; Twyana, S.N.; Paudyal, G.; Khanal, S.; van Nispen, R.; Tan, S.; Thapa, S.S.; van Rens, G. Prevalence and risk factors of diabetic retinopathy among an elderly population with diabetes in Nepal: The Bhaktapur Retina Study. Clin. Ophthalmol. 2018, 12, 561–568. [Google Scholar] [CrossRef]
- Young, R.J.; McCulloch, D.K.; Prescott, R.J.; Clarke, B.F. Alcohol: Another risk factor for diabetic retinopathy? Br. Med. J. Clin. Res. Ed. 1984, 288, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Acan, D.; Calan, M.; Er, D.; Arkan, T.; Kocak, N.; Bayraktar, F.; Kaynak, S. The prevalence and systemic risk factors of diabetic macular edema: A cross-sectional study from Turkey. BMC Ophthalmol. 2018, 18, 91. [Google Scholar] [CrossRef]
- Kawasaki, R.; Kitano, S.; Sato, Y.; Yamashita, H.; Nishimura, R.; Tajima, N.; Japan Diabetes, C.; For the Japan Diabetes Complication and its Prevention prospective (JDCP) study Diabetic Retinopathy working group. Factors associated with non-proliferative diabetic retinopathy in patients with type 1 and type 2 diabetes: The Japan Diabetes Complication and its Prevention prospective study (JDCP study 4). Diabetol. Int. 2019, 10, 3–11. [Google Scholar] [CrossRef]
- Giuffre, G.; Lodato, G.; Dardanoni, G. Prevalence and risk factors of diabetic retinopathy in adult and elderly subjects: The Casteldaccia Eye Study. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 535–540. [Google Scholar] [CrossRef]
- Lee, C.C.; Stolk, R.P.; Adler, A.I.; Patel, A.; Chalmers, J.; Neal, B.; Poulter, N.; Harrap, S.; Woodward, M.; Marre, M.; et al. Association between alcohol consumption and diabetic retinopathy and visual acuity-the AdRem Study. Diabet. Med. 2010, 27, 1130–1137. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E.K. The Association of Alcohol Consumption with the Incidence and Progression of Diabetic Retinopathy. Ophthalmology 1994, 101, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lopez, A.; Babio, N.; Martinez-Gonzalez, M.A.; Corella, D.; Amor, A.J.; Fito, M.; Estruch, R.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2015, 38, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef]
- Das, U.N. Lipoxins, resolvins, and protectins in the prevention and treatment of diabetic macular edema and retinopathy. Nutrition 2013, 29, 1–7. [Google Scholar] [CrossRef]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Tikhonenko, M.; Lydic, T.A.; Opreanu, M.; Li Calzi, S.; Bozack, S.; McSorley, K.M.; Sochacki, A.L.; Faber, M.S.; Hazra, S.; Duclos, S.; et al. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS ONE 2013, 8, e55177. [Google Scholar] [CrossRef]
- Benson, G.; Pereira, R.F.; Boucher, J.L. Rationale for the Use of a Mediterranean Diet in Diabetes Management. Diabetes Spectr. 2011, 24, 36–40. [Google Scholar] [CrossRef][Green Version]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Green Tea Is Neuroprotective in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef]
- Gardener, H.; Moon, Y.P.; Rundek, T.; Elkind, M.S.V.; Sacco, R.L. Diet Soda and Sugar-Sweetened Soda Consumption in Relation to Incident Diabetes in the Northern Manhattan Study. Curr. Dev. Nutr. 2018, 2, nzy008. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.B. Bowman Lecture 1998. Diabetic retinopathy: Some cellular, molecular and therapeutic considerations. Eye 1999, 13 Pt 4, 497–523. [Google Scholar] [CrossRef]
- Skrha, J.; Kunesová, M.; Hilgertová, J.; Weiserová, H.; Krízová, J.; Kotrlíková, E. Short-term very low calorie diet reduces oxidative stress in obese type 2 diabetic patients. Physiol. Res. 2005, 54, 33–39. [Google Scholar] [CrossRef]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Koutnik, A.P.; Azova, S.; Wolfsdorf, J.I.; Ludwig, D.S. Carbohydrate restriction for diabetes: Rediscovering centuries-old wisdom. J. Clin. Investig. 2021, 131, e142246. [Google Scholar] [CrossRef]
- Chiu, C.J.; Taylor, A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 2011, 30, 18–53. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, 2009, Cd006296. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Lee, C.T.; Gayton, E.L.; Beulens, J.W.; Flanagan, D.W.; Adler, A.I. Micronutrients and diabetic retinopathy a systematic review. Ophthalmology 2010, 117, 71–78. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kanwar, M.; Chan, P.S.; Zhang, J.P. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch. Ophthalmol. 2008, 126, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.H.; Ma, Q.; Shen, S.R.; Xu, G.T.; Das, U.N. Effect of α-linolenic acid on streptozotocin-induced diabetic retinopathy indices in vivo. Arch. Med. Res. 2013, 44, 514–520. [Google Scholar] [CrossRef]
- Zhu, W.; Meng, Y.F.; Wu, Y.; Xu, M.; Lu, J. Association of alcohol intake with risk of diabetic retinopathy: A meta-analysis of observational studies. Sci. Rep. 2017, 7, 4. [Google Scholar] [CrossRef]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol and the eye: Activity and molecular mechanisms. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef]
- Albert, M.A.; Glynn, R.J.; Ridker, P.M. Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation 2003, 108, 161–165. [Google Scholar] [CrossRef]
- American Diabetes, A. Professional Practice Committee: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S3. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 786–806. [CrossRef]
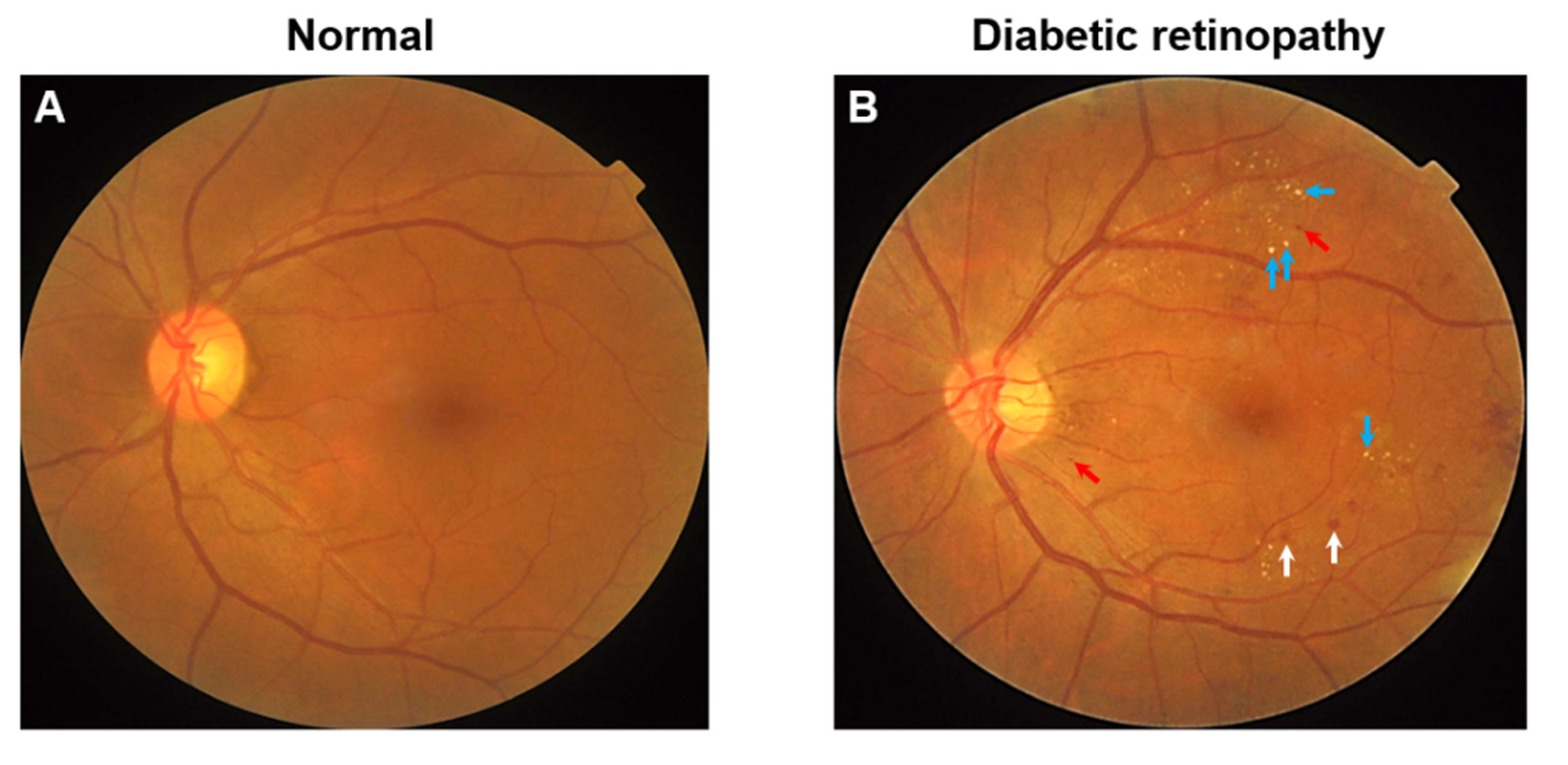


| Study, Year Sample Size | Diabetes Type | Age | Dietary Factor | Diet Evaluation | DR Outcome | DR Evaluation | Classification of DR | Quality Score | |
|---|---|---|---|---|---|---|---|---|---|
| 3 Interventional studies | |||||||||
| Houtsmuller et al., 1979 n = 96 | Any diabetes | Not stated | Saturated fat diet vs. unsaturated fat diet | NA | Incidence and progression | Fundus photograph | None, NPDR, PDR, PRP | High bias | |
| Howard-Williams et al., 1985 n = 149 | Any diabetes | <66 | Saturated fat diet vs. unsaturated fat diet | NA | Incidence | Ophthalmologist examination | None, retinopathy | High bias | |
| Diaz-Lopez et al., 2015 n = 3614 | T2DM | 55–80 | Mediterranean diet | NA | Incidence | Ophthalmologist examination | None, NPDR, PDR | Moderate bias | |
| 17 Prospective studies | |||||||||
| Horikawa et al., 2021 T2DM: 912 | T2DM | 65–85 | Sodium | Validated food frequency questionnaire | Incidence | Ophthalmologist examination | Japanese Diabetes Complication Study Method | 10 | |
| Park et al., 2021 DR: 731 no DR: 1336 | T2DM | DR: 53.1 (9.7) no DR: 55.6 (9.7) | Glutamic acid and aspartic acid | 3-day food record with computer-aided nutritional analysis | Incidence | Fundus photograph, OCT | ETDRS | 10 | |
| Horikawa et al., 2017 n = 936 | T2DM | 40–70 | Carbohydrates | Validated food frequency questionnaire | Incidence and progression | Ophthalmologist examination | International Classification System | 10 | |
| Horikawa et al., 2014 n = 978 | T2DM | 40–70 | Sodium | Validated food frequency questionnaire | Incidence and progression | Ophthalmologist examination | International Classification System | 10 | |
| Tanaka et al., 2013 n = 978 | T2DM | 40–70 | Vitamin C, Vitamin E, carotenoids, fruits, and vegetables, | Validated food frequency questionnaire + 24 h dietary recall | Incidence | Ophthalmologist examination | International Classification System | 10 | |
| Hainsworth et al., 2019 PDR: 379 no PDR: 1061 | T1DM | PDR: 26 (21–32) no PDR: 27 (22–32) | Alcohol beverage | Simple background questionnaire | Incidence and progression | Standardized stereoscopic seven-field fundus photographs | ETDRS | 9 | |
| Horikawa et al., 2019 n = 978 | T2DM | 40–70 | Vitamin B6 | Validated food frequency questionnaire | Incidence | Mydriatic indirect ophthalmoscopic examination and slit lamp biomicroscopic fundus examination, with supplementation of fundus photography and fluorescein angiography | International Clinical Diabetic Retinopathy, DME Severity Scale | 9 | |
| Sala-Vila et al., 2016 n = 3482 | T2DM | 55–80 | Long-chain omega-3 polyunsaturated fatty acids and oily Fish | Validated food frequency questionnaire | Incidence | Clinical and hospital records | None, NPDR, PDR | 9 | |
| Lee et al., 2010 n = 1239 | T2DM | 55–81 | Alcohol | Self-report in a general questionnaire | Progression | Fundus photograph | Modified ETDRS | 9 | |
| Roy et al., 2010 n = 469 | T1DM | Men: 26.7 (10.7) Women: 27.8 (10.8) | MUFA, PUFA, oleic acid, protein, dietary fiber, carbohydrates, sodium, total calories, alcohol | Validated food frequency questionnaire | Incidence and progression | Fundus photograph | Modified ETDRS | 9 | |
| Moss et al., 1993 Young: 439 Older: 478 | Any diabetes | 21–94 | Alcohol | Self-report in a general questionnaire | Incidence and progression | Fundus photograph | Modified ETDRS | 9 | |
| Gupta et al., 2020 Abstainers: 563 Consumers: 93 | Not stated | Abstainers: 58.88 (9.45) Consumers: 58.41 (8.09) | Alcohol | Questionnaire on alcohol consumption | Incidence and progression | Fundus photograph | ETDRS, Airlie House Classification | 8 | |
| Cundiff et al., 2005 n = 1412 | T1DM | 13–39 | MUFA, PUFA, carbohydrates, protein, dietary fiber, sodium, alcohol, high calories | Dietary history interview | Progression | Fundus photograph | Modified ETDRS | 8 | |
| Young et al., 1984 n = 296 | Any diabetes | 20–59 | Alcohol | Self-report in a general questionnaire | Incidence | Direct ophthalmoscopy | Modified ETDRS | 8 | |
| Ghaemi et al., 2021 T1DM with MD: 1669 T1DM without MD: 180 T2DM with MD: 15886 T2DM without MD: 4452 | T1DM or T2DM | T1DM with MD: 50.63 (20.11) T1DM without MD: 51.40 (16.20) T2DM with MD: 59.78 11.00) | Mediterranean diet | 14 item questionnaire | Incidence | Records from the National Program for Prevention and Control of Diabetes of Iran database | International Classification of Diseases, 10th Revision: E10.3, E11.3, E12.3, E13.3, and E14.3 | 7 | |
| Kadri et al., 2021 DR: 106 no DR: 155 | T2DM | 57.73 (11.29) | Alcohol, antioxidants, milk, tea, coffee, fruits, meat, fish, egg, chapathi, rice, total Calories | 24 h dietary recall | Incidence and progression | Dilated fundus examination using slit-lamp biomicroscopy (90D), indirect ophthalmoscopy, fundus photography | Not stated | 7 | |
| Yan et al., 2019 n = 8122 | Not stated | 57.2 (5.2) | Meat, dairy products, wholemeal bread, breakfast cereal, vegetables, fruit, and fruit juice | Self-administered questionnaire | Incidence and progression | Retinal photocoagulation from the Medicare Benefits Schedule data (note: used as a proxy for DR progression) | Not stated | 6 | |
| 29 Cross-Sectional Studies | |||||||||
| Fenwick et al., 2015 n = 395 | T2DM | >18 | Alcohol | Validated food frequency questionnaire | Prevalence | Non-dilated fundus photography | ETDRS | 10 | |
| Ganesan et al., 2012 n = 1261 | Any diabetes | >40 | Dietary fiber | Validated fiber questionnaire | Prevalence | Dilated fundus photograph | Modified ETDRS | 10 | |
| Beulens et al., 2008 n = 1857 | T1DM | 15–60 | Alcohol | Self-report in a general questionnaire | Prevalence | Dilated fundus photograph | None, Background, Proliferative | 10 | |
| Lee et al., 2022 DR: 270 no DR: 1080 | T2DM | DR: 59.9(0.8) no DR: 58.6(0.4) | Coffee | Validated food frequency questionnaire | Prevalence | Fundus photograph | ETDRS, modified Airlie House Classification | 9 | |
| Liu et al., 2021 DR: 378 no DR: 894 | Not stated | >40 | Choline | 24 h dietary recall | Prevalence | Fundus photograph | Not stated | 9 | |
| Millen et al., 2016 n = 1305 | Any diabetes | 45–65 | Vitamin D, fish, milk | Validated food frequency questionnaire | Prevalence | Fundus photograph | Modified Airlie House Classification | 9 | |
| Sahli et al., 2016 n = 1430 | Any diabetes | 45–65 | Carotenoids (lutein) | Validated food frequency questionnaire | Prevalence | Non-dilated fundus photograph | ETDRS | 9 | |
| Mayer-Davis et al., 1998 n = 387 | T2DM | 20–74 | Vitamin C, Vitamin E, beta-carotene | 24 h dietary recall | Prevalence | Dilated fundus photograph | Modified Airlie House Classification | 9 | |
| Moss et al., 1992 Young: 891 Older: 987 | Any diabetes | 2–96 | Alcohol | Self-report in a general questionnaire | Prevalence | Fundus photograph | Modified Airlie House Classification | 9 | |
| Chen et al., 2022 DR: 696 no DR: 4515 | Not stated | DR: 62.43 (11.79) no DR: 58.961 (12.421) | Calcium and potassium | 24 h dietary recall | Prevalence | Fundus photograph | ETDRS | 8 | |
| She et al., 2020 DR: 119 No DR: 336 | T2DM | DR: 63.2 (8.5) no DR: 65.4 (8.8) | Antioxidants | 3-day food record | Prevalence | Fundus photograph | ETDRS | 8 | |
| Chua et al., 2018 n = 357 | T2DM | 58 (52–62) | Fish | Validated food frequency questionnaire | Prevalence | Two-field digital retinal photographs | ETDRS, Airlie House Classification | 8 | |
| Fenwick et al., 2018 n = 609 | T1DM or T2DM | 64.6(11.6) | Diet soft drink | Validated food frequency questionnaire | Prevalence | Two-field (macula and optic disc) dilated fundus photos were captured using a non-mydriatic retinal camera (fundus photography) | ETDRS for DR and the American Academy of Ophthalmology Scale for DME | 8 | |
| Granado-Casas et al., 2018 DR: 103 no DR: 140 | T1DM | DR: 46.2(10.8) no DR: 42.1(10.3) | Fat | Validated food frequency questionnaire | Prevalence | Ophthalmologist examination | International Clinical Classification System for diabetic retinopathy | 8 | |
| Thapa et al., 2018 DM: 1692 no DM: 168 | Not stated | DM: 69.8 (7.4) no DM: 67.9 (6.7) | Alcohol | Simple background questionnaire | Prevalence | Dilated fundus examination by a retina specialist | ETDRS | 8 | |
| Sasaki et al., 2015 n = 379 | Any diabetes | >18 | Vitamin C, Vitamin E, beta-carotene, MUFA, PUFA, carbohydrates, protein | Validated food frequency questionnaire | Prevalence | Fundus photograph | Modified ETDRS | 8 | |
| Kumari et al., 2014 n = 353 | Any diabetes | 21–95 | Coffee | Questionnaire on coffee consumption | Prevalence | Dilated fundus photograph | Modified Airlie House Classification | 8 | |
| Mahoney et al., 2014 n = 155 | Any diabetes | >40 | Fruits and vegetables | Validated food frequency questionnaire | Prevalence | Non-dilated fundus photograph | ETDRS | 8 | |
| Harjutsalo et al., 2013 n = 3608 | T1DM | Median age: 37.4 (28.9–46.8) | Alcohol | Self-report in a general questionnaire | Prevalence | History of laser photocoagulation | Severe DR vs. None | 8 | |
| Millen et al., 2004 n = 1353 | Any diabetes | 45–65 | Vitamin C and Vitamin E | Validated food frequency questionnaire | Prevalence | Non-dilated fundus photograph | Modified Airlie House Classification | 8 | |
| Xu et al., 2020 DM: 614 no DM: 4667 | Not stated | DM: 68.03(6.49) no DM: 67.88(6.64) | Tea | Questionnaire on tea consumption | Prevalence | Fundus photograph | ETDRS | 7 | |
| Engelen et al., 2014 n = 1880 | T1DM | 15–60 | Sodium | Estimated from urinary sodium excretion | Prevalence | Fundus photograph | None, NPDR, PDR | 7 | |
| Shalini et al., 2021 DR: 194 no DR: 150 Control: 151 | T2DM | DR: 55.0(0.6) no DR: 56.0(0.9) Control: 54.0(0.9) | Carotenoids | Validated raw food-based food frequency questionnaire with HPLC of plasma carotenoids | Prevalence | Fundus examination by indirect ophthalmoscopy, slit-lamp biomicroscopy, fundus fluorescein angiography | ETDRS | 6 | |
| Alsbirk et al., 2021 T1DM: 50 T2DM: 460 | T1DM or T2DM | T1DM: 44.5 (13–87) T2DM: 66 (27–92) | Fish food, PUFAs supplements | Questionnaire of self-reported dietary history | Prevalence | Fundus photograph | International Clinical Diabetic Retinopathy, DME Severity Scale | 6 | |
| Mirghani et al., 2021 DR: 66 no DR: 134 | Not stated | 50.74(13.51) | Diet sugar-free carbonated soda beverage | Validated food frequency questionnaire | Prevalence | Fundus examination | Not stated | 5 | |
| Kawasaki et al., 2018 NPDR: 83 no NPDR: 280 | T1DM or T2DM | NPDR: 58.9 no NPDR: 55.6 | Alcohol | Simple background questionnaire | Prevalence | Fundus findings from clinic and hospital records | International Clinical Diabetic Retinopathy | 5 | |
| Lugo-Radillo et al., 2013 n = 88 | Any diabetes | No DR: 58.50 (1.11) DR: 56.82 (1.65) | Fruits and vegetables | Oral questionnaire on fruit and vegetable consumption | Prevalence | Ophthalmologist examination | International Classification System | 5 | |
| Roy et al., 1989 n = 34 | Any diabetes | DR: 37.9 (12) No DR: 37.7 (9) | MUFA, PUFA, carbohydrates, protein, dietary fiber | 3-day food record | Prevalence | Fundus photography | Modified Airlie House Classification | 5 | |
| Acan et al., 2018 DME: 63 no DME: 350 | T1DM or T2DM | DME: 58.86 (11.27) no DME: 56.03 (11.95) | Alcohol | Simple background questionnaire | Prevalence | Dilated fundoscopy by ophthalmologists, central macular thickness analysis with OCT | ETDRS, OCT central macular thickness ≥ 250 μm | 3 | |
| 5 Case–control Studies | |||||||||
| Alcubierre et al., 2016 Case: 146 Control:148 | T2DM | 40–75 | MUFA, PUFA, oleic acid, carbohydrates, protein, dietary fiber | Validated food frequency questionnaire | Prevalence | Ophthalmologist examination | International Classification System | 10 | |
| Zhang et al., 2019 DM with DR: 43 DM without DR: 43 Controls: 40 | T2DM | DM with DR: 59 (49–66) DM without DR: 53 (44–65) Controls: 54(47–67) | Vitamin A | Validated food frequency questionnaire with HPLC of plasma retinol | Prevalence | Not stated | Not stated | 8 | |
| Alcubierre et al., 2015 Case: 139 Control:144 | T2DM | No DR: 58.1 (10.3) DR: 60.3 (8.9) | Vitamin D, calcium | Validated food frequency questionnaire | Prevalence | Ophthalmologist examination | International Classification System | 8 | |
| Ma et al., 2014 Case:100 Control:100 | T2DM | >18 | Green tea | Questionnaire on tea consumption | Prevalence | Fundus photograph | ETDRS | 8 | |
| Giuffre et al., 2004 Case:45 Control:87 | Any diabetes | >40 | Alcohol | Self-report in a general questionnaire | Prevalence | Direct ophthalmoscopy and fundus photograph | ETDRS | 7 | |
| Study, Year Study Design Sample Size (n) | Quality Score | Dietary Factor and Its Association with DR | Adjustment/Matched | Statistical Method Analysis | Key Findings |
|---|---|---|---|---|---|
| Antioxidants | |||||
| Carotenoids | |||||
| Tanaka et al., 2013 Prospective n = 978 | 10 | Carotenoids Protective | Sex, age, BMI, HbA1c, diabetes duration, insulin treatment, oral hypoglycaemic agents without insulin treatment, systolic blood pressure, LDL and HDL cholesterol, triglycerides, physical activity alcohol, smoking, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, omega-6 PUFA and omega-3 PUFA and sodium | Multivariate Cox regression | Highest intake Q4 vs. lowest Intake Q1, HR: 0.52 (0.33–0.81) p < 0.01 |
| Sahli et al., 2016 Cross-sectional n = 1430 | 9 | Lutein carotenoids NS | Diabetes duration, HbA1c, blood pressure, race, total energy consumption, and study center | Multivariable logistic regression | Intake Q4 vs. Q1, OR: 0.89 (0.31–2.50), p = 0.72 |
| Mayer-Davis et al., 1998 Cross-sectional n = 387 | 9 | Beta-Carotene NS | Age, gender, ethnicity, diabetes duration, HbA1c, hypertension, caloric intake, and insulin use | Multivariable logistic regression | No significant associations with DR (data not shown) |
| Zhang et al., 2019 Case–control Type2 DM-86 control-40 | 8 | Retinol carotenoids Protective | Age, sex, smoking, BMI and alcohol consumption | Logistic regression | Intake of retinol (100 μg/day) on DR (OR: 0.88, 95%CI, 0.79–0.98, p = 0.025) |
| Sasaki et al., 2015 Cross-sectional n = 379 | 8 | Beta-carotene NS | Intake of energy | Data not shown | No significant associations with DR (data not shown) |
| Shalini et al., 2021 Cross-sectional n = 495 | 7 | Carotenoids Protective | Nil | One-way analysis of variance F test with a post hoc test of least significant difference | The plasma concentration of carotenoids was significantly lower in the DR group compared to no DR patients and healthy controls (p < 0.001) |
| Vitamin C | |||||
| Tanaka et al., 2013 Prospective n = 978 | 10 | Vitamin C Protective | Sex, age, BMI, HbA1c, diabetes duration, insulin treatment, oral hypoglycaemic agents without insulin treatment, systolic blood pressure, LDL and HDL cholesterol, triglycerides, physical activity alcohol, smoking, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, omega-6 PUFA and omega-3 PUFA and sodium | Multivariate Cox regression | Intake Q4 vs. Q1, HR: 0.61 (0.39–0.96), p = 0.03 |
| Mayer-Davis et al., 1998 Cross-sectional N = 387 | 9 | Vitamin C Risk | Age, gender, ethnicity, diabetes duration, HbA1c, hypertension, caloric intake, and insulin use | Multivariable logistic regression | Intake 9th decile vs. 1st quintile, OR: 2.21, (p = 0.01) |
| She et al., 2020 Cross-sectional n = 455 | 8 | Vitamin C NS | Sex, race, insulin use, HbA1c, hypertension, exercise | Binomial logistic regression multivariate analysis | No significant association with DR (p = 0.413) |
| Sasaki et al., 2015 Cross-sectional n = 379 | 8 | Vitamin C NS | Intake of energy | Data notshown | No significant association with DR (data not shown) |
| Millen et al., 2004 Cross-sectional n = 1353 | 8 | Vitamin C NS | Race, BMI, diabetes duration, serum glucose, total energy intake, hypertension, waist–hip ratio, smoking, alcohol, drinking status, plasma cholesterol, hematocrit value, prevalent coronary heart disease, plasma triacylglycerol, diabetes treatment group, and oral hypoglycaemic treatment or insulin treatment | Multivariable logistic regression | Intake Q4 vs. Q1, OR: 1.4 (0.8–2.4), p = 0.19 |
| Vitamin E | |||||
| Tanaka et al., 2013 Prospective n = 978 | 10 | Vitamin E NS | Sex, age, BMI, HbA1c, diabetes duration, insulin treatment, oral hypoglycaemic agents without insulin treatment, systolic blood pressure, LDL and HDL cholesterol, triglycerides, physical activity alcohol, smoking, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, omega-6 PUFA and omega-3 PUFA and sodium | Multivariate Cox regression | Intake Q4 vs. Q1, HR: 0.84 (0.51–1.40), p = 0.51 |
| Mayer-Davis et al., 1998 Cross-sectional N = 387 | 9 | Vitamin E Risk (in non-insulin taking subjects) | Age, gender, ethnicity, diabetes duration, HbA1c, hypertension, caloric intake, and insulin use | Multivariable logistic regression | No association found in insulin subjects and in non-insulin taking subjects: Intake 10th decile vs. 1st quintile, OR: 3.79, (p < 0.02) |
| She et al., 2020 Cross-sectional n = 455 | 8 | Vitamin E Protective | Sex, race, insulin use, HbA1c, hypertension, exercise | Binomial logistic regression multivariate analysis | Intake in DR vs. No DR (OR: 0.97, 95%CI: 0.95–1.00, p = 0.036) |
| Granado-Casas et al., 2018 Cross-sectional n = 243 | 8 | Vitamin E Protective | Age, sex, educational level, smoking, physical activity, BMI, dyslipidemia, hypertension, diabetes duration, HbA1c | Multivariable conditional logistic regression models | Intake of Vitamin E on DR (OR: 0.85 [0.77–0.95], p = 0.006) |
| Sasaki et al., 2015 Cross-sectional n = 379 | 8 | Vitamin E NS | Intake of energy | Data notshown | No significant associations with DR (data not shown) |
| Millen et al., 2004 Cross-sectional n = 1353 | 8 | Vitamin E NS | Race, BMI, diabetes duration, serum glucose, total energy intake, hypertension, waist–hip ratio, smoking, alcohol, drinking status, plasma cholesterol, hematocrit value, prevalent coronary heart disease, plasma triacylglycerol, diabetes treatment group, and oral hypoglycaemic treatment or insulin treatment | Multivariable logistic regression | Intake Q4 vs. Q1, OR: 1.4 (0.8–2.3), p = 0.76 |
| Selenium | |||||
| She et al., 2020 Cross-sectional n = 455 | 8 | Selenium Protective | Sex, race, insulin use, HbA1c, hypertension, exercise | Binomial logistic regression multivariate analysis | Intake in DR vs. No DR (OR: 0.98, 95%CI: 0.96–1.00, p = 0.017) |
| Riboflavin | |||||
| She et al., 2020 Cross-sectional n = 455 | 8 | Riboflavin NS | Sex, race, insulin use, HbA1c, hypertension, exercise | Binomial logistic regression multivariate analysis | No significant association with DR (p > 0.05) |
| Vitamin D | |||||
| Millen et al., 2016 Cross-sectional n = 1305 | 9 | Vitamin D NS | Race, duration of diabetes, HbA1c and, hypertension | Multivariable logistic regression | Intake Q4 vs. Q1, OR: 1.20 (0.76–1.89), p trend = 0.740 |
| Alcubierre et al., 2015 Case–control Case:139 Ctrl:144 | 8 | Vitamin D NS | NIL | Chi-squared | No significant associations with DR (p = 0.93) |
| Choline | |||||
| Liu et al., 2021 Cross-sectional n = 1272 | 9 | Choline Risk in female | Age, race, diabetes duration, glycaemic control, CVD, CKD * results analyzed in individual sex groups | Multivariable logistic regression | High intake vs. low intake (OR: 2.14, 95%CI: 1.38–3.31; p = 0.001) |
| Calcium | |||||
| Chen et al., 2022 Cross-sectional n = 5321 | 9 | Calcium Protective | Age, sex, race, smoking, serum glucose, serum laboratory data, hemoglobin | Multivariable logistic regression | High intake vs. low intake OR: 0.70, 95%CI: 0.54–0.90, p = 0.05) |
| Alcubierre et al., 2015 Case–control Case:139 Ctrl:144 | 8 | Calcium NS | NIL | Chi-squared | No significant associations with DR (p = 0.65) |
| Potassium | |||||
| Tanaka et al., 2013 Prospective n = 978 | 10 | Potassium NS | Sex, age, BMI, HbA1c, diabetes duration, insulin treatment, oral hypoglycaemic agents without insulin treatment, systolic blood pressure, LDL and HDL cholesterol, triglycerides, physical activity alcohol, smoking, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, omega-6 PUFA and omega-3 PUFA and sodium | Multivariate Cox regression | No significant association with DR (p > 0.05) |
| Chen et al., 2022 Cross-sectional n = 5321 | 9 | Potassium Protective | Age, sex, race, smoking serum glucose, serum laboratory data, hemoglobin | Multivariable logistic regression | High intake vs. low intake OR: 0.761, 95%CI: 0.59–0.97, p = 0.029 |
| Sodium | |||||
| Horikawa et al., 2021 Prospective n = 912 | 10 | Sodium Risk (under low vegetable consumption) | Age, sex, BMI, HbA1c, diabetes duration, LDL cholesterol, HDL cholesterol, log-transformed triglycerides, insulin treatment, smoking, alcohol, energy intake, physical activity, systolic blood pressure, angiotensin II receptor blocker, angiotensin-converting enzyme inhibitor, calcium channel blocker | Multivariate Cox regression analyses | Intake for 2nd, 3rd, and 4th quartile vs. 1st quartile, HRs were 0.87 [95%CI, 0.31–2.41], 2.61 [1.00–6.83], and 3.70 [1.37–10.02], respectively p = 0.010. |
| Horikawa et al., 2014 Prospective n = 978 | 10 | Sodium NS | Sex, age, BMI, HbA1c, duration of diabetes, LDL cholesterol, HDL cholesterol, log-transformed triglycerides, insulin treatment, lipid-lowering agents, smoking, alcohol intake, energy intake, sodium intake, and physical activity | Multivariate Cox regression | Intake Q4 vs. Q1, HR: 1.10 (0.75–1.61), p = 0.55 |
| Roy et al., 2010 Prospective n = 469 | 10 | Sodium Risk (ForDME) NS for DR | Age, sex, HbA1c, hypertension, total caloric intake, protein intake, oleic acid intake, physical exercise, and oleic acid intake | Multivariable logistic regression | Intake Q4 vs. Q1, OR: 1.43 (1.10–1.86), p = 0.008 for DME. No significant associations with DR |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | Sodium NS | Intake of energy | Spearman correlation | Sodium in mg/kcal against DR progression rate, r = 0.02 (p = 0.47) |
| Engelen et al., 2014 Cross-sectional n = 1880 | 7 | Sodium NS | Sex, age, smoking, BMI, urinary potassium excretion, sat fat intake, protein intake antihypertensive medication, total energy intake, physical activity, fiber intake, and alcohol intake | Multivariable logistic regression | Per 1g/day increase in dietary salt intake against NPDR OR: 1.00, (0.96–1.04, p = 0.84. PDR OR: 1.02 (0.95–1.08), p = 0.65 |
| Vitamin B6 | |||||
| Horikawa et al., 2019 Prospective n = 978 | 9 | Vitamin B6 Protective | Age, sex, BMI, HbA1c, diabetes duration, systolic blood pressure, LDL cholesterol, HDL cholesterol, triglycerides, insulin treatment, oral hypoglycemic agents, antihypertensive agents, lipid-lowering agents, urine albumin creatinine ratio, estimated glomerular filtration rate, alcohol, smoking, energy intake, physical activity, retinol, vitamin B1, vitamin B2, vitamin B9, vitamin B12 | Multivariate Cox regression analyses | Intake Q4 vs. Q1 HR: 0.50, 95%CI: 0.30–0.85, p = 0.010) |
| Study, Year Study Design Sample Size (n) | Quality Score | Dietary Factor and Its Association with DR | Adjustment/Matched | Statistical Methods Analysis | Key Findings |
|---|---|---|---|---|---|
| Dietary Fats/lipids | |||||
| Monounsaturated Fatty Acids (MUFA) | |||||
| Alcubierre et al.,2016 Case–control Case:146 Ctrl:148 | 10 | MUFA Protective | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | High MUFA consumption vs. low MUFA consumption, OR: 0.42 (0.18–0.97), p = 0.034 |
| Sasaki et al., 2015 Cross-sectional n = 379 | 10 | MUFA NS | Sex, Age, HbA1c, duration of diabetes, and mean arterial pressure | Multivariable logistic regression models | Per 10 energy-adjusted g/d increase, OR: 1.19 (0.74–1.92) |
| Roy et al., 2010 Prospective n = 469 | 9 | MUFA NS | Total fat, total caloric intake, oleic acid, linoleic acid, fiber, protein, sat fat, cholesterol and sodium intakes | Multivariable logistic regression | No significant associations with DR (data not shown) |
| Granado-Casas et al., 2018 Cross-sectional n = 243 | 8 | MUFA Protective | Age, sex, educational level, smoking, center, physical activity, BMI, dyslipidemia hypertension, diabetes duration, HbA1c | Multivariable conditional logistic regression models | MUFA intake against frequency of DR (OR: 0.95, 95%CI: 0.92–0.99, p = 0.012) |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | MUFA Risk | Intake of energy | Spearmancorrelation | MUFA in %/kcal against DR progression rate, r = 0.12 (p = 0.001) |
| Roy et al., 1989 Cross-sectional n = 34 | 5 | MUFA NS | Intake of energy | t test | No significant associations with DR (data not shown) |
| Oleic acid | |||||
| Alcubierre et al., 2016 Case–control Case:146 Ctrl:148 | 10 | Oleic acid Protective | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | Highest intake tertile (T3) vs. lowest intake tertile (T1), OR: 0.37 (0.16–0.85), p = 0.017 |
| Roy et al., 2010 Prospective n = 469 | 9 | Oleic acid NS | Total fat, total caloric intake, oleic acid, linoleic acid, fiber, protein, sat fat, cholesterol and sodium intake | Multivariable logistic regression | No significant associations with DR (data not reported) |
| Granado-Casas et al., 2018 Cross-sectional n = 243 | 8 | Oleic acid Protective | Age, sex, educational level, smoking, center, physical activity, BMI, dyslipidemia hypertension, diabetes duration, HbA1c | Multivariable conditional logistic regression models | Oleic acid intake against DR (OR: 0.95, CI: 0.92–0.99, p = 0.012) |
| Polyunsaturated Fatty Acids (PUFA) | |||||
| Alcubierre et al., 2016 Case–control Case:146 Ctrl:148 | 10 | PUFA NS | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | High PUFA consumption vs. low MUFA consumption, OR: 0.99 (0.69–1.41) |
| Sasaki et al., 2015 Cross-sectional n = 379 | 10 | PUFA Protective forwell controlleddiabetics | Sex, age, HbA1c, duration of diabetes, and mean arterial pressure | Multivariablelogistic regression models | All subjects: Per 10 energy-adjusted g/d increase, OR: 0.67 (0.37–1.20) Well-controlled diabetics: Per 10 energy adjusted g/d increase, OR: 0.18 (0.06–0.59) |
| Sala-Vila et al., 2016 Prospective n = 3482 | 9 | PUFA (long-chain omega-3 fatty acid) Protective | Age, sex, BMI, intervention group, duration of diabetes, insulin treatment, oral hypoglycemic treatment, smoking, hypertension, systolic blood pressure, physical activity, and adherence to the Mediterranean diet | Cox proportional hazard model | >500 mg/d vs. <500 mg/d, HR: 0.52 (0.31–0.88) p = 0.001 |
| Roy et al., 2010 Prospective n = 469 | 9 | PUFA NS | Total fat, total caloric intake, oleic acid, linoleic acid, fiber, protein, sat fat, cholesterol and sodium intakes | Multivariable logistic regression | No significant associations with DR (data not shown) |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | PUFA Risk | Intake of energy | Spearman correlation | PUFA in %/kcal against DR progression rate, r = 0.09 (p = 0.004) |
| Roy et al., 1989 cross-sectional | 5 | PUFA NS | Intake of energy | t test | No significant associations with DR (data not reported) |
| Interventional studies | |||||
| Howard-Williams et al., 1985 Interventional n = 149 | HighBias | PUFA NS | Age, sex and BMI | Participants on a modified fat diet (PUFA: saturated fat ratio, 0.3) vs. low-carb diet (PUFA: saturated fat ratio, 0.9) No difference between the two groups in all participants (n = 149) (chi-squared, p = 0.69) No difference between the two groups in dietary compliers (n = 58) (chi-squared, p = 0.13) | |
| Houtsmuller et al., 1979 Interventional n = 96 | Highbias | Unsaturatedfats Protective | Gender | Saturated fat diet vs. unsaturated fat diet males (n = 52, 26 on each diet) p < 0.001 females (n = 44, 22 on each diet) p < 0.025 | |
| Carbohydrates | |||||
| Horikawa et al., 2017 Prospective n = 936 | 10 | Carbohydrates NS | Gender, age, BMI, HbA1c, diabetes duration, insulin treatment, systolic blood pressure, LDL cholesterol, HDL cholesterol, antihypertensive agents, lipids lowering drugs, energy intake, triglycerides, current smoker, alcohol consumption, and physical activity | Multivariable Cox regression models | Highest intake tertile (T3) vs. lowest intake tertile (T1), HR: 1.00 (0.72–1.38) |
| Alcubierre et al., 2016 Case–control Case:146 Ctrl:148 | 10 | Carbohydrates NS | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | Highest intake tertile (T3) vs. lowest intake tertile (T1), OR: 1.18 (0.45–3.09) |
| Roy et al., 2010 Prospective n = 469 | 9 | Carbohydrates NS | Total fat, total caloric intake, oleic acid, linoleic acid, fiber, protein, sat fat, cholesterol, and sodium intakes | Multivariable logistic regression | No significant associations with DR (data not shown) |
| Granado-Casas et al., 2018 Cross-sectional n = 243 | 8 | Carbohydrates Risk | Age, sex, educational level smoking, center, physical activity, BMI, dyslipidemia hypertension, diabetes duration, HbA1c | Multivariable conditional logistic regression models | Intake of complex carbohydrates against DR (OR: 1.02, CI: 1.00–1.04, p = 0.031) |
| Sasaki et al., 2015 Cross-sectional n = 379 | 8 | Carbohydrates NS | Intake of energy | Chi-squared | No significant associations with DR (data not shown) |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | Carbohydrates Protective | Intake of energy | Spearman correlation | Carbohydrates in %/kcal against DR progression rate, r = −0.11 (p < 0.001) |
| Roy et al., 1989 cross-sectional n = 34 | 5 | Carbohydrates Protective | Intake of energy | t test | Persons without retinopathy vs. persons with retinopathy (p < 0.05) |
| Protein | |||||
| Park et al., 2021 Prospective n = 2067 | 10 | Protein (glutamic acid and aspartic acid) NS for DR incidence, however aspartic acid protective for PDR | Age, sex, HbA1c, diabetes duration, education income, occupation, creatinine clearance, alanine aminotransferase, other comorbidities | Cox proportional hazard models | No significant association with DR incidence. Intake of aspartic acid highest tertile vs. lowest tertile for PDR (HR: 0.39, 95%CI: 0.16–0.96, p = 0.013) |
| Alcubierre et al., 2016 Case–control Case:146 Ctrl:148 | 10 | Protein NS | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | Highest protein intake tertile (T3) vs lowest protein intake tertile (T1), OR: 1.24 (0.49–3.16) |
| Roy et al., 2010 Prospective n = 469 | 9 | Protein NS | Total fat, total caloric intake, oleic acid, linoleic acid, fiber, protein, sat fat, cholesterol, and sodium intakes | Multivariable logistic regression | No significant associations with DR (data not shown) |
| Sasaki et al., 2015 Cross-sectional n = 379 | 8 | Protein NS | Intake of energy | Chi-squared | No significant associations with DR (data not shown) |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | Protein Protective | Intake of energy | Spearman correlation | Protein in %/kcal against DR progression rate, r = −0.6 (p = 0.018) |
| Roy et al., 1989 Cross-sectional n = 34 | 5 | Protein Risk | Intake of energy | t test | Persons without retinopathy vs. persons with retinopathy (p < 0.02) |
| Study, Year Study Design Sample Size (n) | Quality score | Dietary Factor and Its Association with DR | Adjustment/Matched | Statistical Methods Analysis | Key Findings |
|---|---|---|---|---|---|
| Fruits, vegetables, and dietary fiber | |||||
| Alcubierre et al., 2016 Case–control Case:146 Ctrl:148 | 10 | Dietary fiber NS | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | Highest fiber intake tertile (T3) vs. lowest fiber intake tertile (T1), OR: 0.76 (0.33–0.76) |
| Tanaka et al., 2013 Prospective n = 978 | 10 | Fruits, vegetables, and dietary fiber Protective | Sex, age, BMI, HbA1c, diabetes duration, insulin treatment, oral hypoglycaemic agents without insulin treatment, systolic blood pressure, LDL and HDL cholesterol, triglycerides, physical activity alcohol, smoking, total energy intake, proportions of dietary protein, fat, carbohydrate, saturated fatty acids, omega-6 PUFA and omega-3 PUFA and sodium | Multivariate Cox regression | Veg and fruit intake Q4 vs. Q1, HR: 0.59 (0.37–0.92), p < 0.01. Fruit intake Q4 vs. Q1, HR: 0.48(0.32–0.71), p = 0.01. Dietary fiber intake Q4 vs. Q1, HR: 0.63 (0.38–1.03), p = 0.07. |
| Ganesan et al., 2012 Cross-sectional n = 1261 | 10 | Dietary fiber Protective | Sex, Age, diabetes duration, blood pressure, BMI, Hba1c, serum lipids, smoking, and, socioeconomic status. | Multivariable logistic regression | Low-fiber diet vs. healthy fiber diet for any DR, OR: 1.41 (1.02–1.94), p = 0.039. Low-fiber diet vs. healthy fiber diet for VTDR, OR: 2.24 (1.01–5.02), p = 0.049. |
| Roy et al., 2010 Prospective n = 469 | 9 | Dietary fiber NS | Total fat, total caloric intake, oleic acid, linoleic acid, fiber, protein, sat fat, cholesterol, and sodium intakes | Multivariable logistic regression | No significant associations with DR (Data not shown) |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | Dietary fiber Protective | Intake of energy | Spearman correlation | Dietary fiber in g/1000kcal against DR progression rate, r = −0.10 (p = 0.002) |
| Yan et al., 2019 Prospective n = 8122 | 6 | Fruits, vegetables, and dietary fiber NS | Age, sex, income, educational level, BMI, hypertension, CVD, family history of diabetes, insulin treatment | Cox regression model. | No significant associations with DR (p < 0.05) |
| Roy et al., 1989 Cross-sectional n = 34 | 5 | Dietary fiber Protective | Diabetes duration | t test | Persons without retinopathy vs. persons with retinopathy, (p < 0.01) |
| Rice | |||||
| Kadri et al., 2021 Prospective n = 261 | 8 | Rice Risk | Age, sex, duration, antioxidants, pharmacological treatment, egg, fish, chapathi, rice | Multivariate regression analysis | Rice consumption yes vs. no, OR: 3.19, 95%CI: 1.17–8.69, p = 0.018 |
| Cheese and wholemeal bread | |||||
| Yan et al., 2019 Prospective n = 8122 | 6 | Cheese and wholemeal bread Protective | Age, sex, income, educational level, BMI, hypertension, CVD, family history of diabetes, insulin treatment | Cox regression model. | Cheese intake highest quartiles vs. lowest HR: 0.58, 95%CI: 0.41–0.83, p = 0.007 and wholemeal bread HR: 0.64, CI: 0.4–0.89, p = 0.04 |
| Fish | |||||
| Sala-Vila et al., 2016 Prospective n = 3482 | 9 | Oily fish Protective | Age, sex, BMI, intervention group, duration of diabetes, insulin treatment, oral hypoglycemic treatment, smoking, hypertension, systolic blood pressure, physical activity, and adherence to the Mediterranean diet | Cox proportional hazard model | >2 servings a week vs. <2 servings a week, HR: 0.41 (0.23–0.72), p = 0.002 |
| Kadri et al., 2021 Prospective n = 261 | 8 | Fish Protective | Age, sex, duration, antioxidants, pharmacological treatment, egg, fish, chapathi, rice | Multivariate regression analysis | Fish intake, more frequent vs. less frequent, OR: 0.42, 95%CI: 0.18–0.94, p < 0.05 |
| Chua et al., 2018 Cross-sectional n = 357 | 8 | Fish Protective | Age, sex, race, smoking diabetes duration, diabetic treatment, lipid-lowering medication use, systolic blood pressure, HbA1c, triglycerides | Ordered logistic and linear regression models | Per one serving increase in fish intake per week, OR: 0.91, 95%CI: 0.84–0.99, p = 0.038 |
| Yan et al., 2019 Prospective n = 8122 | 6 | Fish NS | Age, sex, income, educational level, BMI, hypertension, CVD, family history of diabetes, Insulin treatment | Cox regression model | No significant associations with DR (p = 0.22) |
| Alsbirk et al., 2021 Cross-sectional n = 510 | 6 | Fish oil NS | Age, sex, diabetes type, diabetes duration, HbA1c, medication | Logistic regression | No significant association (p > 0.005) |
| Other types of food | |||||
| Yan et al., 2019 Prospective n = 8122 | 6 | Processed meat/breakfast cereal NS | Age, sex, income, educational level, BMI, hypertension, CVD, family history of diabetes, insulin treatment | Cox regression model. | No significant associations with DR (p > 0.05) |
| Study, Year Study Design Sample Size (n) | Quality Score | Dietary Factor and Its Association with DR | Adjustment/Matched | Statistical Methods Analysis | Key Findings |
|---|---|---|---|---|---|
| Coffee | |||||
| Lee at al, 2022 Cross-sectional n = 1350 | 9 | Coffee Protective | Age, sex, education, income, BMI, energy intake, hypertension, dyslipidemia, diabetes duration, HbA1c, smoking, alcohol, physical activity | Multivariable logistic regression models | Consumption ≥ 2 cups coffee/day vs. none for DR (OR: 0.53, 95%CI: 0.28–0.99, p for trend = 0.025) and VTDR (OR: 0.30, 95%CI: 0.10–0.91, p for trend = 0.005) |
| Kumari et al., 2014 Cross-sectional n = 353 | 9 | Coffee NS | Sex, age, HbA1c, smoking, BMI, creatinine, education level, diabetes duration, family history of diabetes, hypertension, stroke, ischemic heart disease, dyslipidemia, and cancer | Multivariable logistic regression | Coffee drinker vs. never/rarely, OR: 1.36 (0.69–2.69) |
| Tea | |||||
| Ma et al., 2014 Case–control Case:100 Ctrl:100 | 8 | Green Tea Protective | Diabetes duration, insulin treatment, family history of diabetes, fasting blood glucose, education, BMI, systolic blood pressure, smoking, alcohol, physical and, activity | Multivariable logistic regression | Regular Chinese green tea drinker vs. non-regular Chinese green tea drinker, OR: 0.48, CI: 0.24–0.97, p = 0.04 |
| Xu et al., 2020 Cross-sectional n = 5,281 | 7 | Tea Protective | Age, sex, individual monthly income, fasting blood glucose, systolic blood pressure, occupation, educational level, smoking, alcohol | Multivariate logistic regression analyses | Tea consumers vs. non-tea consumers, OR: 0:29, 95%CI: 0.09–0.97, p = 0.04 |
| Milk | |||||
| Yan et al., 2019 Prospective n = 8122 | 6 | Milk NS | Age, sex, income, educational level, BMI, hypertension, CVD, family history of diabetes, insulin treatment | Cox regression model | No significant associations with DR (p = 0.74) |
| Diet soda | |||||
| Fenwick et al., 2018 Cross-sectional n = 609 | 8 | Diet soft drink Risk | Age, sex, HbA1c, diabetes duration, insulin use, presence of at least one other diabetes complication, diabetes type, BMI, education antihypertensive medication, hyperlipidaemia, presence of comorbidity, smoking, alcohol energy intake, regular soft drink consumption | Multinomial logistic regression | High-consumption (>4 cans [1.5 liters]/week) vs. no consumption for proliferative DR (OR = 2.62, 95%CI = 1.14–6.06, p = 0.024) |
| Mirghani et al., 2021 Cross-sectional n = 200 | 6 | Diet sugar-free carbonated soda beverage Risk | NIL | Multiple regression analysis | Diet soda was associated with DR (p = 0.043) |
| Alcohol | |||||
| Fenwick et al., 2015 Cross-sectional n = 395 | 10 | Alcohol Protective | Sex, gender, poorly controlled diabetes, diabetes duration, BMI, smoking, systolic blood pressure, insulin therapy, and presence of at least one other diabetic complication | Multivariable logistic regression | Moderate vs. abstainers, OR: 0.47 (0.26–0.85), p = 0.013; moderate white wine vs. abstainers, OR: 0.48 (0.25–0.91), p = 0.024; moderate fortified wine vs. abstainers, OR: 0.15 (0.04–0.62), p = 0.009 |
| Beulens et al., 2008 Cross-sectional n = 1857 | 10 | Alcohol Protective | Sex, Age, smoking, center, smoking, diabetes duration, physical activity, presence of CVD, systolic blood pressure, BMI, and HbA1C | Multivariable logistic regression | Moderate vs. abstainers, OR: 0.60 (0.37–0.99), p = 0.023 |
| Lee et al., 2010 Prospective n = 1239 | 9 | Alcohol NS | Sex, age, ethnicity, smoking, HbA1c, BMI, systolic blood pressure, and duration diabetes | Multivariable logistic regression | Moderate vs. none, OR: 1.08 (0.70–1.67) Heavy vs. none, OR: 1.07 (0.54–2.13), p = 0.8 |
| Moss et al., 1993 Prospective Younger: 439 Older: 478 | 9 | Alcohol NS | Sex, age, HbA1c | Multivariable logistic regression | Younger-onset diabetics per 1oz/day increase in alcohol consumption on DR incidence, OR: 2.09 (0.04–1.07);per 1oz/day increase in alcohol consumption on DR progression, OR: 1.25 (0.75–2.08). Older-onset diabetics per 1oz/day increase in alcohol consumption on DR incidence, OR: 0.75 (0.4–1.42); per 1oz/day increase in alcohol consumption on DR progression, OR: 0.73 (0.4–1.20) |
| Moss et al., 1992 Cross-sectional Younger: 891 Older: 987 | 9 | Alcohol Protective | Diabetes duration, age, HbA1c, diastolic blood pressure, insulin therapy | Multivariable logistic regression | Younger-onset diabetes population per 1oz/day increase in alcohol consumption for PDR, OR: 0.49, (0.27–0.92) Older-onset: no significant associations |
| Gupta et al., 2020 Prospective n = 656 | 8 | Alcohol Protective | Age, sex, BMI, smoking, systolic blood pressure, income, HbA1c, diabetes duration, hyperlipidaemia, CKD, antidiabetic medication | Multivariable analyses | Alcohol consumption vs. non-drinkers, OR: 0.36 (0.13 to 0.98) p = 0.045; occasional drinker (≤2 days/week) vs. non-drinkers, OR:0.17, (0.04–0.69), p = 0.013) |
| Thapa et al., 2018 Cross-sectional n = 1860 | 8 | Alcohol Risk | NIL | Multivariable logistic regression analysis | Alcohol consumption yes vs. no for DR (OR:4.3, 95%CI: 1.6–11.3, p = 0.004) and vision-threatening DR (OR: 8.6, 95%CI: 1.7–47.2, p = 0.010) |
| Harjutsalo et al., 2013 Cross-sectional n = 3608 | 8 | Alcohol Protective | Sex, diabetes duration, age at onset of diabetes, triglycerides, HbA1C, HDL cholesterol, social class, BMI, smoking status, lipid-lowering agents and hypertension | Multivariable logistic regression | Abstainers vs. light consumers, OR: 1.42 (1.11–1.82), p < 0.05; former users vs. light consumers, OR: 1.73 (1.07–2.79), p < 0.05 |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | Alcohol NS | Intake of energy | Spearman correlation | No significant association with DR (p = 0.26) |
| Young et al., 1984 Prospective n = 296 | 8 | Alcohol Risk | Diabetes duration, impotence and glycemic control | Multivariable logistic regression | Heavy consumption vs. none–moderate consumption, RR: 2.25 (1.15–4.42) |
| Giuffre et al., 2004 Case–control Case:45 Ctrl:87 | 7 | Alcohol NS | Diabetes duration, duration of oral treatment and duration of insulin therapy | Multivariable logistic regression | No significant association with DR (data not shown) |
| Kawasaki et al., 2018 Cross-sectional n = 363 | 5 | Alcohol NS | Age, sex, HbA1c, diabetes duration, medication, BMI, lifetime maximum body weight, systolic blood pressure, diastolic blood pressure, non-HDL cholesterol, HDL-cholesterol, LDL, estimated glomerular filtration rate, history of myocardial infarction, history of stroke, alcohol, smoking, number of oral hypoglycemic agents, number of antihypertensive agents | Multiple logistic regression model | No signification was seen (p = 0.759) |
| Acan et al., 2018 Cross-sectional n = 413 | 3 | Alcohol Risk | NIL | t test | p = 0.010 |
| Mediterranean Diet | |||||
| Ghaemi et al., 2021 Prospective n = 22187 | 7 | Mediterranean diet Protective | Age, sex, time, HbA1c, fasting plasma glucose, HDL-cholesterol, total cholesterol, total triglycerides, systolic blood pressure, obesity, smoking, diabetes duration | Pooled logistic regression models | Mediterranean diet against incident retinopathy in type 1 DM (OR: 0.32, 95%CI: 0.24–0.44, p = <0.001) and type 2 DM (OR: 0.68, 95%CI: 0.61–0.71, p = <0.001) |
| Diaz-Lopez et al., 2015 Interventional n = 3614 | ModerateBias | Mediterranean diet Protective | Sex, age, waist circumference, BMI, smoking, physical activity, hypertension, educational level, dyslipidemia, family history of premature coronary heart disease, and baseline adherence | Multivariate Cox regression | Mediterranean diet vs. control diet, HR: 0.60 (0.37–0.96) |
| Caloric Intake | |||||
| Alcubierre et al., 2016 Case–control Case:146 Control:148 | 10 | Caloric intake NS | Sex, age, diabetes duration, energy intake, systolic blood pressure, physical activity, waist circumference, HDL cholesterol, educational level and diabetes treatment | Multivariable logistic regression | Highest energy intake tertile (T3) vs. lowest energy intake tertile (T1), OR: 0.73 (0.37–1.46) |
| Roy et al., 2010 Prospective n = 469 | 10 | Caloric intake Risk | Sex, age, total caloric intake, oleic acid intake, physical exercise, glycated hemoglobin, carbohydrate intake, protein intake, and hypertension | Multivariable logistic regression | Higher caloric intake, OR: 1.48 (1.15–1.92), p = 0.003 |
| Cundiff et al., 2005 Prospective n = 1412 | 8 | Caloric intake Risk | NIL | Spearman correlation | Calories in kcal against DR progression rate, r = 0.07 (p = 0.007) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.; Cheong, Z.Y.; Tan, B.; Wong, D.; Liu, X.; Chua, J. Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients 2022, 14, 5021. https://doi.org/10.3390/nu14235021
Shah J, Cheong ZY, Tan B, Wong D, Liu X, Chua J. Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients. 2022; 14(23):5021. https://doi.org/10.3390/nu14235021
Chicago/Turabian StyleShah, Janika, Zi Yu Cheong, Bingyao Tan, Damon Wong, Xinyu Liu, and Jacqueline Chua. 2022. "Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature" Nutrients 14, no. 23: 5021. https://doi.org/10.3390/nu14235021
APA StyleShah, J., Cheong, Z. Y., Tan, B., Wong, D., Liu, X., & Chua, J. (2022). Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients, 14(23), 5021. https://doi.org/10.3390/nu14235021







