Food Hardness Modulates Behavior, Cognition, and Brain Activation: A Systematic Review of Animal and Human Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Risk Bias Assessment of Included Studies
2.5. Data Collection and Management
3. Results
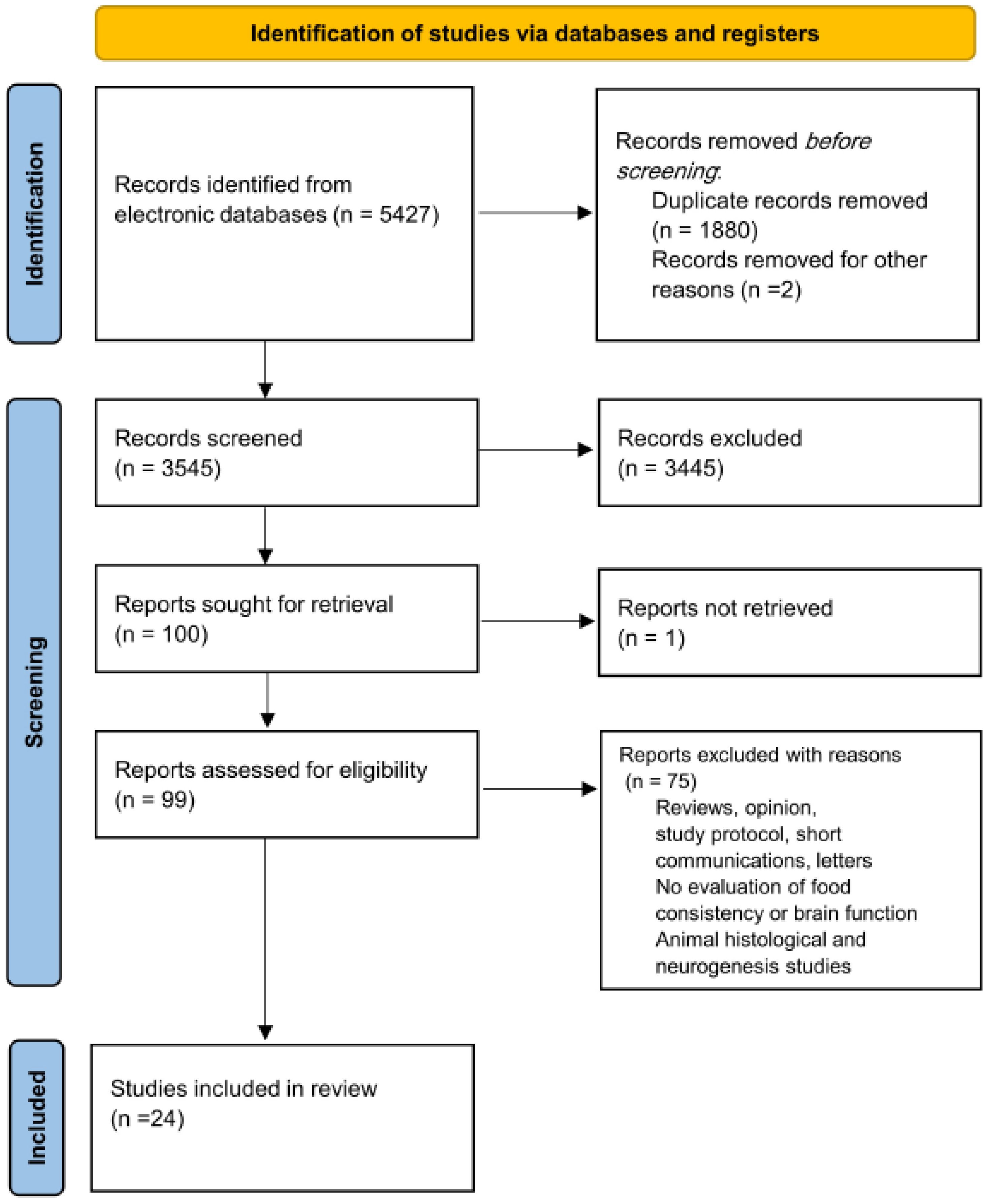
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Animal Studies
3.3.1. Characteristics of Laboratory Animal Species, Strains, Sex, and Age
3.3.2. Intervention: Food Hardness
3.3.3. Housing Conditions and Other Experiment Conditions
3.3.4. Behavioral Test Findings
3.4. Human Studies
3.4.1. Characteristics of Included Participants
3.4.2. Intervention Approaches
3.4.3. Brain Activation and Cognition Assessment Methods
3.4.4. Functional Magnetic Resonance Imaging (fMRI) and Cognition Test Findings
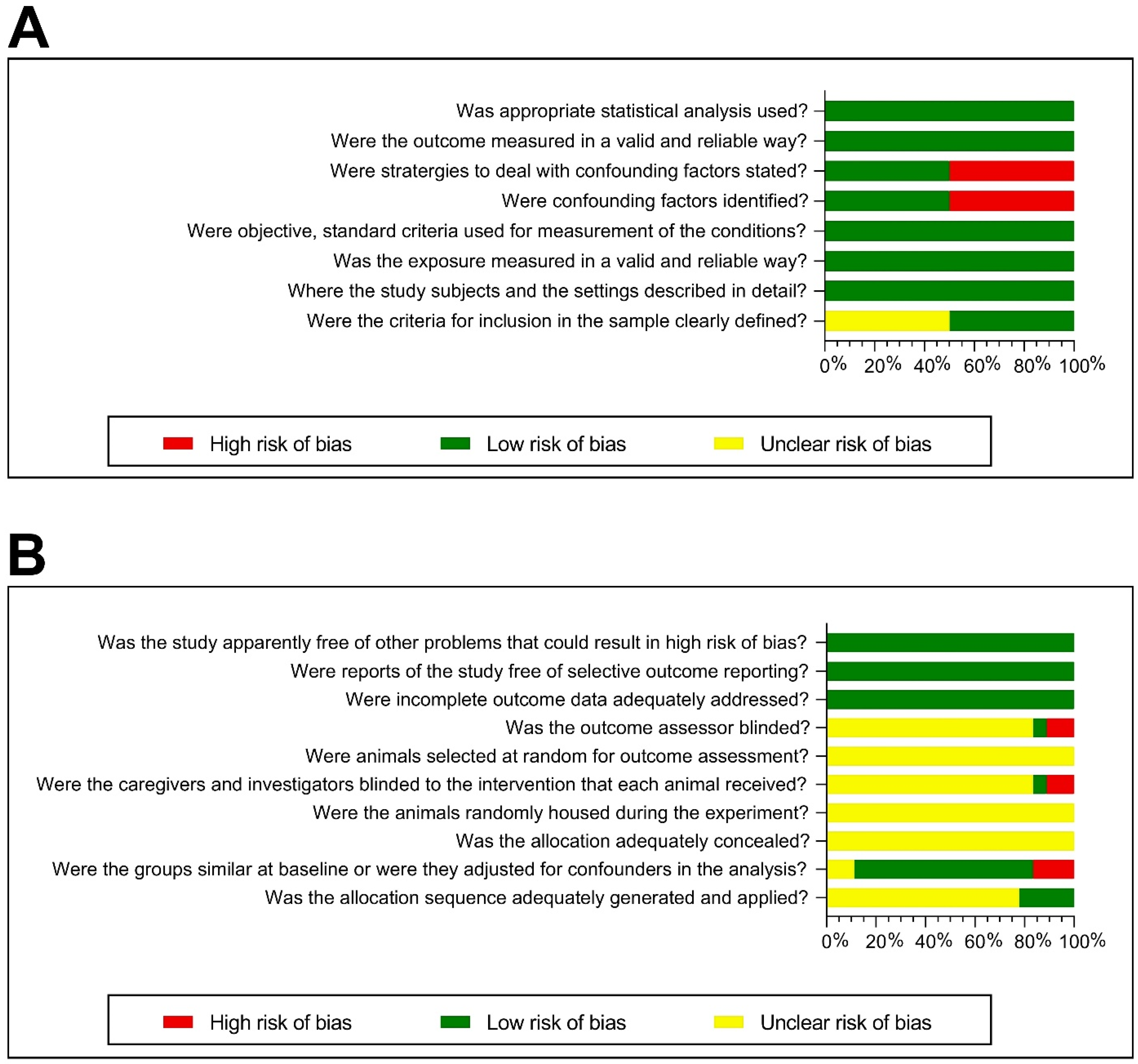
3.4.5. Risk of Bias Assessment
4. Discussion
4.1. Effect of Diet Hardness on Cognitive Functions in Animals
4.2. Effect of Masticatory Rehabilitation on Cognitive Functions in Animals
4.3. Effect of Diet Hardness on Cognitive Functions and Brain Activation in Humans
4.4. Other Factors Affecting the Effect of Hard Food Diets on Brain Function
4.5. Limitations and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Manei, K.; Almotairy, N.; Bostanci, N.; Kumar, A.; Grigoriadis, A. Effect of Chewing on the Expression of Salivary Protein Composition: A Systematic Review. Proteom. Clin. Appl. 2020, 14, e1900039. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.P.; Kolta, A.; Westberg, K.G.; Scott, G. Brainstem mechanisms underlying feeding behaviors. Curr. Opin. Neurobiol. 1998, 8, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Dellow, P.G.; Lund, J.P. Evidence for central timing of rhythmical mastication. J. Physiol. 1971, 215, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.P. Mastication and its control by the brain stem. Crit. Rev. Oral Biol. Med. 1991, 2, 33–64. [Google Scholar] [CrossRef]
- Al-Manei, K.; Almotairy, N.; Al-Manei, K.K.; Kumar, A.; Grigoriadis, A. Oral Fine Motor Control of Teeth Treated with Endodontic Microsurgery: A Single-Blinded Case-control Study. J. Endod. 2021, 47, 226–233. [Google Scholar] [CrossRef]
- Al-Manei, K.; Almotairy, N.; Al-Manei, K.K.; Grigoriadis, A.; Kumar, A. Effect of Apical Microsurgery on Force Regulation of Incisor Teeth during Unpredictable Force Control Task. J. Oral Rehabil. 2022, 49, 788–795. [Google Scholar] [CrossRef]
- Grigoriadis, J.; Kumar, A.; Svensson, P.; Svensson, K.G.; Trulsson, M. Perturbed oral motor control due to anesthesia during intraoral manipulation of food. Sci. Rep. 2017, 7, 46691. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tanaka, Y.; Takahashi, K.; Grigoriadis, A.; Wiesinger, B.; Svensson, P.; Trulsson, M. Vibratory stimulus to the masseter muscle impairs the oral fine motor control during biting tasks. J. Prosthodont. Res. 2019, 63, 354–360. [Google Scholar] [CrossRef]
- Grigoriadis, A.; Kumar, A.; Aberg, M.K.; Trulsson, M. Effect of Sudden Deprivation of Sensory Inputs From Periodontium on Mastication. Front. Neurosci. 2019, 13, 1316. [Google Scholar] [CrossRef]
- Almotairy, N.; Kumar, A.; Grigoriadis, A. Effect of food hardness on chewing behavior in children. Clin. Oral Investig. 2021, 25, 1203–1216. [Google Scholar] [CrossRef]
- Radke, J.C.; Kull, R.S.; Sethi, M.S. Chewing movements altered in the presence of temporomandibular joint internal derangements. Cranio 2014, 32, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lee, E.; Herring, S.W. Cranial sutures and bones: Growth and fusion in relation to masticatory strain. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 276, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Obata, T.; Takahashi, H.; Tachibana, A.; Kuroiwa, D.; Takahashi, T.; Ikehira, H.; Onozuka, M. Effects of chewing on cognitive processing speed. Brain Cogn. 2013, 81, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Lexomboon, D.; Trulsson, M.; Wardh, I.; Parker, M.G. Chewing ability and tooth loss: Association with cognitive impairment in an elderly population study. J. Am. Geriatr. Soc. 2012, 60, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Scholey, A.; Wesnes, K. Chewing gum selectively improves aspects of memory in healthy volunteers. Appetite 2002, 38, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Sesay, M.; Tanaka, A.; Ueno, Y.; Lecaroz, P.; De Beaufort, D.G. Assessment of regional cerebral blood flow by xenon-enhanced computed tomography during mastication in humans. Keio J. Med. 2000, 49 (Suppl. 1), A125–A128. [Google Scholar] [PubMed]
- Seki, M.; Haino, A.; Ishikawa, T.; Inagawa, H.; Soma, G.-I.; Terada, H.; Nashimoto, M. Mastication Affects Transcriptomes of Mouse Microglia. Anticancer Res. 2020, 40, 4719–4727. [Google Scholar] [CrossRef] [PubMed]
- Jaroudi, W.; Garami, J.; Garrido, S.; Hornberger, M.; Keri, S.; Moustafa, A.A. Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev. Neurosci. 2017, 28, 705–714. [Google Scholar] [CrossRef]
- Angeloni, C.; Businaro, R.; Vauzour, D. The role of diet in preventing and reducing cognitive decline. Curr. Opin. Psychiatry 2020, 33, 432–438. [Google Scholar] [CrossRef]
- Kosti, R.I.; Kasdagli, M.I.; Kyrozis, A.; Orsini, N.; Lagiou, P.; Taiganidou, F.; Naska, A. Fish intake, n-3 fatty acid body status, and risk of cognitive decline: A systematic review and a dose-response meta-analysis of observational and experimental studies. Nutr. Rev. 2022, 80, 1445–1458. [Google Scholar] [CrossRef]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 fatty acid, carotenoid and vitamin E supplementation improves working memory in older adults: A randomised clinical trial. Clin. Nutr. 2022, 41, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Kwok, T.; Woo, J. Higher fruit and vegetable variety associated with lower risk of cognitive impairment in Chinese community-dwelling older men: A 4-year cohort study. Eur. J. Nutr. 2022, 61, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.Z.; Chen, M.Q.; Zhang, Z.W.; Wu, T.Y.; Zhao, W.H. Dietary fatty acids and risk for Alzheimer’s disease, dementia, and mild cognitive impairment: A prospective cohort meta-analysis. Nutrition 2021, 90, 111355. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.S.; Gerassimakis, C.; Bygrave, D.; Waldstein, S.R. Dietary Factors and Cognitive Function in Poor Urban Settings. Curr. Nutr. Rep. 2017, 6, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Murakami, K.; Inagaki, H.; Gondo, Y.; Ikebe, K.; Kamide, K.; Masui, Y.; Arai, Y.; Ishizaki, T.; Sasaki, S.; et al. Hardness of the habitual diet and its relationship with cognitive function among 70-year-old Japanese elderly: Findings from the SONIC Study. J. Oral Rehabil. 2019, 46, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Ansai, T.; Soh, I.; Akifusa, S.; Sonoki, K.; Fujisawa, K.; Yoshida, A.; Kagiyama, S.; Hamasaki, T.; Nakamichi, I.; et al. Relationship between chewing ability and high-level functional capacity in an 80-year-old population in Japan. Gerodontology 2008, 25, 147–154. [Google Scholar] [CrossRef]
- Ohkubo, C.; Morokuma, M.; Yoneyama, Y.; Matsuda, R.; Lee, J.S. Interactions between occlusion and human brain function activities. J. Oral Rehabil. 2013, 40, 119–129. [Google Scholar] [CrossRef]
- Watanabe, I.; Ishiyama, N.; Senda, M. Cerebral blood flow during mastication measured with positron emission tomography. Ronen Shika Igaku 1992, 6, 148–150. [Google Scholar]
- Utsugi, C.; Miyazono, S.; Osada, K.; Sasajima, H.; Noguchi, T.; Matsuda, M.; Kashiwayanagi, M. Hard-diet feeding recovers neurogenesis in the subventricular zone and olfactory functions of mice impaired by soft-diet feeding. PLoS ONE 2014, 9, e97309. [Google Scholar] [CrossRef]
- Nose-Ishibashi, K.; Watahiki, J.; Yamada, K.; Maekawa, M.; Watanabe, A.; Yamamoto, G.; Enomoto, A.; Matsuba, Y.; Nampo, T.; Taguchi, T.; et al. Soft-diet feeding after weaning affects behavior in mice: Potential increase in vulnerability to mental disorders. Neuroscience 2014, 263, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Tei, K.; Murata, A.; Yamazaki, Y.; Hata, H.; Muramatsu, M.; Kitagawa, Y.; Inoue, N.; Miura, H. Associations between self-assessed masticatory ability and higher brain function among the elderly. J. Oral Rehabil. 2011, 38, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Makiura, T.; Ikeda, Y.; Hirai, T.; Terasawa, H.; Hamaue, N.; Minami, M. Influence of diet and occlusal support on learning memory in rats behavioral and biochemical studies. Res. Commun. Mol. Pathol. Pharmacol. 2000, 107, 269–277. [Google Scholar]
- Avivi-Arber, L.; Lee, J.C.; Sessle, B.J. Cortical Orofacial Motor Representation: Effect of Diet Consistency. J. Dent. Res. 2010, 89, 1142–1147. [Google Scholar] [CrossRef]
- Endo, Y.; Mizuno, T.; Fujita, K.; Funabashi, T.; Kimura, F. Soft-diet feeding during development enhances later learning abilities in female rats. Physiol. Behav. 1994, 56, 629–633. [Google Scholar] [CrossRef]
- Tsutsui, K.; Kaku, M.; Motokawa, M.; Tohma, Y.; Kawata, T.; Fujita, T.; Kohno, S.; Ohtani, J.; Tenjoh, K.; Nakano, M.; et al. Influences of reduced masticatory sensory input from soft-diet feeding upon spatial memory/learning ability in mice. Biomed. Res. 2007, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kushida, S.; Kimoto, K.; Hori, N.; Toyoda, M.; Karasawa, N.; Yamamoto, T.; Kojo, A.; Onozuka, M. Soft-diet feeding decreases dopamine release and impairs aversion learning in Alzheimer model rats. Neurosci. Lett. 2008, 439, 208–211. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hirayama, A. Effects of soft-diet feeding on synaptic density in the hippocampus and parietal cortex of senescence-accelerated mice. Brain Res. 2001, 902, 255–263. [Google Scholar] [CrossRef]
- Frota de Almeida, M.N.; de Siqueira Mendes Fde, C.; Gurgel Felício, A.P.; Falsoni, M.; Ferreira de Andrade, M.L.; Bento-Torres, J.; da Costa Vasconcelos, P.F.; Perry, V.H.; Picanço-Diniz, C.W.; Kronka Sosthenes, M.C. Spatial memory decline after masticatory deprivation and aging is associated with altered laminar distribution of CA1 astrocytes. BMC Neurosci. 2012, 13, 23. [Google Scholar] [CrossRef]
- Akazawa, Y.; Kitamura, T.; Fujihara, Y.; Yoshimura, Y.; Mitome, M.; Hasegawa, T. Forced mastication increases survival of adult neural stem cells in the hippocampal dentate gyrus. Int. J. Mol. Med. 2013, 31, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.d.C.C.d.S.; de Almeida, M.N.F.; Felicio, A.P.G.; Fadel, A.C.; Silva, D.d.J.; Borralho, T.G.; da Silva, R.P.; Bento-Torres, J.; Vasconcelos, P.F.; Perry, V.H.; et al. Enriched environment and masticatory activity rehabilitation recover spatial memory decline in aged mice. BMC Neurosci. 2013, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Okihara, H.; Ito, J.-I.; Kokai, S.; Ishida, T.; Hiranuma, M.; Kato, C.; Yabushita, T.; Ishida, K.; Ono, T.; Michikawa, M. Liquid diet induces memory impairment accompanied by a decreased number of hippocampal neurons in mice. J. Neurosci. Res. 2014, 92, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Fukushima-Nakayama, Y.; Ono, T.; Hayashi, M.; Inoue, M.; Wake, H.; Ono, T.; Nakashima, T. Reduced Mastication Impairs Memory Function. J. Dent. Res. 2017, 96, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Mitome, M.; Hasegawa, T.; Shirakawa, T. Mastication influences the survival of newly generated cells in mouse dentate gyrus. Neuroreport 2005, 16, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, F.; Tsuchiya, M.; Arai, Y.; Tadano, T.; Tan-No, K. Involvement of catecholaminergic and GABAAergic mediations in the anxiety-related behavior in long-term powdered diet-fed mice. Neurochem. Int. 2019, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anegawa, E.; Kotorii, N.; Ishimaru, Y.; Okuro, M.; Sakai, N.; Nishino, S. Chronic Powder Diet after Weaning Induces Sleep, Behavioral, Neuroanatomical, and Neurophysiological Changes in Mice. PLoS ONE 2015, 10, e0143909. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, M.; Fujita, M.; Watanabe, K.; Hirano, Y.; Niwa, M.; Nishiyama, K.; Saito, S. Mapping brain region activity during chewing: A functional magnetic resonance imaging study. J. Dent. Res. 2002, 81, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Miyamoto, T.; Terao, A.; Yokoyama, A. Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience 2007, 145, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Anastasi, G.; Piancino, M.G.; Frongia, G.; Milardi, D.; Favaloro, A.; Bramanti, P. Hemispheric prevalence during chewing in normal right-handed and left-handed subjects: A functional magnetic resonance imaging preliminary study. Cranio 2010, 28, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Tei, K.; Yamazaki, Y.; Hata, H.; Muramatsu, M.; Kitagawa, Y.; Inoue, N.; Miura, H. Relationships between self-assessed masticatory ability and higher level functional capacity among community-dwelling young-old persons. Int. J. Gerontol. 2012, 6, 33–37. [Google Scholar] [CrossRef]
- Takase, K.; Funabashi, T.; Mogi, K.; Mitsushima, D.; Kimura, F. Feeding with powdered diet after weaning increases visuospatial ability in association with increases in the expression of N-methyl-D-aspartate receptors in the hippocampus of female rats. Neurosci. Res. 2005, 53, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Oue, H.; Okada, S.; Kawano, A.; Koretake, K.; Michikawa, M.; Akagawa, Y.; Tsuga, K. Molar loss and powder diet leads to memory deficit and modifies the mRNA expression of brain-derived neurotrophic factor in the hippocampus of adult mice. BMC Neurosci. 2016, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira Mendes, F.C.C.; da Paixao, L.; Diniz, C.W.P.; Sosthenes, M.C.K. Environmental Impoverishment, Aging, and Reduction in Mastication Affect Mouse Innate Repertoire to Explore Novel Environments and to Assess Risk. Front. Neurosci. 2019, 13, 107. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly Used Animal Models; Principles of Animal Research: Saint Paul, MN, USA, 2017; pp. 117–175. [Google Scholar]
- Fernández, A.; Quintana, E.; Velasco, P.; Moreno-Jimenez, B.; de Andrés, B.; Gaspar, M.L.; Liste, I.; Vilar, M.; Mira, H.; Cano, E. Senescent accelerated prone 8 (SAMP8) mice as a model of age dependent neuroinflammation. J. Neuroinflamm. 2021, 18, 75. [Google Scholar] [CrossRef]
- Benedikz, E.; Kloskowska, E.; Winblad, B. The rat as an animal model of Alzheimer’s disease. J. Cell Mol. Med. 2009, 13, 1034–1042. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Cooper, C.; Li, R.; Lyketsos, C.; Livingston, G. Treatment for mild cognitive impairment: Systematic review. Br. J. Psychiatry 2013, 203, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, P.J.; Scheltens, P. Can nutrients prevent or delay onset of Alzheimer’s disease? J. Alzheimers Dis. 2010, 20, 765–775. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ozono, S.; Nishiyama, K.; Saito, S.; Tonosaki, K.; Fujita, M.; Onozuka, M. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav. Brain Res. 2002, 128, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; de Melo Pereira Fernandes, L.; Noronha, P.A.T.; dos Santos, M.A.R.; Gomes-Leal, W.; do Socorro Ferraz Maia, C.; Lima, R.R. Masticatory Deficiency as a Risk Factor for Cognitive Dysfunction. Int. J. Med. Sci. 2014, 11, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kothari, M.; Grigoriadis, A.; Trulsson, M.; Svensson, P. Bite or Brain: Implication of sensorimotor regulation and neuroplasticity in oral rehabilitation procedures. J. Oral Rehabil. 2018, 45, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef]
- Nakata, M. Masticatory function and its effects on general health. Int. Dent. J. 1998, 48, 540–548. [Google Scholar] [CrossRef]
- Akiyama, Y.; Shikimori, M.; Satov, A.; Motegi, K. The effect of a change in dietary habit upon maze learning ability in rats. J. Oral Rehabil. 1991, 18, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Narita, N.; Kamiya, K.; Yamamura, K.; Kawasaki, S.; Matsumoto, T.; Tanaka, N. Chewing-related prefrontal cortex activation while wearing partial denture prosthesis: Pilot study. J. Prosthodont. Res. 2009, 53, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Levine, D.A.; Gross, A.L.; Briceño, E.M.; Tilton, N.; Giordani, B.J.; Sussman, J.B.; Hayward, R.A.; Burke, J.F.; Hingtgen, S.; Elkind, M.S.V.; et al. Sex Differences in Cognitive Decline among US Adults. JAMA Netw. Open 2021, 4, e210169. [Google Scholar] [CrossRef]
- Lovheim, H.; Sandman, P.O.; Karlsson, S.; Gustafson, Y. Sex differences in the prevalence of behavioral and psychological symptoms of dementia. Int. Psychogeriatr. 2009, 21, 469–475. [Google Scholar] [CrossRef]
- Cooper, R.M.; Zubek, J.P. Effects of enriched and restricted early environments on the learning ability of bright and dull rats. Can. J. Psychol. 1958, 12, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.J.; Mercado, E., 3rd; Orduna, I. Enriched Environments as a Potential Treatment for Developmental Disorders: A Critical Assessment. Front. Psychol. 2019, 10, 466. [Google Scholar] [CrossRef]
- Veena, J.; Srikumar, B.N.; Mahati, K.; Bhagya, V.; Raju, T.R.; Shankaranarayana Rao, B.S. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J. Neurosci. Res. 2009, 87, 831–843. [Google Scholar] [CrossRef]
- Arai, J.A.; Feig, L.A. Long-lasting and transgenerational effects of an environmental enrichment on memory formation. Brain Res. Bull. 2011, 85, 30–35. [Google Scholar] [CrossRef]
- Homberg, J.R.; Kyzar, E.J.; Scattoni, M.L.; Norton, W.H.; Pittman, J.; Gaikwad, S.; Nguyen, M.; Poudel, M.K.; Ullmann, J.F.; Diamond, D.M.; et al. Genetic and environmental modulation of neurodevelopmental disorders: Translational insights from labs to beds. Brain Res. Bull. 2016, 125, 79–91. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, L.; Croucher, K. Housing and dementia care—A scoping review of the literature. Health Soc. Care Community 2005, 13, 570–577. [Google Scholar] [CrossRef]
- van Hoof, J.; Kort, H.S.M.; van Waarde, H. Housing and care for older adults with dementia: A European perspective. J. Hous. Built Environ. 2009, 24, 369–390. [Google Scholar] [CrossRef]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A., 3rd; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhu, Z.; Plassman, B.L.; Wu, B. Dose-Response Meta-Analysis on Tooth Loss with the Risk of Cognitive Impairment and Dementia. J. Am. Med. Dir. Assoc. 2021, 22, 2039–2045. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population (P) | Healthy dentate human and animal subjects | Children below 18 years |
| Intervention (I) | Powder food Soft chewing gums, soft pureed food, liquid/semisolid diet, cooked rice, green leafy vegetables | Food with no indication of softness |
| Comparison (C) | Pellet food Hard chewing gums, all kinds of food, only slightly hard food, dried fish, pork, and fish | Food with no indication of hardness |
| Outcomes (O) | Behavior, cognitive function, and brain activation (animal behavioral tests and human neuroimaging evaluation and cognition evaluation assessments) | No direct and clear findings of behavior, cognitive function, and brain activation (animal histological and neurogenesis studies) |
| Studies design (S) | Peer-reviewed, original studies published in the English language | All reviews (narrative or systematic), meta-analyses, study protocols, conference abstracts, letters to editors, commentaries, preprints, case reports, not peer-reviewed studies, and articles published in a language other than English |
| A. Animal Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Authors (Year), Country | Study Design | Subjects | Age and Sex | Intervention and Comparison Groups | Consumption Time | Outcome Measures and Applied Methods | Main Findings |
| 1 | Endo et al. (1994) [36], Japan. | Animal: Experimental study. | Wistar-Imamichi rats (N = 62). | Age: 3 wk. Sex: 29 M and 33 F. | Intervention: SF (N = NA). Comparison: HF (N = NA). | 16 wk. | Behavioral task: radial eight-arm maze test. | The number of correct choices in the last five–seven trials was greater in rats fed SF than in rats fed HF. Additionally, the number of correct choices was significantly greater in F compared to M. |
| 2 | Yamamoto and Hirayama (2001) [39], Japan. | Animal: Experimental study. | S-A mice (N = 29). | Age: 3 wk. Sex: 20 M. | Intervention: SF: (1) S-AMR1 (N = 5) and (2) S-AMP8 (N = 5). Comparison: HF: (1) S-AMR1 (N = 5) and (2): S-AMP8 (N = 5). | 21 wk. | Behavioral task: radial eight-arm maze test. | Significant impairment in working memory performance resulting from SF feeding was recognized in both S-AMR1 and S-AMP8 mice. |
| 3 | Takase et al. (2005) [52], Japan. | Animal: Experimental study. | Wistar rats (N = 56). | Age:3 wk. Sex: 28 M and 28 F. | Intervention: SF (N = 28). Comparison: HF (N = 28). | 7–11 wk. | Behavioral task: radial eight-arm maze test, open-field test. | No significant differences were observed in the behavioral task (the spatial ability) between rats fed SF or HF. In rats fed HF, M performed better than F in the radial 8-arm maze task. |
| 4 | Mitome et al. (2005) [45], Japan | Animal: Experimental study. | C57BL/6 mice (N = 54). | Age: 4 wk. Sex: 54 F. | Intervention: (1) SF (N = 18); (2) SETF (N = 18). Comparison: (3) HF (N = 18). | 15 wk. | Behavioral task: locomotor activity test. | No significant differences were observed in the behavioral task (locomotor activity test) between mice fed SF of HF. |
| 5 | Tsutsui et al. (2007) [37], Japan. | Animal: Experimental study. | B6C3Fe-a/a mice (N = 109). | Age: 3 wk. Sex: 109 M. | Intervention: SF: (1) for 180 days (N = 26) and (2) for 360 days (N = 29). Comparison: HF: (1) for 180 days (N = 24) and for 360 days (N = 30). | 23 and 48 wk. | Behavioral task: Morris water maze test. | No significant difference in the escape latency was found between the 180-day-old HF group and 180-day-old SF group. However, a tendency to prolong the escape latency was observed in the 360-day-old SF group compared with the 360-day-old HF group. |
| 6 | Kushida et al. (2008) [38], Japan. | Animal: Experimental study. | Wistar Aβ-infused rats (N = 38). | Age: 3 wk. Sex: 38 M. | Intervention: SF (N = 28). Comparison: HF (N = 28). | 7 wk. | Behavioral task: passive avoidance test. | STL time of rats fed SF was significantly shorter than rats fed HF indicating that SF feeding impairs learning ability. |
| 7 | Avivi-Arbwe et al. (2010) [35], Canada. | Animal: Experimental study. | Sprague Dawley rats (N = 12). | Age: NA. Sex: 12 M. | Intervention: SF (N = 6). Comparison: HF (N = 6). | 2–23 wk. | Behavioral task: ICMS-induced EMG recordings. | No significant differences between the HF and SF groups in orofacial motor representations of the jaw and tongue within the face-M1 and adjacent face-S1. |
| 8 | Frota de Almeida et al. (2012) [40], Brazil. | Animal: Experimental study. | Albino Swiss mice (N = 66). | Age: 3 wk. Sex: 66 F. | Intervention: SF (N = 30). Comparison: HF (N = 36). | 9, 21, and 69 wk. | Behavioral task: Morris water maze test. | Escape latencies of 6-month-old mice fed HF were significantly shorter than age-matched mice fed SF. However, no significant changes in escape latencies were observed between SF and HF groups at the age of 3 months or 18 months. |
| 9 | Mendes et al. (2013) [42], Brazil. | Animal: Experimental study. | Albino Swiss mice (N = 222). | Age: 3 wk. Sex: 222 F. | Intervention: HF/SF (N = 62). Comparison: (1) HF (N = 92) and (2) HF/SF/HF (N = 68). Under two conditions: IE or EE and two ages: 6- and 18- Mon- old. | 24 and 74 wk. | Behavioral task: Morris water maze test. | For learning rate, and independent of age and condition, (HF/SF) was associated with lower learning rate and performance values compared with control (HF) or masticatory rehabilitated (HF/SF/HF) mice. Similar findings in swim speed and distance traveled, 6-month-HF/SF traveled longer distances than 6-month-HF and 6-month-HF/SF/HF, but shorter than 18-month-HF/SF and 18-month-HF/SF/HF. |
| 10 | Akazawa et al. (2013) [41], Japan. | Animal: Experimental study. | C57BL/6 mice (N = NA). | Age: 6 wk. Sex: NA. | Intervention: SF (N = NA). Comparison: HF (N = NA). | 14 wk. | Behavioral task: Morris water maze test. | Mice fed HF required significantly less time to reach the platform than mice fed SF. |
| 11 | Nose-Ishibashi et al. (2014) [31], Japan. | Animal: Experimental study. | C57BL6/J mice (N = 21–30). | Age: 3 wk. Sex: 21–30 M. | Intervention: SF (N = 7–10). Comparison: (1) SF/HF (N = 7–10) and (2) HF (N = 7–10). | 4–10 wk. | Behavioral task: home cage activity test (4 wk.), elevated plus-maze test and open-field test (5 wk.), Y-maze test (7 wk.), Morris water maze test (8 wk.), fear conditioning test (10 wk.). | Elevated plus maze test, Y-maze test, Morris water maze test, and classical fear conditioning test did not show any differences in the SF and SF/HF as compared to the HF. In the open field test, the total distance of locomotion in 15 min was significantly greater in SF than in HF. In the home cage activity test, the SF showed significantly lower activity levels per day than the HF. No differences in the behavioral tests were noted between HF and SF/HF. |
| 12 | Okihara et al. (2014) [43], Japan. | Animal: Experimental study. | C57BL/6J mice (N = 14). | Age: 3 wk. Sex: 14 M. | Intervention: SF (N = 7). Comparison: HF (N = 7). | 11 wk. | Behavioral task: passive avoidance test. | In the HF group, the latency 24 h after one trial training significantly increased compared with that of training, but not in the SF group indicating an impairment in memory. |
| 13 | Utsugi et al.(2014) [30], Japan. | Animal: Experimental study. | C57BL/6 mice(N = 131). | Age: 24–28 wk. Sex:131 F | Intervention: SF (N = 32). Comparison: (1) HF (N = 31); SF/HF (N = 43). | 4 and 12 wk. | Behavioral task: Y-maze odor preference apparatus. | In the HF group and SF/HF group, the preference ratio significantly increased compared with the SF group after 4 wk. |
| 14 | Anegawa et al. (2015) [47], USA. | Animal: Experimental study. | C57BL/6J mice (N = 20). | Age: 3 wk. Sex: 20 M. | Intervention: SF (N = 10). Comparison: HF (N = 10). | 15 wk. and 19 wk. | Behavioral task: marble burying test (15 wk.), food-deprivation test (19 wk.). | No significant difference in the marble burying test between SF and HF. SF induced attenuated diurnal sleep/wake rhythm. SF showed less enhancement of wake/locomotor activity compared to HF. |
| 15 | Takeda et al. (2016) [53], Japan. | Animal: Experimental study. | C57BL/6K mice (N = 48). | Age: 28 wk. Sex: 28 M | Intervention: SF (N = 12). Comparison: HF (N = 12). With two conditions: IT and ET. | 4 wk. and 16 wk. | Behavioral task: passive avoidance test. | No significant difference in latency times between the groups in the acquisition trial after 4 wk. The latency time of the ET/SF group was shorter than the IT/HF group after 16 wk. |
| 16 | Fukushima-Nakayama et al. (2017) [44], Japan. | Animal: Experimental study. | C57BL/6J mice (N = 63). | Age: 3 wk. Sex: 63 M. | Intervention: SF (N = 32). Comparison: HF (N = 31). | 11 wk. | Behavioral task: passive avoidance, object location tests, and open-field test. | The frequency to sniff the moving object was lower in the mice fed with SF than in HF, suggesting impaired spatial memory. |
| 17 | Mendes et al. (2019) [54], Brazil. | Animal: Experimental study. | Albino Swiss mice (N = 180). | Age: 3 wk. Sex: 180 F. | Intervention: HF/SF (N = 60). Comparison: (1) HF (N = 60) and (2) HF/SF/HF (N = 60). Under two conditions: IE or EE and two ages: 6-, 12-, and 18-month-old. | 24 wk., 48 wk. and 75 wk. | Behavioral task: open field test. | Outcomes were significantly influenced by interactions between environment, age, and diet. The locomotor and exploratory activities in open field tasks declined with age and SF. |
| 18 | Yaoita et al. (2019) [46], Japan. | Animal: Experimental study. | BALB/c mice (N = 28–36). | Age: 3 wk. Sex: 28–36 M. | Intervention: SF (N = 10). Comparison: HF (N = 10). | 17 wk. | Behavioral task: elevated plus-maze test. | SF increased the % of the open-arm time and the total number of arm entries, indicating that the mice have low anxiety, hyperactivity, and impulsive behaviors. The % of open-arm time in HF was increased by treatment with an anxiolytic agent but not in SF. |
| B. Human Studies | ||||||||
| No. | Authors (Year), Country | Study Design | Subjects | Age and Sex | Intervention and Comparison Groups | Consumption Time | Outcome Measures and Applied Methods | Main Findings |
| 1 | Onozuka et al. (2002) [48], Japan. | Human: Cross-sectional study. | Young adults (N = 17). | Age: 20–31 y. Sex: 10 M and 7 F. | Intervention: moderately HF (N = 17). Comparison: HF (N = 17). | half min | Radiographic evaluation: fMRI. | Chewing of HF produced a stronger BOLD signal than the chewing of moderately HF in the cerebellum, whereas the converse was true for the primary cortical area and non-primary cortical areas, except for the thalamus, in which no difference was seen between the types of the food. |
| 2 | Takahashi et al. (2007) [49], Japan. | Human: Cross-sectional study. | Young adults (N = 15). | Age: 22–32 y. Sex: 6 M and 7 F. | Intervention: change in the food hardness (N = 15). Comparison: hardest food (N = 15). | half min | Radiographic evaluation: fMRI. | With the changes in the food hardness, selective activation was noted in the SMA, DLPFC, and STG of the left hemisphere, and the PM and inferior parietal lobule. |
| 3 | Bracco et al. (2010) [50], Italy. | Human: Cross-sectional study. | Young adults (N = 10). | Age: 23–32 y. Sex: 7 M and 3 F. | Intervention: SF (N = 10). Comparison: HF (N = 10). | 3 min | Radiographic evaluation: fMRI. | Chewing of HF produced a weaker BOLD signal than the chewing of SF in the primary motor and premotor cortical areas the ascending parietal gyrus of the primary somatic sensory cortex and non-primary cortical areas. |
| 4 | Moriya et al. (2011) [32], Japan. | Human: Cross-sectional study. | Old adults (N = 208). | Age: 70–74 y. Sex: 79 M and 129 F. | Intervention: (1) only SF (N = 20), (2) only slightly HF (N = 56). Comparison: chew all kinds of food (N = 132). | NA | Self-assessed chewing ability: 4 neuropsychological tests: (a) RCPM, (b) VerPA, (c) VisPA, (d) Block Design. | Significant and positive correlations were found between the RCPM test, the VerPA task, the Block Design test, and the ability to chew all kinds of food compared to the other groups. |
| 5 | Moriya et al. (2012) [51], Japan. | Human: Cross-sectional study. | Old adults (N = 366). | Age: 67–74 y. Sex: 138 M and 228 F. | Intervention: (1) only SF (N = 27), (2) only slightly HF (N = 94). Comparison: chew all kinds of food (N = 245). | NA | Self-assessed chewing ability: TMIG-Index: (a) instrumental self-maintenance, (b) intellectual activity, and (c) social role. | No significant differences in the instrumental self-maintenance scale among the three groups, but significant differences were found in the total score, intellectual activity, and social role. |
| 6 | Okubo et al. (2019) [26], Japan. | Human: Cross-sectional study. | Old adults (N = 635). | Age: 69–71 y. Sex: 292 M and 343 F. | Intervention: 38 food items (N = NA). Comparison: hardest food within the list (N = NA). | NA | Self-assessed chewing ability: MoCA-J Assessment. | Food hardness was positively associated with the MoCA-J score. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Manei, K.; Jia, L.; Al-Manei, K.K.; Ndanshau, E.L.; Grigoriadis, A.; Kumar, A. Food Hardness Modulates Behavior, Cognition, and Brain Activation: A Systematic Review of Animal and Human Studies. Nutrients 2023, 15, 1168. https://doi.org/10.3390/nu15051168
Al-Manei K, Jia L, Al-Manei KK, Ndanshau EL, Grigoriadis A, Kumar A. Food Hardness Modulates Behavior, Cognition, and Brain Activation: A Systematic Review of Animal and Human Studies. Nutrients. 2023; 15(5):1168. https://doi.org/10.3390/nu15051168
Chicago/Turabian StyleAl-Manei, Khaled, Leming Jia, Kholod Khalil Al-Manei, Elisande Lindström Ndanshau, Anastasios Grigoriadis, and Abhishek Kumar. 2023. "Food Hardness Modulates Behavior, Cognition, and Brain Activation: A Systematic Review of Animal and Human Studies" Nutrients 15, no. 5: 1168. https://doi.org/10.3390/nu15051168
APA StyleAl-Manei, K., Jia, L., Al-Manei, K. K., Ndanshau, E. L., Grigoriadis, A., & Kumar, A. (2023). Food Hardness Modulates Behavior, Cognition, and Brain Activation: A Systematic Review of Animal and Human Studies. Nutrients, 15(5), 1168. https://doi.org/10.3390/nu15051168






