Stimulation of GHRH Neuron Axon Growth by Leptin and Impact of Nutrition during Suckling in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Arcuate Explant Culture Experiments
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
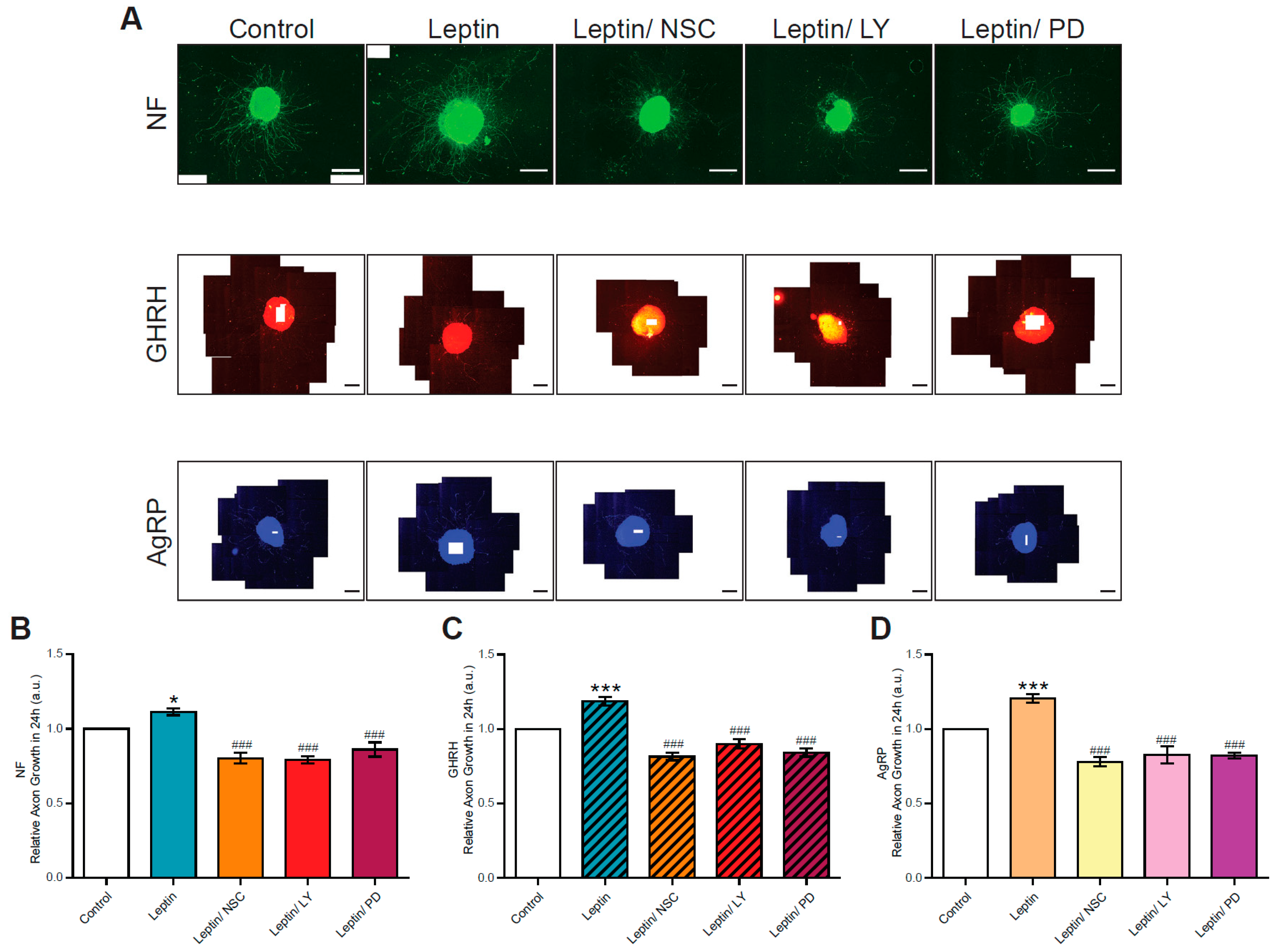
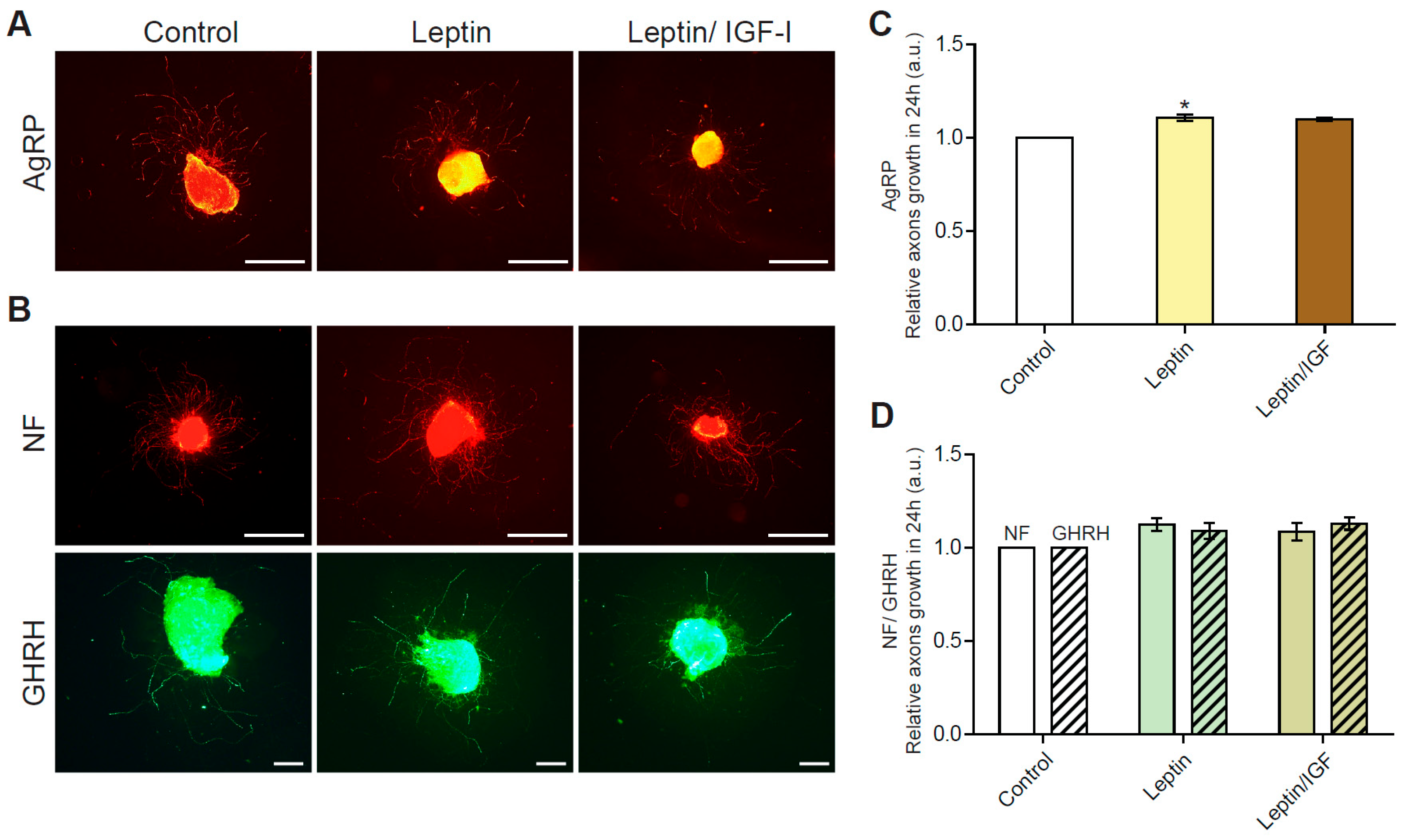
3.1. Leptin Stimulates Axon Growth in GHRH Neurons in Arcuate Nucleus Explants from Normally Fed Pups
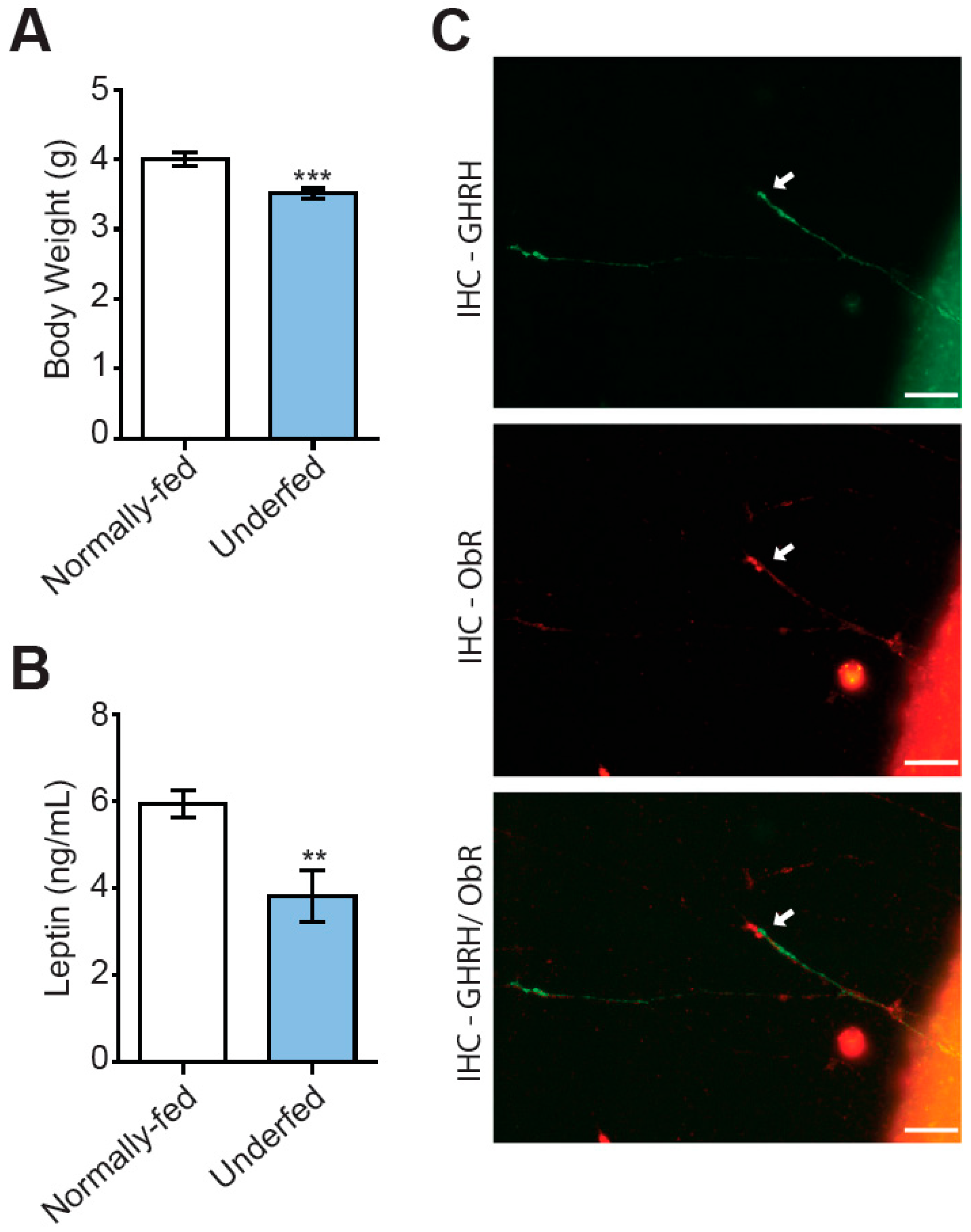
3.2. GHRH Neurons in Arcuate Nucleus Explants from Underfed Pups Do Not Respond to Leptin Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, L.; De Magalhaes Filho, C.; Leneuve, P.; Xu, J.; Brunel, N.; Chatziantoniou, C.; Le Bouc, Y.; Holzenberger, M. Early postnatal nutrition determines somatotropic function in mice. Endocrinology 2009, 150, 314–323. [Google Scholar] [CrossRef]
- Ong, K.K.; Emmett, P.; Northstone, K.; Golding, J.; Rogers, I.; Ness, A.R.; Wells, J.C.; Dunger, D.B. Infancy weight gain predicts childhood body fat and age at menarche in girls. J. Clin. Endocrinol. Metab. 2009, 94, 1527–1532. [Google Scholar] [CrossRef]
- Akitake, Y.; Katsuragi, S.; Hosokawa, M.; Mishima, K.; Ikeda, T.; Miyazato, M.; Hosoda, H. Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutr. Res. 2015, 35, 76–87. [Google Scholar] [CrossRef]
- Castro, C.A.; Tracy, M.; Rudy, J.W. Early-life undernutrition impairs the development of the learning and short-term memory processes mediating performance in a conditional-spatial discrimination task. Behav. Brain Res. 1989, 32, 255–264. [Google Scholar] [CrossRef]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Paz-Filho, G.J.; Babikian, T.; Asarnow, R.; Delibasi, T.; Esposito, K.; Erol, H.K.; Wong, M.L.; Licinio, J. Leptin replacement improves cognitive development. PLoS ONE 2008, 3, e3098. [Google Scholar] [CrossRef]
- Valerio, A.; Ghisi, V.; Dossena, M.; Tonello, C.; Giordano, A.; Frontini, A.; Ferrario, M.; Pizzi, M.; Spano, P.; Carruba, M.O.; et al. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J. Biol. Chem. 2006, 281, 12950–12958. [Google Scholar] [CrossRef]
- Caron, E.; Ciofi, P.; Prevot, V.; Bouret, S.G. Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function. J. Neurosci. 2012, 32, 11486–11494. [Google Scholar] [CrossRef]
- Castellano, J.M.; Bentsen, A.H.; Sanchez-Garrido, M.A.; Ruiz-Pino, F.; Romero, M.; Garcia-Galiano, D.; Aguilar, E.; Pinilla, L.; Dieguez, C.; Mikkelsen, J.D.; et al. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology 2011, 152, 3396–3408. [Google Scholar] [CrossRef]
- Hiney, J.K.; Srivastava, V.K.; Pine, M.D.; Les Dees, W. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology 2009, 150, 376–384. [Google Scholar] [CrossRef]
- Roa, J.; Tena-Sempere, M. Energy balance and puberty onset: Emerging role of central mTOR signaling. Trends Endocrinol. Metab. 2010, 21, 519–528. [Google Scholar] [CrossRef]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef]
- Coupe, B.; Grit, I.; Darmaun, D.; Parnet, P. The timing of “catch-up growth” affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R813–R824. [Google Scholar] [CrossRef]
- Steculorum, S.M.; Collden, G.; Coupe, B.; Croizier, S.; Lockie, S.; Andrews, Z.B.; Jarosch, F.; Klussmann, S.; Bouret, S.G. Neonatal ghrelin programs development of hypothalamic feeding circuits. J. Clin. Investig. 2015, 125, 846–858. [Google Scholar] [CrossRef]
- Juan De Solis, A.; Baquero, A.F.; Bennett, C.M.; Grove, K.L.; Zeltser, L.M. Postnatal undernutrition delays a key step in the maturation of hypothalamic feeding circuits. Mol. Metab. 2016, 5, 198–209. [Google Scholar] [CrossRef]
- Decourtye, L.; Mire, E.; Clemessy, M.; Heurtier, V.; Ledent, T.; Robinson, I.C.; Mollard, P.; Epelbaum, J.; Meaney, M.J.; Garel, S.; et al. IGF-1 Induces GHRH Neuronal Axon Elongation during Early Postnatal Life in Mice. PLoS ONE 2017, 12, e0170083. [Google Scholar]
- Kappeler, L.; Clemessy, M.; Saget, S.; Decourtye, L.; Le Bouc, Y. Regulation of growth: Epigenetic mechanisms? Ann. D’endocrinologie 2017, 78, 92–95. [Google Scholar] [CrossRef]
- Solloso, A.; Barreiro, L.; Seoane, R.; Nogueira, E.; Canibano, C.; Alvarez, C.V.; Zalvide, J.; Dieguez, C.; Pombo, C.M. GHRH proliferative action on somatotrophs is cell-type specific and dependent on Pit-1/GHF-1 expression. J. Cell. Physiol. 2008, 215, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, L.; De Magalhaes Filho, C.; Dupont, J.; Leneuve, P.; Cervera, P.; Perin, L.; Loudes, C.; Blaise, A.; Klein, R.; Epelbaum, J.; et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008, 6, e254. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Bates, S.H.; Chen, S.; Myers, M.G., Jr.; Simerly, R.B. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J. Neurosci. 2012, 32, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Thornton, J.E.; Nurani, S.D.; Clifton, D.K.; Steiner, R.A. A reassessment of leptin’s role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology 2001, 74, 12–21. [Google Scholar] [CrossRef]
- Allison, M.B.; Myers, M.G., Jr. 20 years of leptin: Connecting leptin signaling to biological function. J. Endocrinol. 2014, 223, T25–T35. [Google Scholar] [CrossRef]
- Clement, K.; Vaisse, C.; Lahlou, N.; Cabrol, S.; Pelloux, V.; Cassuto, D.; Gourmelen, M.; Dina, C.; Chambaz, J.; Lacorte, J.M.; et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998, 392, 398–401. [Google Scholar] [CrossRef]
- Rupp, A.C.; Allison, M.B.; Jones, J.C.; Patterson, C.M.; Faber, C.L.; Bozadjieva, N.; Heisler, L.K.; Seeley, R.J.; Olson, D.P.; Myers, M.G., Jr. Specific subpopulations of hypothalamic leptin receptor-expressing neurons mediate the effects of early developmental leptin receptor deletion on energy balance. Mol. Metab. 2018, 14, 130–138. [Google Scholar] [CrossRef]
- Furigo, I.C.; Teixeira, P.D.S.; de Souza, G.O.; Couto, G.C.L.; Romero, G.G.; Perello, M.; Frazao, R.; Elias, L.L.; Metzger, M.; List, E.O.; et al. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat. Commun. 2019, 10, 662. [Google Scholar] [CrossRef]
- Decourtye, L.; Clemessy, M.; Mire, E.; Ledent, T.; Perin, L.; Robinson, I.C.; Le Bouc, Y.; Kappeler, L. Impact of insulin on primary arcuate neurons culture is dependent on early-postnatal nutritional status and neuronal subpopulation. PLoS ONE 2018, 13, e0193196. [Google Scholar] [CrossRef]
- Balthasar, N.; Mery, P.F.; Magoulas, C.B.; Mathers, K.E.; Martin, A.; Mollard, P.; Robinson, I.C. Growth hormone-releasing hormone (GHRH) neurons in GHRH-enhanced green fluorescent protein transgenic mice: A ventral hypothalamic network. Endocrinology 2003, 144, 2728–2740. [Google Scholar] [CrossRef]
- Fiorotto, M.L.; Burrin, D.G.; Perez, M.; Reeds, P.J. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am. J. Physiol. 1991, 260, R1104–R1113. [Google Scholar] [CrossRef]
- Lopez-Bendito, G.; Cautinat, A.; Sanchez, J.A.; Bielle, F.; Flames, N.; Garratt, A.N.; Talmage, D.A.; Role, L.W.; Charnay, P.; Marin, O.; et al. Tangential neuronal migration controls axon guidance: A role for neuregulin-1 in thalamocortical axon navigation. Cell 2006, 125, 127–142. [Google Scholar] [CrossRef]
- Mire, E.; Thomasset, N.; Jakeman, L.B.; Rougon, G. Modulating Sema3A signal with a L1 mimetic peptide is not sufficient to promote motor recovery and axon regeneration after spinal cord injury. Mol. Cell. Neurosci. 2008, 37, 222–235. [Google Scholar] [CrossRef]
- Bouyer, K.; Simerly, R.B. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J. Neurosci. 2013, 33, 840–851. [Google Scholar] [CrossRef]
- Robertson, S.A.; Leinninger, G.M.; Myers, M.G., Jr. Molecular and neural mediators of leptin action. Physiol. Behav. 2008, 94, 637–642. [Google Scholar] [CrossRef]
- Donato, J., Jr.; Cravo, R.M.; Frazao, R.; Gautron, L.; Scott, M.M.; Lachey, J.; Castro, I.A.; Margatho, L.O.; Lee, S.; Lee, C.; et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Investig. 2011, 121, 355–368. [Google Scholar] [CrossRef]
- Egan, O.K.; Inglis, M.A.; Anderson, G.M. Leptin Signaling in AgRP Neurons Modulates Puberty Onset and Adult Fertility in Mice. J. Neurosci. 2017, 37, 3875–3886. [Google Scholar] [CrossRef]
- Saini, H.K.; Enright, A.J.; Griffiths-Jones, S. Annotation of mammalian primary microRNAs. BMC Genom. 2008, 9, 564. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.; Surolia, A.; Banerjee, K. Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS ONE 2011, 6, e26674. [Google Scholar] [CrossRef]
- Baquero, A.F.; de Solis, A.J.; Lindsley, S.R.; Kirigiti, M.A.; Smith, M.S.; Cowley, M.A.; Zeltser, L.M.; Grove, K.L. Developmental switch of leptin signaling in arcuate nucleus neurons. J. Neurosci. 2014, 34, 9982–9994. [Google Scholar] [CrossRef]
- Zeltser, L.M. Developmental influences on circuits programming susceptibility to obesity. Front. Neuroendocrinol. 2015, 39, 17–27. [Google Scholar] [CrossRef] [PubMed]


| Target | Name of Antibody | Manufacturer, Catalog Number | Species Raised in, Antibody Type | Dilution Used |
|---|---|---|---|---|
| AgRP | Agouti-Related Protein (AGRP) (82–131) Amide (Mouse) Antibody | Phoenix Pharmaceuticals, H-003-57 | Rabbit, polyclonal | 1/500 |
| AgRP # | Agouti-Related Protein (AGRP) (25–131) (Mouse) Antibody | R&D systems, AF634 | Goat, polyclonal | 1/250 |
| GFP | Anti-GFP antibody | Abcam, ab6556 | Rabbit, polyclonal | 1/1000 |
| GHRH # | Anti-GHRH antibody | Proteogenix, VC-15 | Rabbit, polyclonal | 1/500 |
| Leptin R | Goat anti-mouse leptin receptor | R&D system, AF497 | Goat polyclonal | 1/250 |
| Neurofilament | Anti-160 kD Neurofilament Medium antibody | Abcam, ab72998 | Chicken, polyclonal | 1/500 |
| Chicken IgY | Goat Anti-Chicken IgY H&L (DyLight 594) | Abcam, ab96949 | Goat, polyclonal | 1/400 |
| Chicken IgY # | Donkey Anti-Chicken IgY H&L (FITC) | Abcam, ab63507 | Donkey, polyclonal | 1/400 |
| Goat IgG | Donkey anti-goat (DyLight 550) | Abcam, ab96932 | Donkey, polyclonal | 1/400 |
| Goat IgG # | Donkey anti-goat (DyLight 405) | Abcam, ab175665 | Donkey, polyclonal | 1/400 |
| Rabbit IgG | Donkey Anti-Rabbit IgG H&L (DyLight 550) preadsorbed | Abcam, ab96920 | Donkey, polyclonal | 1/400 |
| Rabbit IgG | Goat Anti-Rabbit IgG H&L (DyLight 488) | Abcam, ab96883 | Goat, polyclonal | 1/400 |
| Target | Name of Antibody | Manufacturer, Catalog Number | Species Raised in, Antibody Type | Dilution Used |
|---|---|---|---|---|
| Actin | anti-βactin (D6A8) HRP Conjugate | Cell Signaling, #12620 | Rabbit, polyclonal | 1/2000 |
| Akt | Akt HRP Conjugate mAb | Cell Signaling, #8596 | Rabbit, monoclonal | 1/2000 |
| Phospho Akt | Phospho-Akt (Ser473) (D9E) XP Rabbit mAb | Cell Signaling Technology, #4060 | Rabbit, monoclonal | 1/2000 |
| Erk1/2 | p44/42 MAPK (Erk1/2) (137F5) Rabbit mAb | Cell Signaling Technology, #4695 | Rabbit, monoclonal | 1/1000 |
| Phospho Erk1/2 | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP Rabbit mAb | Cell Signaling Technology, #4370 | Rabbit, monoclonal | 1/2000 |
| Jak2 | Jak2(D2E12) XP | Cell Signaling 3230 | Rabbit, monoclonal | 1/1000 |
| Phospho Jak2 | Phospho Jak2 (Tyr 1007/1008) (C80C3) Rabbit mAb | Cell Signaling, #3776 | Rabbit, monoclonal | 1/1000 |
| Mek1 | Anti- Dual specificity mitogen-activated protein kinase kinase 1, MAP2K1 | Boster Biological Technology, PA1376 | Rabbit, polyclonal | 1/3000 |
| Phospho Mek1 | Anti-Mek1 (phospho S298) antibody [EPR3338] | Abcam, ab96379 | Rabbit, monoclonal | 1/3000 |
| Stat3 | Stat3 (79D7) | Cell Signaling, #4904 | Rabbit, monoclonal | 1/2000 |
| phospho Stat3 | Phospho Stat3 (Tyr705) | Cell Signaling, #9131 | Rabbit, polyclonal | 1/1000 |
| Rabbit IgG | Anti-Rabbit IgG (whole molecule)-HRP linked | Sigma-Aldrich, A0545 | Goat, polyclonal | 1/20,000 |
| Rabbit IgG | Anti-Rabbit IgG (whole molecule)-HRP linked | Cell Signaling 7074 | Goat, polyclonal | 1/20,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decourtye-Espiard, L.; Clemessy, M.; Leneuve, P.; Mire, E.; Ledent, T.; Le Bouc, Y.; Kappeler, L. Stimulation of GHRH Neuron Axon Growth by Leptin and Impact of Nutrition during Suckling in Mice. Nutrients 2023, 15, 1077. https://doi.org/10.3390/nu15051077
Decourtye-Espiard L, Clemessy M, Leneuve P, Mire E, Ledent T, Le Bouc Y, Kappeler L. Stimulation of GHRH Neuron Axon Growth by Leptin and Impact of Nutrition during Suckling in Mice. Nutrients. 2023; 15(5):1077. https://doi.org/10.3390/nu15051077
Chicago/Turabian StyleDecourtye-Espiard, Lyvianne, Maud Clemessy, Patricia Leneuve, Erik Mire, Tatiana Ledent, Yves Le Bouc, and Laurent Kappeler. 2023. "Stimulation of GHRH Neuron Axon Growth by Leptin and Impact of Nutrition during Suckling in Mice" Nutrients 15, no. 5: 1077. https://doi.org/10.3390/nu15051077
APA StyleDecourtye-Espiard, L., Clemessy, M., Leneuve, P., Mire, E., Ledent, T., Le Bouc, Y., & Kappeler, L. (2023). Stimulation of GHRH Neuron Axon Growth by Leptin and Impact of Nutrition during Suckling in Mice. Nutrients, 15(5), 1077. https://doi.org/10.3390/nu15051077






