Effectiveness of Dietary Management for Moderate Wasting among Children > 6 Months of Age—A Systematic Review and Meta-Analysis Exploring Different Types, Quantities, and Durations
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
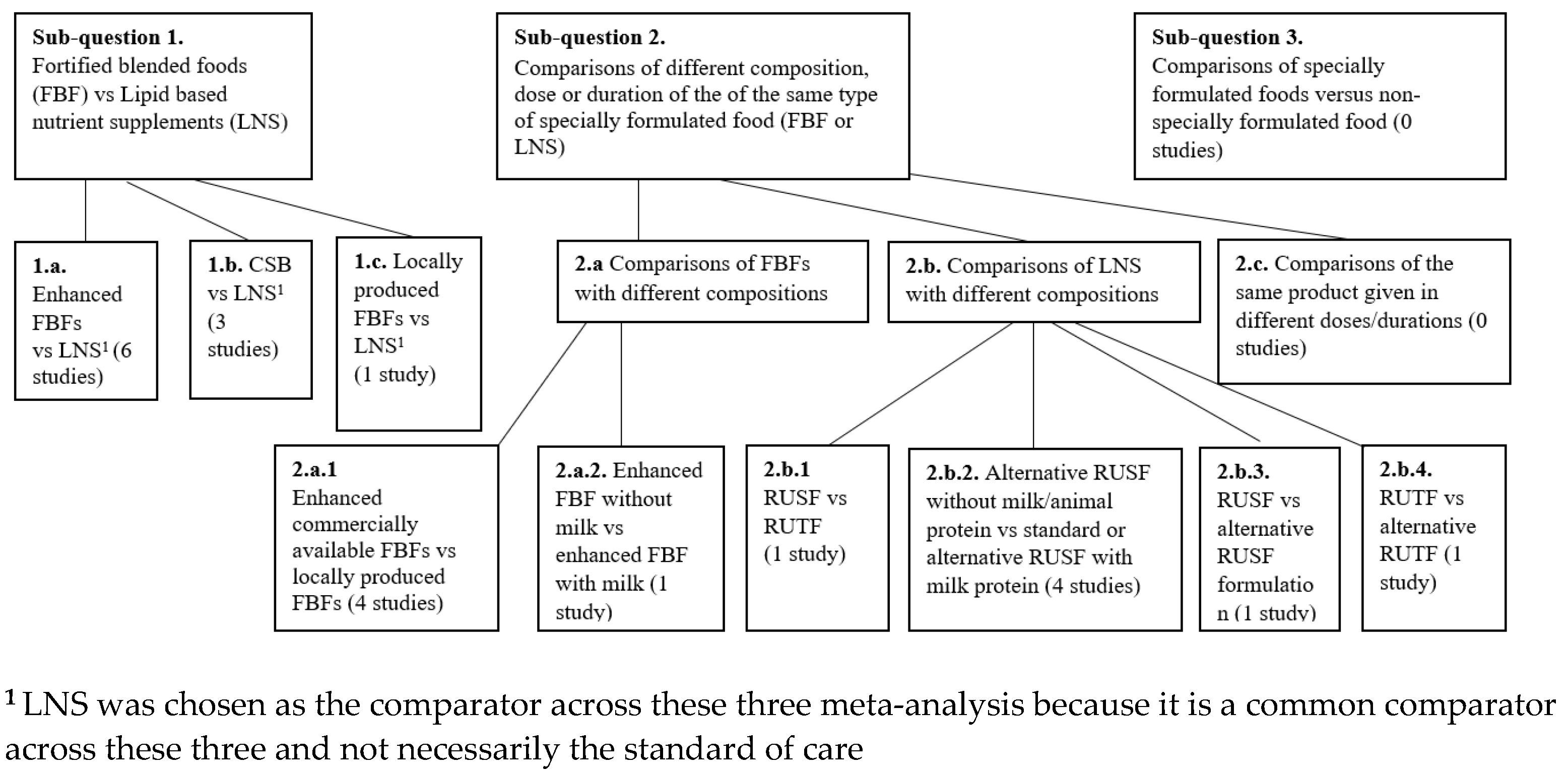
- Sub-question 1: Effectiveness of fortified blended foods (FBFs) versus lipid-based nutrient supplements (LNS):
- -
- Comparison 1.a. Enhanced FBFs vs. LNSs;
- -
- Comparison 1.b. CSB vs. LNS;
- -
- Comparison 1.c. Locally produced FBFs versus LNS.
- Sub-question 2: Effectiveness of different compositions, doses or durations among different variations of the same type of specially formulated food (FBFs or LNS):
- ◦
- Comparison 2.a. Comparisons of FBFs:
- -
- 2.a.1. Enhanced FBFs vs. locally produced FBFs;
- -
- 2.a.2. Supercereal without milk vs. Supercereal with milk.
- ◦
- Comparison 2.b. Comparisons of LNS:
- -
- 2.b.1. RUSF vs. RUTF;
- -
- 2.b.2. RUSF without animal protein vs. RUSF with animal protein;
- -
- 2.b.3. RUSF vs. alternative RUSF;
- -
- 2.b.4. RUTF vs. alternative RUTF.
- Sub-question 3. Effectiveness of specially formulated foods versus non-specially formulated food.
2.2. Search Methods for Identification of Studies
2.3. Selection of Studies
2.4. Data Extraction
2.5. Quality Assessment of Included Studies
2.6. Data Analysis
2.7. Sub-Group Analysis
3. Results
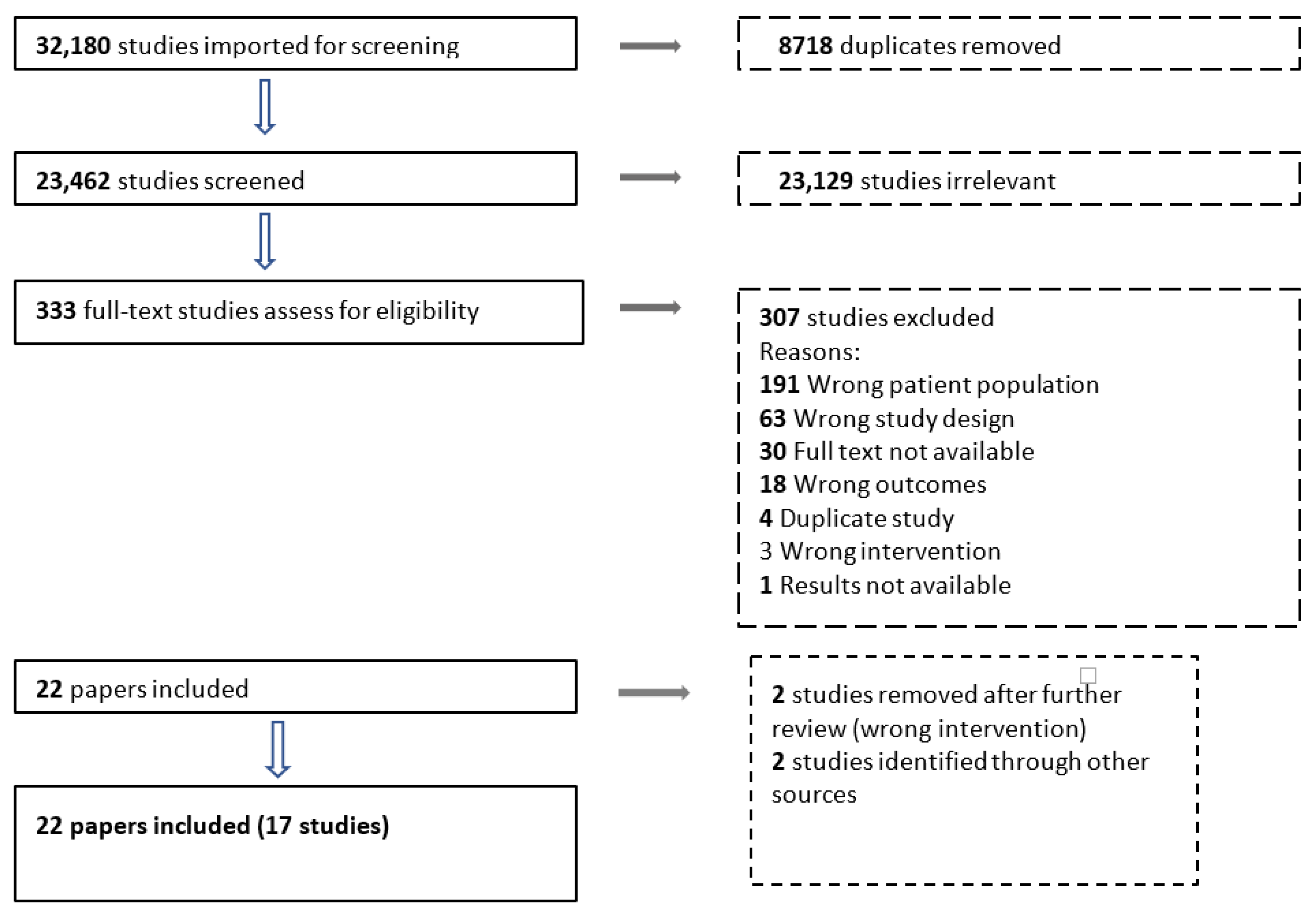
3.1. Results of the Search
3.2. Characteristics of Included Studies
3.3. Effect of Intervention
3.3.1. Sub-Question 1: Effectiveness of Fortified Blended Foods (FBFs) Versus Lipid-Based Nutrient Supplements (LNS)
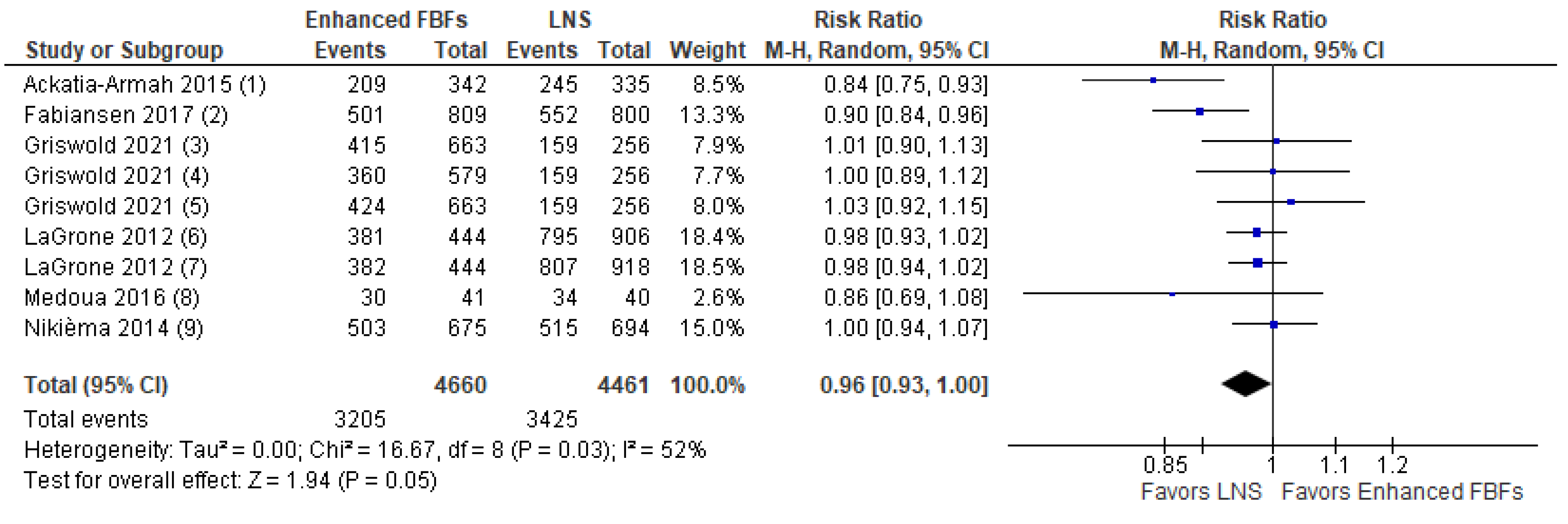
- Comparison 1.a. Enhanced FBFs compared to LNS
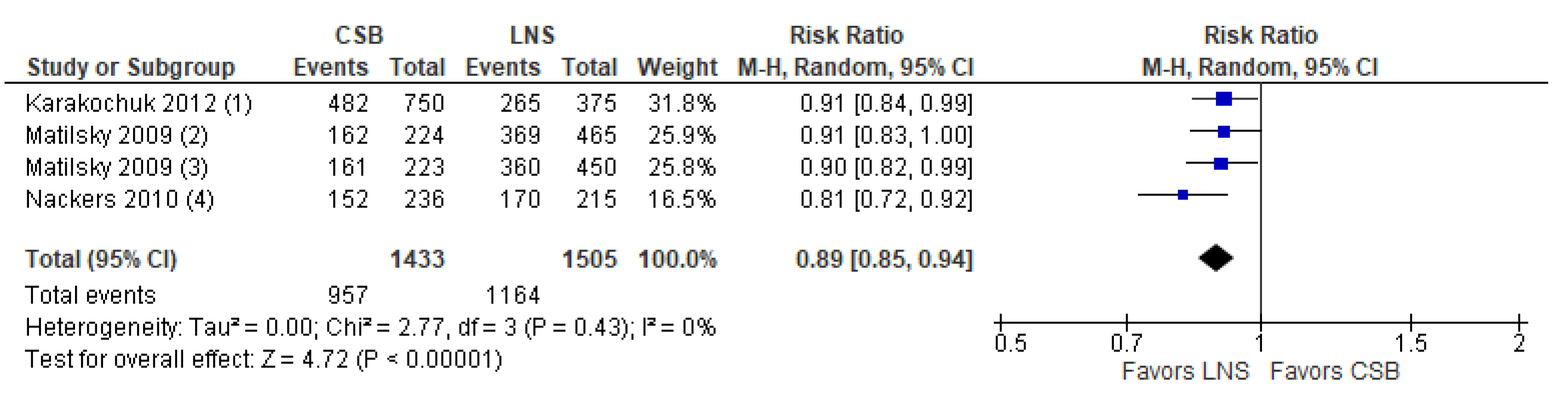
- Comparison 1.b. CSB compared to LNS
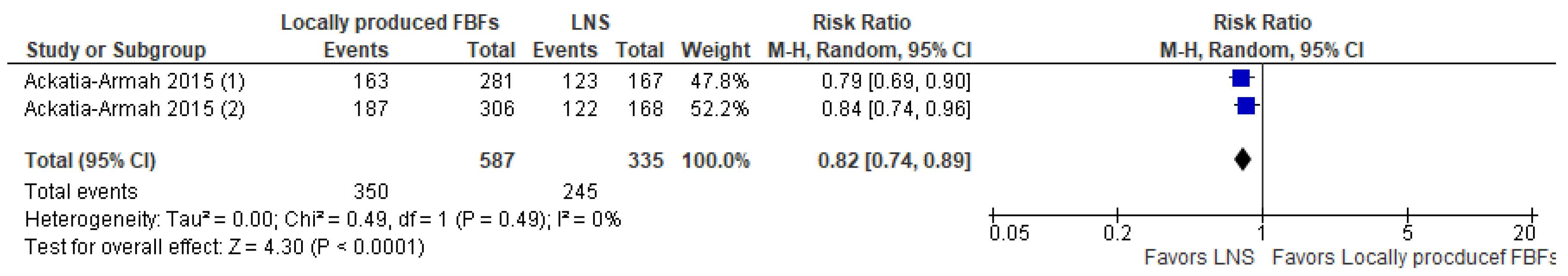
- Comparison 1.c. Locally produced FBFs compared to LNS
3.3.2. Sub-Question 2: Effectiveness of Different Compositions, Doses or Durations among Different Variations of the Same Type of specially Formulated Food (FBFs or LNSs)
- Comparison 2.a. Comparisons of FBFs
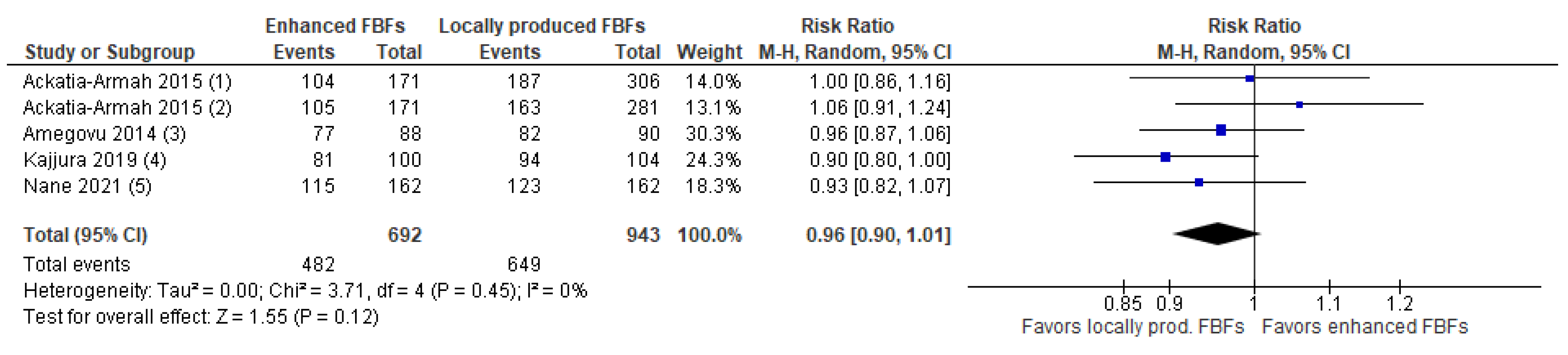
- Comparison 2.a.1. Enhanced FBFs compared to locally produced FBFs
- Comparison 2.a.2. Enhanced FBFs without milk vs. enhanced FBFs with milk
- Comparison 2.b. Comparisons of different variations of LNSs
- Comparison 2.b.1. RUTF vs. RUSF
- Comparison 2.b.2. RUSF without animal-based protein compared to RUSF with animal-based protein
- Comparison 2.b.3. RUSF vs. alternative (high protein) RUSF
- Comparison 2.b.4. RUTF vs. alternative RUTF
3.3.3. Sub-Question 3. Effectiveness of Specially Formulated Foods Versus Non-Specially Formulated Food
3.3.4. Dose and Duration of Specially Formulated Food
4. Discussion
4.1. Summary of Results
4.2. Overall Completeness and Applicability
4.3. Agreements and Disagreements with Other Studies or Reviews
4.4. Implications for Practice
4.5. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- United Nations Children’s Fund (UNICEF); World Health Organization; International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Roberton, T.; Carter, E.D.; Chou, V.B.; Stegmuller, A.R.; Jackson, B.D.; Tam, Y.; Sawadogo-Lewis, T.; Walker, N. Early Estimates of the Indirect Effects of the COVID-19 Pandemic on Maternal and Child Mortality in Low-Income and Middle-Income Countries: A Modelling Study. Lancet Glob. Health 2020, 8, e901–e908. [Google Scholar] [CrossRef] [PubMed]
- Black, R.; Victora, C.; Walker, S.; Bhutta, Z.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low and Middle Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; World Food Programme; United Nations System Standing Committee on Nutrition; United Nations Children’s Fund. Community-Based Management of Severe Acute Malnutrition: A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. WHO Guideline on the Dairy Protein Content in Ready-to-Use Therapeutic Foods for Treatment of Uncomplicated Severe Acute Malnutrition; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Essential Nutrition Actions: Mainstreaming Nutrition through the Life-Course; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Ciliberto, M.; Sandige, H.; Nheka, M.; Ashorn, P.; Briend, A.; Ciliberto, H.; Manary, M. Comparison of Home-Based Therapy with Ready-to-Use Therapeutic Food with Standard Therapy in the Treatment of Malnourished Malawian Children: A Controlled, Clinical Effectiveness Trial. Am. J. Clin. Nutr. 2005, 81, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Defourny, I.; Minetti, A.; Harczi, G.; Doyon, S.; Shepherd, S.; Tectonidis, M.; Bradol, J.; Golden, M. A Large-Scale Distribution of Milk-Based Fortified Spreads: Evidence for a New Approach in Regions with High Burden of Acute Malnutrition. PLoS ONE 2009, 4, e5455. [Google Scholar] [CrossRef]
- Nackers, F.; Broillet, F.; Oumarou, D.; Djibo, A.; Gaboulaud, V.; Guerin, P.J.; Rusch, B.; Grais, R.F.; Captier, V. Effectiveness of Ready-to-Use Therapeutic Food Compared to a Corn/Soy-Blend-Based Pre-Mix for the Treatment of Childhood Moderate Acute Malnutrition in Niger. J. Trop. Pediatr. 2010, 56, 407–413. [Google Scholar] [CrossRef]
- World Health Organization. Technical Note: Supplementary Foods for the Management of Moderate Acute Malnutrition in Infants and Children Aged 6–59 Months of Age; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Annan, R.; Webb, P.; Brown, R. Management of Moderate Acute Malnutrition (MAM): Current Knowledge and Practice; CMAM Forum: London, UK, 2014. [Google Scholar]
- Fabiansen, C.; Yaméogo, C.W.; Iuel-Brockdorf, A.-S.; Cichon, B.; Rytter, M.J.H.; Kurpad, A.; Wells, J.C.; Ritz, C.; Ashorn, P.; Filteau, S.; et al. Effectiveness of Food Supplements in Increasing Fat-Free Tissue Accretion in Children with Moderate Acute Malnutrition: A Randomised 2 × 2 × 3 Factorial Trial in Burkina Faso. PLoS Med. 2017, 14, e1002387. [Google Scholar] [CrossRef]
- LaGrone, L.; Trehan, I.; Meuli, G.; Wang, R.; Thakwalakwa, C.; Maleta, K.; Manary, M. A Novel Fortified Blended Flour, Corn-Soy Blend “plus-plus,” Is Not Inferior to Lipid-Based Ready-to-Use Supplementary Foods for the Treatment of Moderate Acute Malnutrition in Malawian Children. Am. J. Clin. Nutr. 2012, 95, 212–219. [Google Scholar] [CrossRef]
- Stobaugh, H.C.; Ryan, K.N.; Kennedy, J.A.; Grise, J.B.; Crocker, A.H.; Thakwalakwa, C.; Litkowski, P.E.; Maleta, K.M.; Manary, M.J.; Trehan, I. Including Whey Protein and Whey Permeate in Ready-to-Use Supplementary Food Improves Recovery Rates in Children with Moderate Acute Malnutrition: A Randomized, Double-Blind Clinical Trial. Am. J. Clin. Nutr. 2016, 103, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Noriega, K.; Lindshield, B. Is the Inclusion of Animal Source Foods in Fortified Blended Foods Justified? Nutrients 2014, 6, 3516–3535. [Google Scholar] [CrossRef]
- Lazzerini, M.; Rubert, L.; Pani, P. Specially Formulated Foods for Treating Children with Moderate Acute Malnutrition in Low- and Middle-Income Countries (Review). Cochrane Database Syst. Rev. 2013, 6, CD009584. [Google Scholar]
- Emergency Nutrition Network (ENN). Emergency Nutrition Network Technical Brief: Simplified Approaches to the Treatment of Wasting; Emergency Nutrition Network (ENN): Kidlington, UK, 2020. [Google Scholar]
- Covidence Systematic Review Software; V.H.I.: Melbourne, Australia, 2020.
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.4.; Cochrane: London, UK, 2020. [Google Scholar]
- Amegovu, A.K.; Ochola, S.; Ogwok, P.; Yiga, P.; Musalima, J.; Mandha, J. Efficacy of Sorghum Peanut Blend and Corn Soy Blend plus in the Treatment of Moderate Acute Malnutrition in Children Aged 6– 59 Months in Karamoja, Uganda: A Cluster Randomized Trial. Nutr. Diet. Suppl. 2014, 2014, 75–84. [Google Scholar] [CrossRef]
- Nikièma, L.; Huybregts, L.; Kolsteren, P.; Lanou, H.; Tiendrebeogo, S.; Bouckaert, K.; Kouanda, S.; Sondo, B.; Roberfroid, D. Treating Moderate Acute Malnutrition in First-Line Health Services: An Effectiveness Cluster-Randomized Trial in Burkina Faso. Am. J. Clin. Nutr. 2014, 100, 241–249. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. Br. Med. J. 2008, 336, 924. [Google Scholar] [CrossRef] [PubMed]
- Ackatia-Armah, R.; McDonald, C.; Doumbia, S.; Erhardt, J.; Hamer, D.; Brown, K. Malian Children with Moderate Acute Malnutrition Who Are Treated with Lipid-Based Dietary Supplements Have Greater Weight Gains and Recovery Rates than Those Treated with Locally Produced Cereal-Legume Products: A Community-Based, Cluster-Randomized Trial. Am. J. Clin. Nutr. 2015, 101, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Opondo, C.; Lelijveld, N.; Marron, B.; Onyo, P.; Musyoki, E.N.; Adongo, S.W.; Manary, M.; Briend, A.; Kerac, M. A Simplified, Combined Protocol versus Standard Treatment for Acute Malnutrition in Children 6-59 Months (ComPAS Trial): A Cluster-Randomized Controlled Non-Inferiority Trial in Kenya and South Sudan. PLoS Med. 2020, 17, e1003192. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Lelijveld, N.; Khara, T.; Dolan, C.; Stobaugh, H.; Sadler, K.; Lino Lako, R.; Briend, A.; Opondo, C.; Kerac, M.; et al. Response to Malnutrition Treatment in Low Weight-for-Age Children: Secondary Analyses of Children 6-59 Months in the ComPAS Cluster Randomized Controlled Trial. Nutrients 2021, 13, 1054. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Trehan, I.; Wang, R.J.; Thakwalakwa, C.; Maleta, K.; Deitchler, M.; Manary, M.J. Children Successfully Treated for Moderate Acute Malnutrition Remain at Risk for Malnutrition and Death in the Subsequent Year after Recovery. J. Nutr. 2013, 143, 215–220. [Google Scholar] [CrossRef]
- Chen, R.Y.; Mostafa, I.; Hibberd, M.C.; Das, S.; Mahfuz, M.; Naila, N.N.; Islam, M.M.; Huq, S.; Alam, M.A.; Zaman, M.U.; et al. A Microbiota-Directed Food Intervention for Undernourished Children. N. Engl. J. Med. 2021, 384, 1517–1528. [Google Scholar] [CrossRef]
- Fabiansen, C.; Phelan, K.; Cichon, B.; Ritz, C.; Briend, A.; Michaelsen, K.; Friis, H.; Shepherd, S. Short Children with a Low Midupper Arm Circumference Respond to Food Supplementation: An Observational Study from Burkina Faso. Am. J. Clin. Nutr. 2016, 103, 415–421. [Google Scholar] [CrossRef]
- Griswold, S.P.; Langlois, B.K.; Shen, Y.; Cliffer, I.R.; Suri, D.J.; Walton, S.; Chui, K.; Rosenberg, I.H.; Koroma, A.S.; Wegner, D.; et al. Effectiveness and Cost-Effectiveness of 4 Supplementary Foods for Treating Moderate Acute Malnutrition: Results from a Cluster-Randomized Intervention Trial in Sierra Leone. Am. J. Clin. Nutr. 2021, 114, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Kajjura, R.B.; Veldman, F.J.; Kassier, S.M. Effect of a Novel Supplementary Porridge on the Nutritional Status of Infants and Young Children Diagnosed with Moderate Acute Malnutrition in Uganda: A Cluster Randomised Control Trial. J. Hum. Nutr. Diet. 2019, 32, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Karakochuck, C.; Van den Briel, T.; Stephens, D.; Zlotkin, S. Treatment of Moderate Acute Malnutrition with Ready-to-Use Supplementary Food Results in Higher Overall Recovery Rates Compared with a Corn-Soya Blend in Children in Southern Ethiopia: An Operations Research Trial. Am. J. Clin. Nutr. 2012, 96, 911–916. [Google Scholar] [CrossRef]
- Kohlmann, K.; Callaghan-Gillespie, M.; Gauglitz, J.M.; Steiner-Asiedu, M.; Saalia, K.; Edwards, C.; Manary, M.J. Alternative Ready-To-Use Therapeutic Food Yields Less Recovery Than the Standard for Treating Acute Malnutrition in Children From Ghana. Glob. Health Sci. Pr. 2019, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Lelijveld, N.; Musyoki, E.; Adongo, S.W.; Mayberry, A.; Wells, J.C.; Opondo, C.; Kerac, M.; Bailey, J. Relapse and Post-Discharge Body Composition of Children Treated for Acute Malnutrition Using a Simplified, Combined Protocol: A Nested Cohort from the ComPAS RCT. PLoS ONE 2021, 16, e0245477. [Google Scholar] [CrossRef]
- Matilsky, D.K.; Maleta, K.; Castleman, T.; Manary, M.J.; Matilsky, D.K.; Maleta, K.; Castleman, T.; Manary, M.J. Supplementary Feeding with Fortified Spreads Results in Higher Recovery Rates than with a Corn/Soy Blend in Moderately Wasted Children. J. Nutr. 2009, 139, 773–778. [Google Scholar] [CrossRef]
- Medoua, G.; Ntsama, P.; Ndzana, A.; Essa’a, V.; Tsafack, J.; Dimodi, H. Recovery Rate of Children with Moderate Acute Malnutrition Treated with Ready-to-Use Supplementary Food (RUSF) or Improved Corn-Soya Blend (CSB plus): A Randomized Controlled Trial. Public Health Nutr. 2016, 19, 363–370. [Google Scholar] [CrossRef]
- Roediger, R.; Stein, H.H.; Callaghan-Gillespie, M.; Blackman, J.K.; Kohlmann, K.; Maleta, K.; Manary, M. Protein Quality in Ready-to-Use Supplementary Foods for Moderate Wasting. Matern. Child Nutr. 2020, 16, e13019. [Google Scholar] [CrossRef]
- Trehan, I.; Banerjee, S.; Murray, E.; Ryan, K.; Thakwalakwa, C.; Maleta, K.; Manary, M. Extending Supplementary Feeding for Children Younger Than 5 Years With Moderate Acute Malnutrition Leads to Lower Relapse Rates. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 544–549. [Google Scholar] [CrossRef]
- Nane, D.; Hatløy, A.; Lindtjørn, B. Development and Nutritional Evaluation of Local Ingredients-based Supplements to Treat Moderate Acute Malnutrition among Children Aged below Five Years: A Descriptive Study from Rural Wolaita, Southern Ethiopia. Food Sci. Nutr. 2020, 8, 6287–6295. [Google Scholar] [CrossRef]
- Gluning, I.; Kerac, M.; Bailey, J.; Bander, A.; Opondo, C. The Management of Moderate Acute Malnutrition in Children Aged 6-59 Months in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1317–1329. [Google Scholar] [CrossRef]
- Karakochuck, C.; Whitfield, K.; Rappaport, A.; Barr, S.; Vercauteren, S.; McLean, J.; Prak, S.; Hou, K.; Talukder, A.; Devenish, R.; et al. The Homozygous Hemoglobin EE Genotype and Chronic Inflammation Are Associated with High Serum Ferritin and Soluble Transferrin Receptor Concentrations among Women in Rural Cambodia. J. Nutr. 2015, 145, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Lenters, L.M.; Wazny, K.; Webb, P.; Ahmed, T.; Bhutta, Z.A. Treatment of Severe and Moderate Acute Malnutrition in Low- and Middle-Income Settings: A Systematic Review, Meta-Analysis and Delphi Process. BMC Public Health 2013, 13, S23. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Sachdev, H. Effect of Iron Supplementation on Incidence of Infectious Illness in Children: Systematic Review. Bristish Med. J. 2002, 325, 1142. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Salam, R.; Hadi, Y.; Sadiq, S.S.; Bhutta, A.Z.; Weise Prinzo, Z.; Bhutta, Z.A. Preventive Lipid-Based Nutrient Supplements given with Complementary Foods to Infants and Young Children 6 to 23 Months of Age for Health, Nutrition, and Developmental Outcomes (Review). Cochrane Database Syst. Rev. 2019, 2019, CD012611. [Google Scholar]
- Oakley, E.; Reinking, J.; Sandige, H.; Trehan, I.; Kennedy, G.; Maleta, K.; Manary, M. A Ready-To-Use Therapeutic Food Containing 10% Milk Is Less Effective Than One with 25% Milk in the Treatment of Severely Malnourished Children. J. Nutr. 2010, 140, 2248–2252. [Google Scholar] [CrossRef]
- Iuel-Brockdorf, A.-S.; Draebel, T.A.; Ritz, C.; Fabiansen, C.; Cichon, B.; Brix Christensen, V.; Yameogo, C.; Oummani, R.; Briend, A.; Michaelsen, K.F.; et al. Evaluation of the Acceptability of Improved Supplementary Foods for the Treatment of Moderate Acute Malnutrition in Burkina Faso Using a Mixed Method Approach. Appetite 2016, 99, 34–45. [Google Scholar] [CrossRef]
- Isanaka, S.; Barnhart, D.A.; McDonald, C.; Ackatia-Armah, R.; Kupka, R.; Seydou, D.; Brown, K.; Menzies, N.A. Cost-Effectiveness of Community-Based Screening and Treatment of Moderate Acute Malnutrition in Mali. MBJ Glob. Health 2019, 4, e001227. [Google Scholar] [CrossRef]
- McDonald, C.; Olofin, I.; Flaxman, S.; Fawzi, W.; Spiegelman, D.; Caulfield, L.; Black, R.; Ezzati, M.; Danaei, G. The Effect of Multiple Anthropometric Deficits on Child Mortality: Meta-Analysis of Individual Data in 10 Propective Studies from Developing Countries. Am. J. Clin. Nutr. 2013, 97, 896–901. [Google Scholar] [CrossRef]
- Cichon, B.; Fabiansen, C.; Iuel-Brockdorf, A.-S.; Yaméogo, C.W.; Ritz, C.; Christensen, V.B.; Filteau, S.; Briend, A.; Michaelsen, K.F.; Friis, H. Impact of Food Supplements on Hemoglobin, Iron Status, and Inflammation in Children with Moderate Acute Malnutrition: A 2 × 2 × 3 Factorial Randomized Trial in Burkina Faso. Am. J. Clin. Nutr. 2018, 107, 278–286. [Google Scholar] [CrossRef]
- Friis, H.; Cichon, B.; Fabiansen, C.; Iuel-Brockdorff, A.-S.; Yaméogo, C.W.; Ritz, C.; Frikke-Schmidt, R.; Briend, A.; Michaelsen, K.F.; Christensen, V.B.; et al. Serum Cobalamin in Children with Moderate Acute Malnutrition in Burkina Faso: Secondary Analysis of a Randomized Trial. PLOS Med. 2022, 19, e1003943. [Google Scholar] [CrossRef]
- Yaméogo, C.W.; Cichon, B.; Fabiansen, C.; Rytter, M.J.H.; Faurholt-Jepsen, D.; Stark, K.D.; Briend, A.; Shepherd, S.; Traoré, A.S.; Christensen, V.B.; et al. Correlates of Whole-Blood Polyunsaturated Fatty Acids among Young Children with Moderate Acute Malnutrition. Nutr. J. 2017, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Caiafa, C.; Dewey, K.G.; Michaelsen, K.; De Pee, S.; Collins, S.; Rogers, B.; El-Kour, T.; Walton, S.; Webb, P. Food Aid for Nutrition: Narrative Review of Major Research Topics Presented at a Scientific Symposium Held October 21, 2017, at the 21st International Congress of Nutrition in Buenos Aires, Argentina. Food Nutr. Bull. 2019, 40, 111–123. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, D. Research Gaps in the Use of Dairy Ingredients in Food Aid Products. Food Nutr. Bull. 2016, 37, S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Andersen, G.; Jacobsen, S. The Use of Whey or Skimmed Milk Powder in Fortified Blended Foods for Vulnerable Groups. J. Nutr. 2008, 138, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.F.; Iuel-Brockdorff, A.-S.; Yaméogo, C.W.; Cichon, B.; Fabiansen, C.; Filteau, S.; Phelan, K.; Ouédraogo, A.; Michaelsen, K.F.; Gladstone, M.; et al. Impact of Food Supplements on Early Child Development in Children with Moderate Acute Malnutrition: A Randomised 2 × 2 × 3 Factorial Trial in Burkina Faso. PLOS Med. 2020, 17, e1003442. [Google Scholar] [CrossRef]
- Purwestri, R.C.; Scherbaum, V.; Inayati, D.A.; Wirawan, N.N.; Suryantan, J.; Bloem, M.A.; Pangaribuan, R.V.; Stuetz, W.; Hoffmann, V.; Qaim, M.; et al. Impact of Daily versus Weekly Supply of Locally Produced Ready-to-Use Food on Growth of Moderately Wasted Children on Nias Island, Indonesia. ISRN Nutr. 2013, 2013, 412145. [Google Scholar] [CrossRef]
- Korachais, C.; Nkurunziza, S.; Nimpagaritse, M.; Meessen, B. Impact of the Extension of a Performance-Based Financing Scheme to Nutrition Services in Burundi on Malnutrition Prevention and Management among Children below Five: A Cluster-Randomized Control Trial. PLoS ONE 2020, 15, e0239036. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Parameter | Criteria |
|---|---|
| Population | Children aged > 6 months with moderate wasting, defined as a weight-for-height z-score of ≥−3 and <−2 and/or a mid-upper arm circumference of ≥ 11.5 cm and <12.5 cm or a weight-for-height between >70% and <80% of the median and no oedema, treated either as inpatients or outpatients. |
| Intervention | Dietary treatment in addition to standard clinical care. |
| Comparator | No specific dietary treatment in addition to standard clinical care and/or comparing different dietary treatment approaches to each other. |
| Outcomes | Anthropometric recovery, anthropometric outcomes (weight-for-length, weight-for-age, mid-upper arm circumference, weight and height gain), sustained recovery, deterioration to severe wasting, time to recovery, non-response, relapse. |
| Study design | Included: Randomized or non-randomized controlled trials. Excluded: Cross-sectional or observational studies, case reports, animal studies, case studies, opinions, editorials, commentaries, letters, conference abstracts, studies with external comparison groups and reviews. |
| Study Number | Author, Year | Study Design and Setting | Participants, Admission and Recovery Criteria | Intervention/Control and Dose and Duration of Supplementation | Outcomes |
|---|---|---|---|---|---|
| 1. | Ackatia-Armah et al., 2015 [25] | cRCT Twelve community health centres in a rural setting in Diola Health District, Bamako, Mali | 1264 children aged 6–35 months Admission criteria: -WHZ <−2 and ≥−3 (WHO Growth Standards) or MUAC <12.5 cm and ≥11.5 cm -WHZ <80% and ≥70% of the NCHS median or MUAC <12.0 and ≥11.0 cm (National criteria in Mali at the time) Recovery from MAM was defined as WHZ >−2.0 and MUAC >12.5 cm during at least 2 consecutive follow-up visits | Group 1: 500 kcal/day of ready-to-use supplementary food (RUSF) (n = 344) Group 2: 500 kcal/day of corn–soy blend (CSB++) n = 349) Group 3: 500 kcal/day of Misola (n = 307) Group 4: 500 kcal/day of locally milled flours + micronutrient powder (n = 284) Duration: 12 weeks | Adherence, MUAC, weight, length, WHZ, LAZ, haemoglobin, serum ferritin, retinol-binding protein, transferrin receptor, body iron stores, and plasma zinc |
| 2. | Amegovu et al., 2014 [22] | cRCT Two health centres Kakamongole and Namalu in Nakapiripirit district, Karamoja, Uganda | 440 children aged 6–59 months Admission criteria: -WHZ <−2 and ≥−3 (WHO Growth Standards) Recovery criteria: Children were defined as having recovered when they reached a WHZ> –2 and had attained 10% of their admission weight for two consecutive visits | Group 1: 1200 kcal/day of corn–soy blend plus (CSB+) mixed with vegetable oil and sugar Group 2: 1228 kcal per day of sorghum peanut blend mixed with ghee and honey Duration: Up to 3 months | Recovery rate, time to recovery |
| 3. | Bailey et al., 2020 [26] (Plus a linked nested cohort study (Lelijveld et al., 2021 [35]) and a secondary data analysis (Bailey et al., 2021 [27])) | cRCT Kenya and South Sudan (The nested cohort study reported only on the Kenya subsample) | 4,110 Children aged 6–59 months, 2858 of these were MAM, the rest SAM. Admission criteria: MUAC < 12.5 cm and/or oedema (+/++, i.e., mild or moderate). MAM was defined as MUAC between 115 mm and 125 mm Recovery criteria: MUAC measurement of ≥12.5 cm for 2 consecutive visits | Group 1: Standard protocol Children with MAM received 500 kcal/day of RUSF Group 2: Combined protocol children with MAM received 500 kcal RUTF/day (1 sachet/day). Duration: 17 weeks | Primary outcome: Nutritional recovery. Secondary outcomes: cost-effectiveness, coverage, defaulting, death, length of stay, average daily weight and MUAC gains, WHZ, WAZ, LAZ, and bioelectrical impedance analysis |
| 4. | Chen et al., 2021 [29] | RCT Mirpur, Bangladesh | 123 children aged 12–18 months Admission criteria: WHZ <−2 and ≥−3 without bipedal oedema Recovery from MAM was defined as a WHZ ≥−2 | Group 1: 204 kcal/day of microbiota-directed complementary food prototype (MDCF-2) Group 2: 247 kcal/day of ready-to-use supplementary food (RUSF) Duration: 3 months intervention, 1 month follow-up period | Weekly rate of change in the WHZ, WAZ, MUAC, and LAZ medical complications, plasma proteomic profile, and gut microbiota configuration |
| 5. | Fabiansen et al., 2017 [13] (Plus a nested observational cohort study (Fabiansen 2016 [30])) | Randomised 2 × 2 × 3 factorial trial Province du Passoré in the Northern Region of Burkina Faso | 1609 children aged 6–23 months Admission criteria: WHZ <−2 and ≥−3 (WHO Growth Standards) or MUAC <12.5 cm and ≥11.5 cm Recovery from MAM was defined as WHZ >−2.0 and MUAC >12.5 cm during at least 2 consecutive follow-up visits The nested observational cohort study included a subgroup of children who were MAM by MUAC only but had a WHZ > −2 | Children received one of 500 kcal/day of one 12 food products. Products were either CSB or LNS, with either soy isolate or dehulled soy and either 0%, 20% or 50% of protein from milk. Duration: 12 weeks | The primary outcome was a fat-free mass index. Other outcomes included: weight, length, knee-heel length gain, MUAC, triceps skinfold, nutritional recovery, and weight MUAC, micronutrient status, acceptability, development, and physical activity |
| 6. | Griswold et al., 2021 [31] | cRCT Sierra Leone | 2691 children with MAM aged 6–59 months Admission criteria: MUAC <12.5 cm and ≥11.5 cm Recovery from MAM was defined as achieving a MUAC >12.5 cm by 12 weeks | Group 1: 550 kcal/d of CSB+ w/oil Group 2: 550 kcal/d of CSWB w/oil Group 3: 550 kcal/d RUSF Group 4: 550 kcal/d SC+ w/A Duration: ∼12 weeks | Recovery, deterioration to SAM, death, default, and cost-effectiveness |
| 7. | Kajjura et al., 2019 [32] | cRCT 24 rural parishes Arua district, North Western Uganda | 220 mother–child pairs aged 6–18 months Admission criteria: WHZ <−2 and ≥−3 (WHO Growth Standards) Recovery from MAM was defined as WHZ >−2.0 | Intervention group: 675 kcal/day of an active malt, extruded maize and soy sorghum-based porridge Control group: 600 kcal/day of CSB Plus Duration: 3 months | Weight, length, WAZ Score, LAZ score, WLZ, and haemoglobin level |
| 8. | Karakochuk et al., 2012 [33] | cRCT Ten health centres and health posts in the northern region of the Sidama zone, Ethiopia | 1125 children aged 6–60 months Admission criteria: WFH ≥70 to <80% according to NCHS growth standards. Recovery from MAM was defined as WFH ≥85% on 2 consecutive visits | Group 1: 500 kcal/day of supplementary Plumpy (n = 375) Group 2: CSB 1413 kcal/day of CSB and vegetable oil (300 g CSB and 32 g oil) was given biweekly (n = 750) Duration: 16 weeks | Recovery, default, transport, non-response, and mortality |
| 9. | Kohlmann et al., 2019 [34] | Randomised, double-blind controlled study 29 clinics, Brong Ahafo region of Ghana | 1270 children 6–59 months Admission criteria: Children experiencing acute malnutrition and passing the appetite test. MAM was defined as not having SAM and having WHZ <−2 and ≥−3 and/or MUAC of < 12.5 cm >11.5 cm Recovery criteria were defined as MUAC >12.4 cm and WHZ > −2 | Intervention group: 75 kcal/kg/day2 alternative RUTF (A-RUTF), where half the amount of peanut was replaced with local soybean and sorghum flour and 50% of protein from dairy came from a combination of whey protein concentrate and non-fat dried milk. Control: 75 kcal/kg/day standard RUTF Duration: 12 weeks | Primary outcome: Recovery Secondary outcomes: rates of weight and MUAC gain, number of visits, cost of RUTF per child recovered, and adverse events |
| 10. | La Grone et al., 2012 [14] (Plus two linked observational follow-up studies (Chang et al., 2013 [28]; Trehan et al., 2015 [39])) | RCT Rural setting in South TFC, Malawi | 2890 children aged 6–59 month Admission criteria: WHZ <−2 and ≥−3 without bipedal oedema Recovery from MAM was defined as a WHZ ≥−2 Linked observational studies followed up 2349 recovered children for 12 months. Chang et al. followed up on 1967 children who were treated until recovery (treat-to-goal), while Trehan compared these children with a smaller sample of 382 children who were treated for a fixed 12 weeks | Group 1: 75 kcal/kg/day of CSB++ (n = 948) 2 Group 2: 75 kcal/kg/day soy RUSF (n = 964) Group 3: 75 kcal/kg/day soy/whey RUSF (n = 978) Duration: Until recovery up to 12 weeks | Recovered, developed SAM, remained MAM, died, defaulted time to recovery, rate of adverse events, and rates of gain in weight, length, and MUAC, relapse, and sustained recovery |
| 11. | Matilsky et al., 2009 [36] | RCT Rural setting in the southern region of Malawi | 1362 children aged 6–60 months Admission criteria: WHZ <−2 and ≥−3 without bipedal oedema Recovery from MAM was defined as a WHZ ≥−2 | Group 1: 75 kcal/kg/day of a milk/peanut fortified spread (Nutriset) (n = 465) 2 Group 2: 75 kcal/kg/day of a soy/peanut fortified spread (Nutriset) (n = 450) Group 3: 75 kcal/kg/day of a corn–soy blend (n = 447) Duration: Up to 8 weeks | Recovery, rates of gain in weight, stature, MUAC, and adverse outcomes |
| 12. | Medoua et al., 2015 [37] | RCT Mvog-Beti and Evodoula health districts in the Centre region of Cameroon | 81 children aged 25–59 months Admission criteria: WHZ <−2 and ≥−3 without bipedal oedema Recovery from MAM was defined as a WHZ ≥−2 | Group 1: 40 kcal/kg/day CSB+ with soy oil (n = 41) 3 Group 2: 40 kcal/kg/day RUSF (n = 40) Duration: 8 weeks | Recovery rate, time to recovery, and rates of gain in weight and MUAC |
| 13. | Nackers et al., 2010 [10] | RCT Two supplementary feeding centres in the remote villages of Mallawa and Bangaza, Magaria department, Zinder region, South of Niger | 807 children aged 6–59 months Admission criteria: WHM from 70% to <80% NCHS reference, without oedema and with a MUAC ≥110 mm and less than 135 mm. Recovery was defined as WHM% ≥ 85% for 2 consecutive weeks | Group 1: 1231 kcal/day of CSB with vegetable oil and sugar (n = 406) Group 2: 1000 kcal/day of RUTF Plumpy’nut) (n = 401) Duration: 16 weeks | Weight gain, recovery rate, mortality, non-responder and defaulter rates, length of stay, MUAC and height gain, haemoglobin, and relapse |
| 14. | Nane et al., 2021 [40] | RCT Damot Pulassa district, Wolaita, Southern Ethiopia | 324 children aged 6–59 months Admission criteria: WHZ <−2 and ≥−3 (WHO Growth Standards) or MUAC <12.5 cm and ≥11.5 cm Recovery percentage of children who attained a MUAC ≥12.5 cm and/or WHZ ≥−2 without bipedal edema at the end of 12 weeks. | Group 1: 699 kcal/day of Local ingredients base supplement with 8 mL of refined deodorized and cholesterol-free sunflower oil/day Group 2: 751 kcal/day of CSB+ with 16 mL of refined deodorized and cholesterol- free sunflower oil/day Duration: 12 weeks | Recovery rate, weight gain, time to recovery, MUAC gain, and length gain |
| 15. | Nikiema et al., 2014 [23] | cRCT Rural setting in Hounde, Burkina Faso | 1974 children aged 6–24 months of age Admission criteria: WHZ < −2 and ≥−3 without bipedal oedema Recovery from MAM was defined as a WHZ ≥ −2 | Group 1: Child-centred nutrition counselling only (n = 605). Group 2: 273 kcal/day of corn–soy blend ++ (CSB++) (n = 675) Group 3: 258 kcal/day of a locally produced peanut and soy-based ready-to-use supplementary food (RUSF) (n = 694) Duration: 12 weeks | Recovered, died or dropped out, attendance, time to recovery, weight, length, and daily MUAC gains |
| 16. | Roediger et al., 2020 [38] | RCT Stable setting, southern region of Malawi. 27 Feeding sites | 1737 children aged 6–59 months Admission criteria: WHZ < −2 and ≥−3 (WHO Growth Standards) or MUAC < 12.5 cm and ≥11.5 cm Recovery from MAM was defined as WHZ > −2.0 and MUAC > 12.5 cm during at least 2 consecutive follow-up visits | Intervention group: 75 kcal/kg/day of a high protein RUSF (HiPro-RUSF)2 Control: 75 kcal/kg/day of a standard control RUSF. The two RUSFs were isonitrogenous, but the HiPro-RUSF contained non-fat dried skim milk and whey permeate, whereas C-RUSF contained whey permeate and whey protein concentrate Duration: Until recovery up to 12 weeks | Recovery, deterioration to SAM, lost to follow-up, average weight and MUAC gain, and time to recovery |
| 17. | Stobaugh et al., 2016 [15] | RCT Stable setting in Rural Malawi (South) | 2230 children aged 6–59 months Admission: MAM, as defined by MUAC of 11.5–12.4 cm without bipedal oedema Recovery: MUAC of 12.5 cm without bipedal oedema within 12 weeks of therapy | Group 1: 75 kcal/kg/day of soy RUSF2 Group 2: 75 kcal/kg/d of a novel whey RUSF (combination of 4.9% WPC80 and 18.7% whey permeate) Duration: 12 weeks | Recovery, deterioration to SAM, default, died, MUAC, WHZ, weight gain, and height gain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichon, B.; Das, J.K.; Salam, R.A.; Padhani, Z.A.; Stobaugh, H.C.; Mughal, M.; Pajak, P.; Rutishauser-Perera, A.; Bhutta, Z.A.; Black, R.E. Effectiveness of Dietary Management for Moderate Wasting among Children > 6 Months of Age—A Systematic Review and Meta-Analysis Exploring Different Types, Quantities, and Durations. Nutrients 2023, 15, 1076. https://doi.org/10.3390/nu15051076
Cichon B, Das JK, Salam RA, Padhani ZA, Stobaugh HC, Mughal M, Pajak P, Rutishauser-Perera A, Bhutta ZA, Black RE. Effectiveness of Dietary Management for Moderate Wasting among Children > 6 Months of Age—A Systematic Review and Meta-Analysis Exploring Different Types, Quantities, and Durations. Nutrients. 2023; 15(5):1076. https://doi.org/10.3390/nu15051076
Chicago/Turabian StyleCichon, Bernardette, Jai K. Das, Rehana A. Salam, Zahra A. Padhani, Heather C. Stobaugh, Muzna Mughal, Patrizia Pajak, Alexandra Rutishauser-Perera, Zulfiqar A. Bhutta, and Robert E. Black. 2023. "Effectiveness of Dietary Management for Moderate Wasting among Children > 6 Months of Age—A Systematic Review and Meta-Analysis Exploring Different Types, Quantities, and Durations" Nutrients 15, no. 5: 1076. https://doi.org/10.3390/nu15051076
APA StyleCichon, B., Das, J. K., Salam, R. A., Padhani, Z. A., Stobaugh, H. C., Mughal, M., Pajak, P., Rutishauser-Perera, A., Bhutta, Z. A., & Black, R. E. (2023). Effectiveness of Dietary Management for Moderate Wasting among Children > 6 Months of Age—A Systematic Review and Meta-Analysis Exploring Different Types, Quantities, and Durations. Nutrients, 15(5), 1076. https://doi.org/10.3390/nu15051076






