ATP and NAD+ Deficiency in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
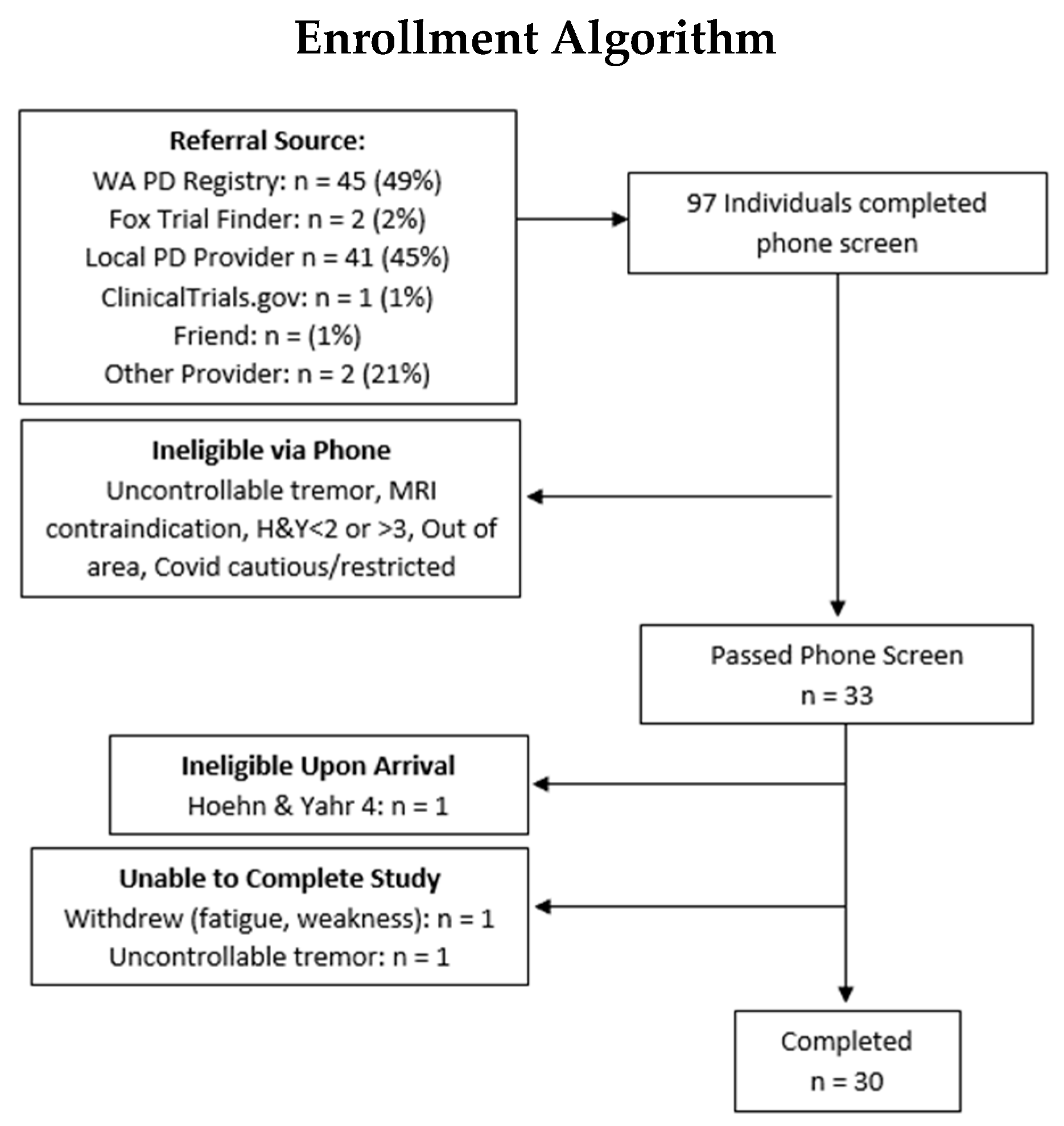
2.1. Participants and Study Design
2.2. Magnetic Resonance Spectroscopy
2.3. NMR Data Processing
2.4. Muscle Size by Magnetic Resonance Imaging
2.5. ATPmax
2.6. Statistical Analysis
3. Results
3.1. Mitochondrial ATP Production
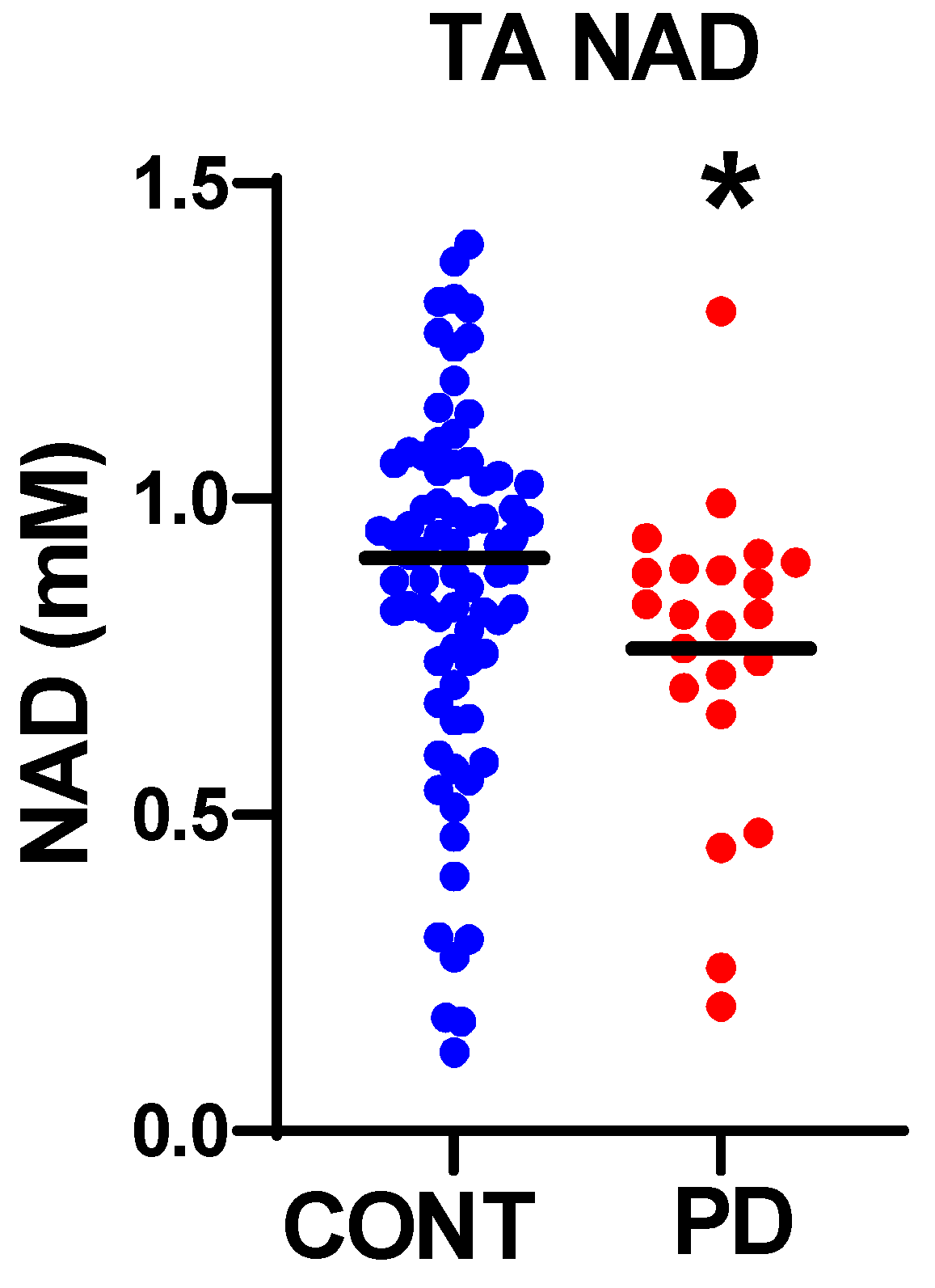
3.2. Nicotinamide Metabolites
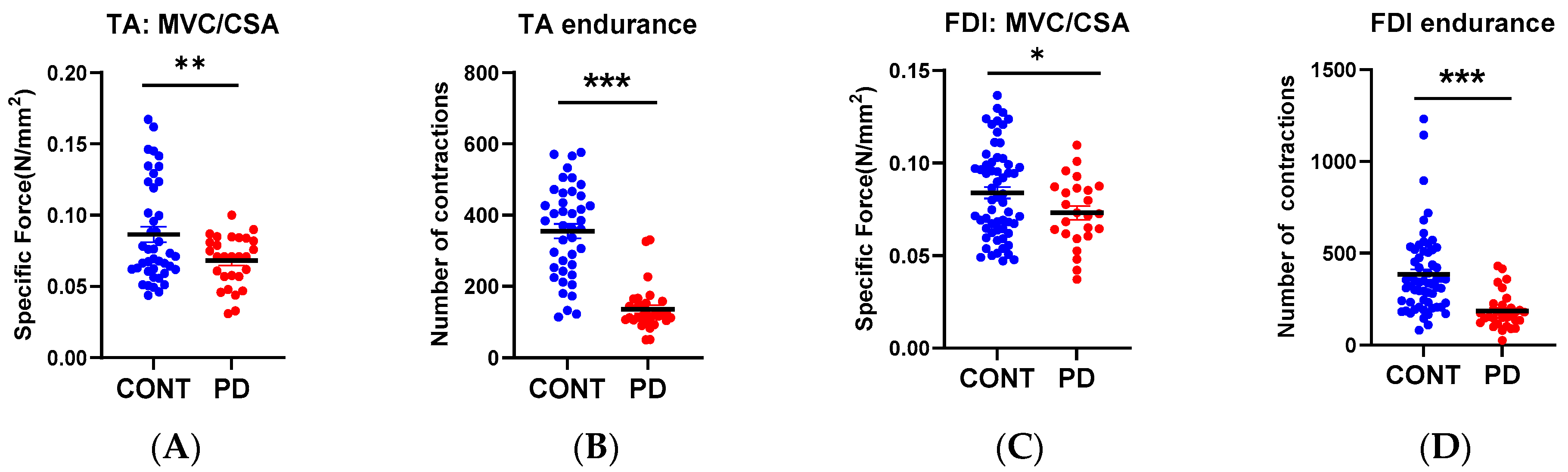
3.3. Muscle Function
3.4. Relationship to PD Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1989, 1, 1269. [Google Scholar] [CrossRef]
- Malpartida, A.B.; Williamson, M.; Narendra, D.P.; Wade-Martins, R.; Ryan, B.J. Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef]
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Li, J.L.; Lin, T.Y.; Chen, P.L.; Guo, T.N.; Huang, S.Y.; Chen, C.H.; Lin, C.H.; Chan, C.C. Mitochondrial Function and Parkinson’s Disease: From the Perspective of the Electron Transport Chain. Front. Mol. Neurosci. 2021, 14, 797833. [Google Scholar] [CrossRef]
- Holper, L.; Ben-Shachar, D.; Mann, J.J. Multivariate meta-analyses of mitochondrial complex I and IV in major depressive disorder, bipolar disorder, schizophrenia, Alzheimer disease, and Parkinson disease. Neuropsychopharmacology 2019, 44, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Long, X.; Xiong, J.; Jia, M.; Chen, C.; Huang, J.; Ghoorah, D.; Kong, X.; Lin, Z.; Wang, T. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit. Rev. Toxicol. 2012, 42, 613–632. [Google Scholar] [CrossRef] [PubMed]
- Blei, M.L.; Conley, K.E.; Kushmerick, M.J. Separate measures of ATP utilization and recovery in human skeletal muscle. J. Physiol. 1993, 465, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Amara, C.E.; Marcinek, D.J.; Shankland, E.G.; Schenkman, K.A.; Arakaki, L.S.; Conley, K.E. Mitochondrial function in vivo: Spectroscopy provides window on cellular energetics. Methods 2008, 46, 312–318. [Google Scholar] [CrossRef]
- Campbell, M.D.; Marcinek, D.J. Evaluation of in vivo mitochondrial bioenergetics in skeletal muscle using NMR and optical methods. Biochim. Biophys. Acta 2016, 1862, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Prompers, J.J.; Wessels, B.; Kemp, G.J.; Nicolay, K. MITOCHONDRIA: Investigation of in vivo muscle mitochondrial function by 31P magnetic resonance spectroscopy. Int. J. Biochem. Cell Biol. 2014, 50, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hattingen, E.; Magerkurth, J.; Pilatus, U.; Mozer, A.; Seifried, C.; Steinmetz, H.; Zanella, F.; Hilker, R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 2009, 132, 3285–3297. [Google Scholar] [CrossRef]
- Taylor, D.J.; Krige, D.; Barnes, P.R.; Kemp, G.J.; Carroll, M.T.; Mann, V.M.; Cooper, J.M.; Marsden, C.D.; Schapira, A.H. A 31P magnetic resonance spectroscopy study of mitochondrial function in skeletal muscle of patients with Parkinson’s disease. J. Neurol. Sci. 1994, 125, 77–81. [Google Scholar] [CrossRef]
- Coen, P.M.; Jubrias, S.A.; Distefano, G.; Amati, F.; Mackey, D.C.; Glynn, N.W.; Manini, T.M.; Wohlgemuth, S.E.; Leeuwenburgh, C.; Cummings, S.R.; et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 447–455. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Scalzo, P.; D’Agostino, J.; Moore, Z.A.; Diaz-Ruiz, A.; Fabbri, E.; Zane, A.; Chen, B.; Becker, K.G.; Lehrmann, E.; et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 2018, 17, e12725. [Google Scholar] [CrossRef]
- Weiss, K.; Schär, M.; Panjrath, G.S.; Zhang, Y.; Sharma, K.; Bottomley, P.A.; Golozar, A.; Steinberg, A.; Gerstenblith, G.; Russell, S.D.; et al. Fatigability, Exercise Intolerance, and Abnormal Skeletal Muscle Energetics in Heart Failure. Circ. Heart Fail. 2017, 10, e004129. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Gamboa, J.; Liu, S.; Ali, A.S.; Shankland, E.; Jue, T.; Giulivi, C.; Smith, L.R.; Himmelfarb, J.; de Boer, I.H.; et al. Impaired skeletal muscle mitochondrial bioenergetics and physical performance in chronic kidney disease. JCI Insight 2020, 5, e133289. [Google Scholar] [CrossRef]
- Janssens, G.E.; Houtkooper, R.H.; Hoeks, J. NAD+ to assess health in aging humans. Aging 2022, 14, 5962–5963. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.T.; Segall, J. The SPS4 gene of Saccharomyces cerevisiae encodes a major sporulation-specific mRNA. Mol. Cell Biol. 1986, 6, 4478–4485. [Google Scholar] [CrossRef] [PubMed]
- Whitson, J.A.; Bitto, A.; Zhang, H.; Sweetwyne, M.T.; Coig, R.; Bhayana, S.; Shankland, E.G.; Wang, L.; Bammler, T.K.; Mills, K.F.; et al. SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging Cell 2020, 19, e13213. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Daneshgar, N.; Chu, Y.; Chen, B.; Hefti, M.; Vikram, A.; Irani, K.; Song, L.S.; Brenner, C.; Abel, E.D.; et al. Metabolic rescue ameliorates mitochondrial encephalo-cardiomyopathy in murine and human iPSC models of Leigh syndrome. Clin. Transl. Med. 2022, 12, e954. [Google Scholar] [CrossRef]
- Jensen, J.B.; Dollerup, O.L.; Møller, A.B.; Billeskov, T.B.; Dalbram, E.; Chubanava, S.; Damgaard, M.V.; Dellinger, R.W.; Trošt, K.; Moritz, T.; et al. A randomized placebo-controlled trial of nicotinamide riboside+pterostilbene supplementation in experimental muscle injury in elderly subjects. JCI Insight 2022, 7, e158314. [Google Scholar] [CrossRef]
- Song, M.; Armenian, S.H.; Bhandari, R.; Lee, K.; Ness, K.; Putt, M.; Lindenfeld, L.; Manoukian, S.; Wade, K.; Dedio, A.; et al. Exercise training and NR supplementation to improve muscle mass and fitness in adolescent and young adult hematopoietic cell transplant survivors: A randomized controlled trial {1}. BMC Cancer 2022, 22, 795. [Google Scholar] [CrossRef]
- Freeberg, K.A.; Craighead, D.H.; Martens, C.R.; You, Z.; Chonchol, M.; Seals, D.R. Nicotinamide Riboside Supplementation for Treating Elevated Systolic Blood Pressure and Arterial Stiffness in Midlife and Older Adults. Front. Cardiovasc. Med. 2022, 9, 881703. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Baden, P.; Deleidi, M. Progresses in both basic research and clinical trials of NAD+ in Parkinson’s disease. Mech. Ageing Dev. 2021, 197, 111499. [Google Scholar] [CrossRef]
- Schöndorf, D.C.; Ivanyuk, D.; Baden, P.; Sanchez-Martinez, A.; De Cicco, S.; Yu, C.; Giunta, I.; Schwarz, L.K.; Di Napoli, G.; Panagiotakopoulou, V.; et al. The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson’s Disease. Cell Rep. 2018, 23, 2976–2988. [Google Scholar] [CrossRef]
- Brakedal, B.; Dölle, C.; Riemer, F.; Ma, Y.; Nido, G.S.; Skeie, G.O.; Craven, A.R.; Schwarzlmüller, T.; Brekke, N.; Diab, J.; et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 2022, 34, 396–407.e396. [Google Scholar] [CrossRef]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Liu, S.Z.; Ali, A.S.; Shankland, E.G.; Goss, C.; Amory, J.K.; Robertson, H.T.; Marcinek, D.J.; Conley, K.E. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PLoS ONE 2021, 16, e0253849. [Google Scholar] [CrossRef]
- Liu, S.Z.; Ali, A.S.; Campbell, M.D.; Kilroy, K.; Shankland, E.G.; Roshanravan, B.; Marcinek, D.J.; Conley, K.E. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachexia Sarcopenia Muscle 2018, 9, 826–833. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Valencia, A.P.; VanDoren, M.P.; Shankland, E.G.; Roshanravan, B.; Conley, K.E.; Marcinek, D.J. Astaxanthin supplementation enhances metabolic adaptation with aerobic training in the elderly. Physiol. Rep. 2021, 9, e14887. [Google Scholar] [CrossRef] [PubMed]
- Layec, G.; Gifford, J.R.; Trinity, J.D.; Hart, C.R.; Garten, R.S.; Park, S.Y.; Le Fur, Y.; Jeong, E.K.; Richardson, R.S. Accuracy and precision of quantitative 31P-MRS measurements of human skeletal muscle mitochondrial function. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E358–E366. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, X.H.; Zhang, Y.; Chen, W. Intracellular redox state revealed by in vivo (31) P MRS measurement of NAD(+) and NADH contents in brains. Magn. Reson. Med. 2014, 71, 1959–1972. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cohen, B.M.; Chen, X.; Lukas, S.E.; Shinn, A.K.; Yuksel, A.C.; Li, T.; Du, F.; Ongur, D. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr. Bull. 2017, 43, 197–204. [Google Scholar] [CrossRef]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grünewald, A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526 Pt 1, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD(+) in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef]
- Grange, R.M.H.; Sharma, R.; Shah, H.; Reinstadler, B.; Goldberger, O.; Cooper, M.K.; Nakagawa, A.; Miyazaki, Y.; Hindle, A.G.; Batten, A.J.; et al. Hypoxia ameliorates brain hyperoxia and NAD+ deficiency in a murine model of Leigh syndrome. Mol. Genet. Metab. 2021, 133, 83–93. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Chakraborty, A.D.; Light, C.M.; Tian, R.; Sadoshima, J.; Shi, X.; Gu, H.; Lee, C.F. NAD+ Redox Imbalance in the Heart Exacerbates Diabetic Cardiomyopathy. Circ. Heart Fail. 2021, 14, e008170. [Google Scholar] [CrossRef]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Ali, A.S.; Flores, B.; Jubrias, S.A.; Shankland, E.G. Mitochondrial NAD(P)H In vivo: Identifying Natural Indicators of Oxidative Phosphorylation in the (31)P Magnetic Resonance Spectrum. Front. Physiol. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Lee, B.Y.; Tuite, P.; Coles, L.; Sathe, A.G.; Chen, C.; Cloyd, J.; Low, W.C.; Steer, C.J.; Chen, W. Quantitative Assessment of Occipital Metabolic and Energetic Changes in Parkinson’s Patients, Using In Vivo. Metabolites 2021, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Lu, M.; Lee, B.Y.; Ugurbil, K.; Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhu, X.H.; Chen, W. In vivo (31) P MRS assessment of intracellular NAD metabolites and NAD(+)/NADH redox state in human brain at 4 T. NMR Biomed. 2016, 29, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Malloy, C.R.; Sherry, A.D. Quantitative measurement of redox state in human brain by. Magn. Reson. Med. 2020, 84, 2338–2351. [Google Scholar] [CrossRef] [PubMed]
- White, A.T.; Schenk, S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E308–E321. [Google Scholar] [CrossRef] [PubMed]
- Langley, M.R.; Choi, C.I.; Peclat, T.R.; Guo, Y.; Simon, W.L.; Yoon, H.; Kleppe, L.; Lucchinetti, C.F.; Chini, C.C.S.; Chini, E.N.; et al. Critical Role of Astrocyte NAD(+) Glycohydrolase in Myelin Injury and Regeneration. J. Neurosci. 2021, 41, 8644–8667. [Google Scholar] [CrossRef]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. 1), 216–231. [Google Scholar] [CrossRef]
- Yang, B.; Dan, X.; Hou, Y.; Lee, J.H.; Wechter, N.; Krishnamurthy, S.; Kimura, R.; Babbar, M.; Demarest, T.; McDevitt, R.; et al. NAD(+) supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell 2021, 20, e13329. [Google Scholar] [CrossRef]

| N = 33 (100%) | |
|---|---|
| Characteristics | |
| Male, n (%) | 24 (73%) |
| Female, n (%) | 9 (27%) |
| Age, mean | 72.5 |
| Symptom Severity | |
| Years since PD diagnosis, mean | 7.56 |
| Hoehn & Yahr 2, n (%) | 6 (22%) |
| Hoehn & Yahr 2.5, n (%) | 11 (40.7%) |
| Hoehn & Yahr 3, n (%) | 10 (37%) |
| UPDRS-Part 1, mean ± SD | 2.85 ± 1.94 |
| UPDRS-Part 2, mean ± SD | 10.93 ± 4.98 |
| UPDRS-Part 3, mean ± SD | 16.74 ± 7.07 |
| UPDRS-Part 4, mean ± SD | 4.04 ± 2.69 |
| UPDRS total, mean ± SD | 34.59 ± 12.62 |
| PRO-PD | |
| Mean (SD) | 815 (423) |
| Minimum | 275 |
| Maximum | 1763 |
| Quality of Life | |
| Excellent | 5 (18.5%) |
| Very Good | 10 (37%) |
| Good | 9 (33.3%) |
| Fair | 3 (11.1%) |
| Poor | 0 |
| PD (Mean ± SE) | Control | |
|---|---|---|
| Hand | ||
| Muscle Size | 259 ± 12.59 (n = 25) * | 218 ± 6.8 (n = 60) |
| Muscle Force | 18.81 ± 1.1 (n = 30) | 19.79 ± 0.5 (n = 135) |
| ATPmax | 0.61 ± 0.047 (n = 30) | 0.71 ± 0.02 (n = 134) |
| Leg | ||
| Muscle Size | 1189 ± 44.9 (n = 28) ** | 1021 ± 19.07 (n = 124) |
| Muscle Force | 76.99 ± 4.07 (n = 30) ** | 88.5 ± 1.9 (n = 145) |
| NADt [ATP = 8.2] | 1.060 ± 0.056 (n = 22) | 1.186 ± 0.0039 (n = 79) |
| NAD [ATP = 8.2] | 0.766 ± 0.051 (n = 22) * | 0.907 ± 0.038 (n = 79) |
| ATPmax | 0.656 ± 0.03 (n = 29) ** | 0.76 ± 0.017(n = 112) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mischley, L.K.; Shankland, E.; Liu, S.Z.; Bhayana, S.; Fox, D.J.; Marcinek, D.J. ATP and NAD+ Deficiency in Parkinson’s Disease. Nutrients 2023, 15, 943. https://doi.org/10.3390/nu15040943
Mischley LK, Shankland E, Liu SZ, Bhayana S, Fox DJ, Marcinek DJ. ATP and NAD+ Deficiency in Parkinson’s Disease. Nutrients. 2023; 15(4):943. https://doi.org/10.3390/nu15040943
Chicago/Turabian StyleMischley, Laurie K., Eric Shankland, Sophia Z. Liu, Saakshi Bhayana, Devon J. Fox, and David J. Marcinek. 2023. "ATP and NAD+ Deficiency in Parkinson’s Disease" Nutrients 15, no. 4: 943. https://doi.org/10.3390/nu15040943
APA StyleMischley, L. K., Shankland, E., Liu, S. Z., Bhayana, S., Fox, D. J., & Marcinek, D. J. (2023). ATP and NAD+ Deficiency in Parkinson’s Disease. Nutrients, 15(4), 943. https://doi.org/10.3390/nu15040943









