An Oxylipin-Related Nutrient Pattern and Risk of Type 1 Diabetes in the Diabetes Autoimmunity Study in the Young (DAISY)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. IA and T1D Case Definition
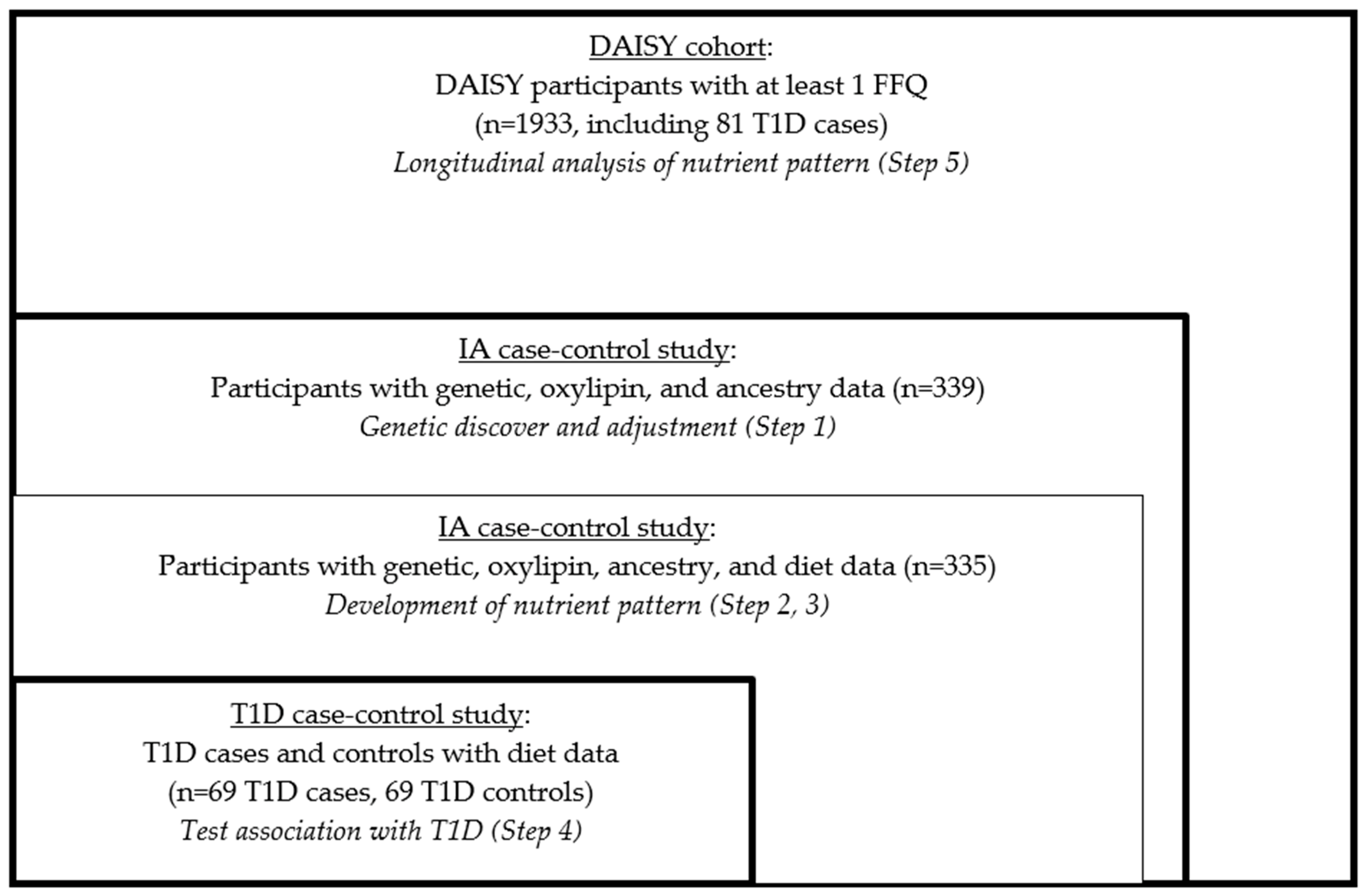
2.3. Nested Case-Control Study
- The earliest sample (at 9–15 months)
- The sample collected just prior to seroconversion (or age-matched visit for the IA controls)
- The sample collected just after seroconversion (i.e., the sample in which autoantibodies were first detected)
- The sample collected just prior to T1D diagnosis (for the T1D case-control study)
2.4. Measurement of Oxylipins
2.5. Genotyping
2.6. Dietary Assessment
2.7. Overview of Statistical Analysis
- We derived the genetically adjusted oxylipin PCs in the nested IA case-control study.
- We derived the average nutrient measures in the nested IA case-control study.
- We developed the genetically adjusted nutrient patterns in the nested IA case-control study using RRR.
- We tested the associations of the nutrient patterns with incident T1D risk in the nested T1D case-control study.
- We examined the validity of the findings in the full DAISY cohort.
- (1)
- Derivation of Genetically Adjusted Oxylipin PCs.
- (2)
- Derivation of Average Nutrient Measures
- (3)
- Nutrient Pattern Development.
- (4)
- Testing the association in the nested case-control study.
- (5)
- Nutrient patterns and risk of T1D longitudinally within the full DAISY cohort.
3. Results
3.1. T1D Nested Case-Control Characteristics
3.2. Development of Oxylipin Patterns
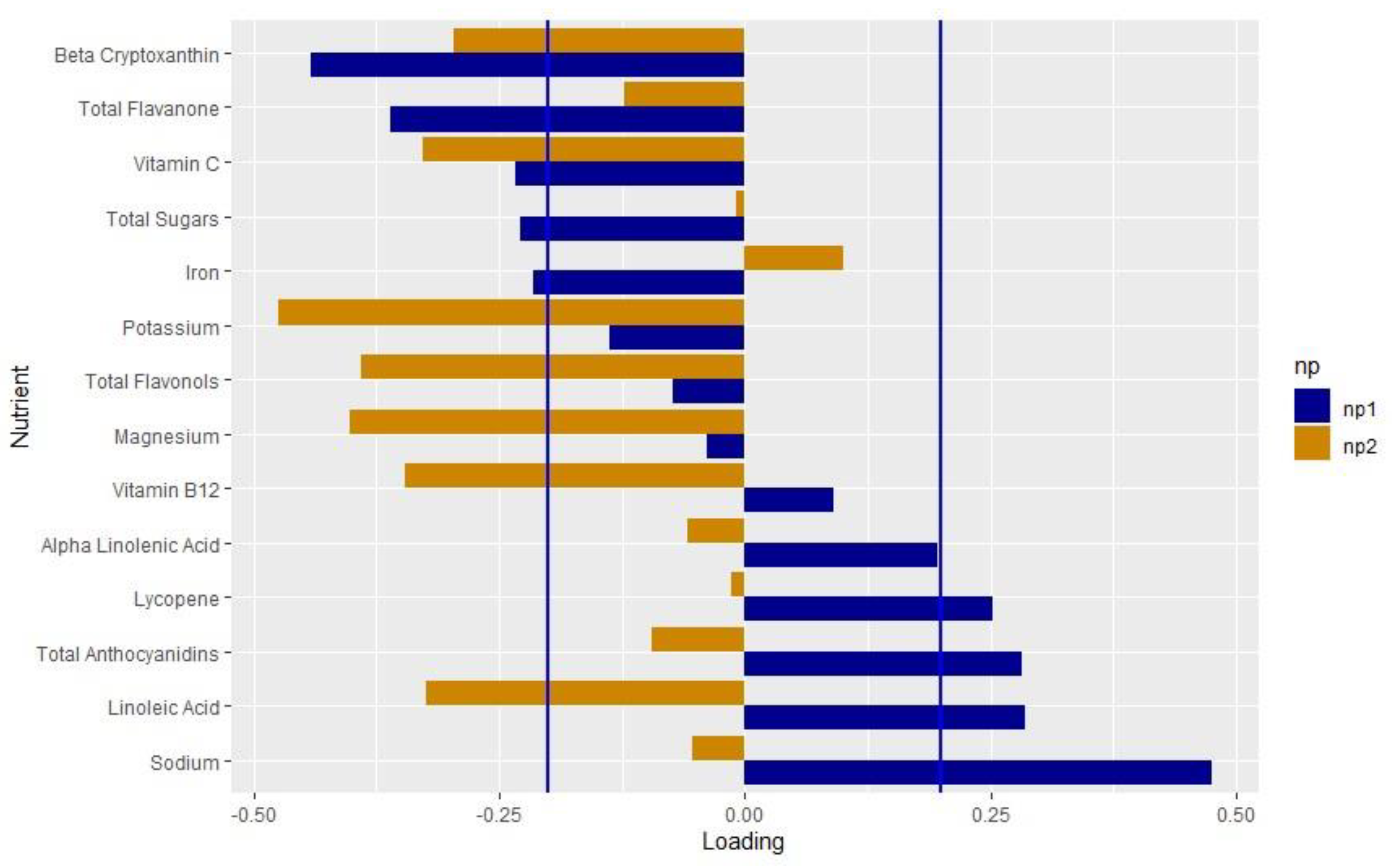
3.3. Development of Nutrient Pattern
3.4. Nested Case-Control Association with T1D
3.5. Longitudinal Association with T1D
4. Discussion
4.1. Interpretation of the Findings for Nutrient Pattern 1 (NP1)
4.2. Interpretation of Findings for Nutrient Pattern 2 (NP2)
4.3. Replication in the Full DAISY Cohort
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bach, J.F. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr. Rev. 1994, 15, 516–542. [Google Scholar] [CrossRef]
- Willcox, A.; Richardson, S.J.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009, 155, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Vehik, K.; Lynch, K.F.; Wong, M.C.; Tian, X.; Ross, M.C.; Gibbs, R.A.; Ajami, N.J.; Petrosino, J.F.; Rewers, M.; Toppari, J.; et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 2019, 25, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Stene, L.C.; Oikarinen, S.; Hyoty, H.; Tian, X.; Ross, M.C.; Gibbs, R.A.; Ajami, N.J.; Petrosino, J.F.; Rewers, M.; Toppari, J.; et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: The Diabetes and Autoimmunity Study in the Young (DAISY). Diabetes 2010, 59, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, V.S.E.; Xia, C.Q.; Clare-Salzler, M.J.; Horwitz, M.S. Type 1 Diabetes and Type 1 Interferonopathies: Localization of a Type 1 Common Thread of Virus Infection in the Pancreas. EBioMedicine 2017, 22, 10–17. [Google Scholar] [CrossRef]
- Mejia-Leon, M.E.; Barca, A.M. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients 2015, 7, 9171–9184. [Google Scholar] [CrossRef] [PubMed]
- Henschel, A.M.; Cabrera, S.M.; Kaldunski, M.L.; Jia, S.; Geoffrey, R.; Roethle, M.F.; Lam, V.; Chen, Y.G.; Wang, X.; Salzman, N.H.; et al. Modulation of the diet and gastrointestinal microbiota normalizes systemic inflammation and β-cell chemokine expression associated with autoimmune diabetes susceptibility. PLoS ONE 2018, 13, e0190351. [Google Scholar] [CrossRef]
- Needell, J.C.; Brown, M.N.; Zipris, D. Involvement of adipose tissue inflammation and dysfunction in virus-induced type 1 diabetes. J. Endocrinol. 2018, 238, 61–75. [Google Scholar] [CrossRef]
- Roep, B.O.; Kleijwegt, F.S.; van Halteren, A.G.; Bonato, V.; Boggi, U.; Vendrame, F.; Marchetti, P.; Dotta, F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin. Exp. Immunol. 2010, 159, 338–343. [Google Scholar] [CrossRef]
- Bergmann, L.; Kroncke, K.D.; Suschek, C.; Kolb, H.; Kolb-Bachofern, V. Cytotoxic action of IL-1 beta against pancreatic islets is mediated via nitric oxide formation and is inhibited by NG-monomethyl-L-arginine. FEBS Lett. 1992, 299, 103–106. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2017, 127, 1589. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Molteni, A.; Fuchtenbusch, M.; Naserke, H.E.; Ziegler, A.G.; Bonifacio, E. Reduced IL-4 associated antibody responses to vaccine in early pre-diabetes. Diabetologia 2002, 45, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Waugh, K.; Snell-Bergeon, J.; Michels, A.; Dong, F.; Steck, A.K.; Frohnert, B.I.; Norris, J.M.; Rewers, M. Increased inflammation is associated with islet autoimmunity and type 1 diabetes in the Diabetes Autoimmunity Study in the Young (DAISY). PLoS ONE 2017, 12, e0174840. [Google Scholar] [CrossRef]
- Buckner, T.; Vanderlinden, L.A.; DeFelice, B.C.; Carry, P.M.; Kechris, K.; Dong, F.; Fiehn, O.; Frohnert, B.I.; Clare-Salzler, M.; Rewers, M.; et al. The oxylipin profile is associated with development of type 1 diabetes: The Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 2021, 64, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Buckner, T.; Vanderlinden, L.A.; Johnson, R.K.; DeFelice, B.C.; Carry, P.M.; Seifert, J.; Waugh, K.; Dong, F.; Fiehn, O.; Clare-Salzler, M.; et al. Predictors of oxylipins in a healthy pediatric population. Pediatr. Res. 2021, 89, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Kroehl, M.; Fingerlin, T.E.; Frederiksen, B.N.; Seifert, J.; Wong, R.; Clare-Salzler, M.; Rewers, M. Erythrocyte membrane docosapentaenoic acid levels are associated with islet autoimmunity: The Diabetes Autoimmunity Study in the Young. Diabetologia 2014, 57, 295–304. [Google Scholar] [CrossRef]
- Norris, J.M.; Yin, X.; Lamb, M.M.; Barriga, K.; Seifert, J.; Hoffman, M.; Orton, H.D.; Baron, A.E.; Clare-Salzler, M.; Chase, H.P.; et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007, 298, 1420–1428. [Google Scholar] [CrossRef]
- Niinisto, S.; Takkinen, H.M.; Erlund, I.; Ahonen, S.; Toppari, J.; Ilonen, J.; Veijola, R.; Knip, M.; Vaarala, O.; Virtanen, S.M.; et al. Fatty acid status in infancy is associated with the risk of type 1 diabetes-associated autoimmunity. Diabetologia 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Hypponen, E.; Laara, E.; Reunanen, A.; Jarvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Cadario, F.; Savastio, S.; Rizzo, A.M.; Carrera, D.; Bona, G.; Ricordi, C. Can Type 1 diabetes progression be halted? Possible role of high dose vitamin D and omega 3 fatty acids. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1604–1609. [Google Scholar]
- Virtanen, S.M.; Niinisto, S.; Nevalainen, J.; Salminen, I.; Takkinen, H.M.; Kaaria, S.; Uusitalo, L.; Alfthan, G.; Kenward, M.G.; Veijola, R.; et al. Serum fatty acids and risk of advanced beta-cell autoimmunity: A nested case-control study among children with HLA-conferred susceptibility to type I diabetes. Eur. J. Clin. Nutr. 2010, 64, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Kosova, E.C.; Auinger, P.; Bremer, A.A. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J. Acad. Nutr. Diet 2013, 113, 219–227. [Google Scholar] [CrossRef] [PubMed]
- de Koning, L.; Malik, V.S.; Kellogg, M.D.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012, 125, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bielohuby, M.; Fleming, T.; Grabner, G.F.; Foppen, E.; Bernhard, W.; Guzman-Ruiz, M.; Layritz, C.; Legutko, B.; Zinser, E.; et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 2017, 6, 897–908. [Google Scholar] [CrossRef]
- Lamb, M.M.; Yin, X.; Barriga, K.; Hoffman, M.R.; Baron, A.E.; Eisenbarth, G.S.; Rewers, M.; Norris, J.M. Dietary glycemic index, development of islet autoimmunity, and subsequent progression to type 1 diabetes in young children. J. Clin. Endocrinol. Metab. 2008, 93, 3936–3942. [Google Scholar] [CrossRef]
- Lamb, M.M.; Frederiksen, B.; Seifert, J.A.; Kroehl, M.; Rewers, M.; Norris, J.M. Sugar intake is associated with progression from islet autoimmunity to type 1 diabetes: The Diabetes Autoimmunity Study in the Young. Diabetologia 2015, 58, 2027–2034. [Google Scholar] [CrossRef]
- Rewers, M.; Bugawan, T.L.; Norris, J.M.; Blair, A.; Beaty, B.; Hoffman, M.; McDuffie, R.S., Jr.; Hamman, R.F.; Klingensmith, G.; Eisenbarth, G.S.; et al. Newborn screening for HLA markers associated with IDDM: Diabetes autoimmunity study in the young (DAISY). Diabetologia 1996, 39, 807–812. [Google Scholar] [CrossRef]
- Barker, J.M.; Barriga, K.J.; Yu, L.; Miao, D.; Erlich, H.A.; Norris, J.M.; Eisenbarth, G.S.; Rewers, M. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J. Clin. Endocrinol. Metab. 2004, 89, 3896–3902. [Google Scholar] [CrossRef]
- Norris, J.M.; Barriga, K.; Klingensmith, G.; Hoffman, M.; Eisenbarth, G.S.; Erlich, H.A.; Rewers, M. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. Jama 2003, 290, 1713–1720. [Google Scholar] [CrossRef]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl 1), S17–S38. [CrossRef]
- Pedersen, T.L.; Newman, J.W. Establishing and Performing Targeted Multi-residue Analysis for Lipid Mediators and Fatty Acids in Small Clinical Plasma Samples. Methods Mol. Biol. 2018, 1730, 175–212. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Hyndman, R.; Athanasopoulos, G.; Bergmeir, C.; Caceres, G.; Chhay, L.; O’Hara-Wild, M.; Petropoulos, F.; Razbash, S.; Wang, E.; Yasmeen, F. _Forecast: Forecasting Functions for Time Series and Linear Models_. R Package Version 8.15. 2021. Available online: https://pkg.robjhyndman.com/forecast/ (accessed on 12 January 2023).
- Martínez-Arranz, I.; Mayo, R.; Pérez-Cormenzana, M.; Mincholé, I.; Salazar, L.; Alonso, C.; Mato, J.M. Data in support of enhancing metabolomics research through data mining. Data Brief 2015, 3, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Taliun, D.; Harris, D.N.; Kessler, M.D.; Carlson, J.; Szpiech, Z.A.; Torres, R.; Taliun, S.A.G.; Corvelo, A.; Gogarten, S.M.; Kang, H.M.; et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 2021, 590, 290–299. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Fuchsberger, C.; Abecasis, G.R.; Hinds, D.A. minimac2: Faster genotype imputation. Bioinformatics 2015, 31, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Shea, S.; Basch, C.E.; Contento, I.R.; Zybert, P. Consistency of the Willett semiquantitative food frequency questionnaire and 24-hour dietary recalls in estimating nutrient intakes of preschool children. Am. J. Epidemiol. 1992, 135, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Parrish, L.A.; Marshall, J.A.; Krebs, N.F.; Rewers, M.; Norris, J.M. Validation of a food frequency questionnaire in preschool children. Epidemiology (Camb. Mass.) 2003, 14, 213–217. [Google Scholar] [CrossRef]
- Rockett, H.R.; Breitenbach, M.; Frazier, A.L.; Witschi, J.; Wolf, A.M.; Field, A.E.; Colditz, G.A. Validation of a youth/adolescent food frequency questionnaire. Prev. Med. 1997, 26, 808–816. [Google Scholar] [CrossRef]
- Lamb, M.M.; Ross, C.A.; Brady, H.L.; Norris, J.M. Comparison of children’s diets as reported by the child via the Youth/Adolescent Questionnaire and the parent via the Willett food-frequency questionnaire. Public Health Nutr. 2007, 10, 663–670. [Google Scholar] [CrossRef]
- Appannah, G.; Pot, G.K.; O’Sullivan, T.A.; Oddy, W.H.; Jebb, S.A.; Ambrosini, G.L. The reliability of an adolescent dietary pattern identified using reduced-rank regression: Comparison of a FFQ and 3 d food record. Br. J. Nutr. 2014, 112, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. 4), 1220S–1228S, discussion 29S–31S. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br. J. Nutr. 2006, 95, 860–869. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Steffen, L.M. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am. J. Clin. Nutr. 2003, 78 (Suppl. 3), 508s–513s. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nothlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Weikert, C.; Schulze, M.B. Evaluating dietary patterns: The role of reduced rank regression. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 341–346. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T. An Introduction to Multivariate Statistical Analysis; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Rizopoulos, D.; Takkenberg, J.J. Tools & techniques--statistics: Dealing with time-varying covariates in survival analysis--joint models versus Cox models. EuroIntervention 2014, 10, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Manson, J.E.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; Colditz, G.A.; Hu, F.B. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am. J. Clin. Nutr. 2005, 82, 675–684. [Google Scholar] [CrossRef]
- Johnson, R.K.; Vanderlinden, L.A.; DeFelice, B.C.; Uusitalo, U.; Seifert, J.; Fan, S.; Crume, T.; Fiehn, O.; Rewers, M.; Kechris, K.; et al. Metabolomics-related nutrient patterns at seroconversion and risk of progression to type 1 diabetes. Pediatr. Diabetes 2020, 21, 1202–1209. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, Y.M.; Shin, M.H.; Lee, Y.H.; Om, A.S.; Kim, M.K. Development and validation of dietary atherogenic index using common carotid artery-intima-media thickness: A food frequency questionnaire-based longitudinal study in Korean adults. Nutr. Res. 2022, 104, 55–65. [Google Scholar] [CrossRef]
- Gu, Y.; Manly, J.J.; Mayeux, R.P.; Brickman, A.M. An Inflammation-related Nutrient Pattern is Associated with Both Brain and Cognitive Measures in a Multiethnic Elderly Population. Curr. Alzheimer Res. 2018, 15, 493–501. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Crandell, J.; Mendez, M.A.; Lamichhane, A.P.; Liu, W.; Ji, L.; Du, S.; Rosamond, W.; Popkin, B.M.; Mayer-Davis, E.J. Dietary patterns associated with HbA1c and LDL cholesterol among individuals with type 1 diabetes in China. J. Diabetes Its Complicat. 2015, 29, 343–349. [Google Scholar] [CrossRef]
- Liese, A.D.; Nichols, M.; Hodo, D.; Mellen, P.B.; Schulz, M.; Goff, D.C.; D’Agostino, R.B. Food intake patterns associated with carotid artery atherosclerosis in the Insulin Resistance Atherosclerosis Study. Br. J. Nutr. 2010, 103, 1471–1479. [Google Scholar] [CrossRef]
- Seah, J.Y.H.; Ong, C.N.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. A Dietary Pattern Derived from Reduced Rank Regression and Fatty Acid Biomarkers Is Associated with Lower Risk of Type 2 Diabetes and Coronary Artery Disease in Chinese Adults. J. Nutr. 2019, 149, 2001–2010. [Google Scholar] [CrossRef]
- Ishimiya, M.; Nakamura, H.; Kobayashi, Y.; Noguchi-Shinohara, M.; Abe, C.; Dohmoto, C.; Ikeda, Y.; Tokuno, K.; Ooi, K.; Yokokawa, M.; et al. Tooth loss-related dietary patterns and cognitive impairment in an elderly Japanese population: The Nakajima study. PLoS ONE 2018, 13, e0194504. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Fuzo, C.A.; Ued, F.V.; Kaput, J. Dietary patterns related to zinc and polyunsaturated fatty acids intake are associated with serum linoleic/dihomo-γ-linolenic ratio in NHANES males and females. Sci. Rep. 2021, 11, 12215. [Google Scholar] [CrossRef]
- Jacobs, S.; Kroeger, J.; Schulze, M.B.; Frank, L.K.; Franke, A.A.; Cheng, I.; Monroe, K.R.; Haiman, C.A.; Kolonel, L.N.; Wilkens, L.R.; et al. Dietary Patterns Derived by Reduced Rank Regression Are Inversely Associated with Type 2 Diabetes Risk across 5 Ethnic Groups in the Multiethnic Cohort. Curr. Dev. Nutr. 2017, 1, e000620. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Monsen, E.R. Vitamin C, the common cold, and iron absorption. Am. J. Clin. Nutr. 1977, 30, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Reddy, M.B. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am. J. Clin. Nutr. 2001, 73, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Brune, M.; Rossander, L. Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum. Nutr. Appl. Nutr. 1986, 40, 97–113. [Google Scholar] [PubMed]
- Sayers, M.H.; Lynch, S.R.; Charlton, R.W.; Bothwell, T.H.; Walker, R.B.; Mayet, F. Iron absorption from rice meals cooked with fortified salt containing ferrous sulphate and ascorbic acid. Br. J. Nutr. 1974, 31, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Størdal, K.; McArdle, H.J.; Hayes, H.; Tapia, G.; Viken, M.K.; Lund-Blix, N.A.; Haugen, M.; Joner, G.; Skrivarhaug, T.; Mårild, K.; et al. Prenatal iron exposure and childhood type 1 diabetes. Sci. Rep. 2018, 8, 9067. [Google Scholar] [CrossRef]
- Thorsen, S.U.; Halldorsson, T.I.; Bjerregaard, A.A.; Olsen, S.F.; Svensson, J. Maternal and Early Life Iron Intake and Risk of Childhood Type 1 Diabetes: A Danish Case-Cohort Study. Nutrients 2019, 11, 734. [Google Scholar] [CrossRef]
- Mattila, M.; Hakola, L.; Niinistö, S.; Tapanainen, H.; Takkinen, H.M.; Ahonen, S.; Ilonen, J.; Toppari, J.; Veijola, R.; Knip, M.; et al. Maternal Vitamin C and Iron Intake during Pregnancy and the Risk of Islet Autoimmunity and Type 1 Diabetes in Children: A Birth Cohort Study. Nutrients 2021, 13, 928. [Google Scholar] [CrossRef]
- Yip, L.; Alkhataybeh, R.; Taylor, C.; Fuhlbrigge, R.; Fathman, C.G. Identification of Novel Disease-Relevant Genes and Pathways in the Pathogenesis of Type 1 Diabetes: A Potential Defect in Pancreatic Iron Homeostasis. Diabetes 2022, 71, 1490–1507. [Google Scholar] [CrossRef]
- Dahlquist, G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia 2006, 49, 20–24. [Google Scholar] [CrossRef]
- Croden, J.; Silva, J.R.; Huang, W.; Gupta, N.; Fu, W.; Matovinovic, K.; Black, M.; Li, X.; Chen, K.; Wu, Y.; et al. Cyanidin-3-O-Glucoside improves the viability of human islet cells treated with amylin or Aβ1-42 in vitro. PLoS ONE 2021, 16, e0258208. [Google Scholar] [CrossRef]
- Li, C.; Yang, B.; Xu, Z.; Boivin, E.; Black, M.; Huang, W.; Xu, B.; Wu, P.; Zhang, B.; Li, X.; et al. Protective effect of cyanidin-3-O-glucoside on neonatal porcine islets. J. Endocrinol. 2017, 235, 237–249. [Google Scholar] [CrossRef]
- Zhang, B.; Buya, M.; Qin, W.; Sun, C.; Cai, H.; Xie, Q.; Xu, B.; Wu, Y. Anthocyanins from Chinese bayberry extract activate transcription factor Nrf2 in β cells and negatively regulate oxidative stress-induced autophagy. J. Agric. Food Chem. 2013, 61, 8765–8772. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.Y.; Zhang, Q.; Sha, W.; Kay, C.D.; Chandra, P.; Kay, K.L.; Lila, M.A. Blueberry and/or Banana Consumption Mitigate Arachidonic, Cytochrome P450 Oxylipin Generation During Recovery From 75-Km Cycling: A Randomized Trial. Front. Nutr. 2020, 7, 121. [Google Scholar] [CrossRef]
- Dreisbach, A.W.; Rice, J.C.; Japa, S.; Newman, J.W.; Sigel, A.; Gill, R.S.; Hess, A.E.; Cemo, A.C.; Fonseca, J.P.; Hammock, B.D.; et al. Salt loading increases urinary excretion of linoleic acid diols and triols in healthy human subjects. Hypertension 2008, 51, 755–761. [Google Scholar] [CrossRef]
- Das, U.N. Molecular biochemical aspects of salt (sodium chloride) in inflammation and immune response with reference to hypertension and type 2 diabetes mellitus. Lipids Health Dis. 2021, 20, 83. [Google Scholar] [CrossRef]
- He, W.; Zhang, M.; Zhao, M.; Davis, L.S.; Blackwell, T.S.; Yull, F.; Breyer, M.D.; Hao, C.M. Increased dietary sodium induces COX2 expression by activating NFκB in renal medullary interstitial cells. Pflugers Arch. 2014, 466, 357–367. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, M.; He, W.; Milne, G.L.; Howard, J.R.; Morrow, J.; Hébert, R.L.; Breyer, R.M.; Chen, J.; Hao, C.M. Increased dietary NaCl induces renal medullary PGE2 production and natriuresis via the EP2 receptor. Am. J. Physiol. Renal. Physiol. 2008, 295, F818–F825. [Google Scholar] [CrossRef]
- Niinistö, S.; Erlund, I.; Lee, H.S.; Uusitalo, U.; Salminen, I.; Aronsson, C.A.; Parikh, H.M.; Liu, X.; Hummel, S.; Toppari, J.; et al. Children’s erythrocyte fatty acids are associated with the risk of islet autoimmunity. Sci. Rep. 2021, 11, 3627. [Google Scholar] [CrossRef]
- Zhao, J.V.; Schooling, C.M. The role of linoleic acid in asthma and inflammatory markers: A Mendelian randomization study. Am. J. Clin. Nutr. 2019, 110, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H. Role of linoleic acid in autoimmune disorders: A Mendelian randomisation study. Ann. Rheum. Dis. 2018, 78, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Andres Contreras, G.; De Koster, J.; de Souza, J.; Laguna, J.; Mavangira, V.; Nelli, R.K.; Gandy, J.; Lock, A.L.; Sordillo, L.M. Lipolysis modulates the biosynthesis of inflammatory lipid mediators derived from linoleic acid in adipose tissue of periparturient dairy cows. J. Dairy Sci. 2019, 103, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.Y.; Yeh, T.S.; Chiu, Y.H.; Lee, Y.C.; Cheng, H.H.; Hsieh, R.H. Linoleic acid promotes mitochondrial biogenesis and maintains mitochondrial structure for prevention of streptozotocin damage in RIN-m5F cells. Biosci. Biotechnol. Biochem. 2009, 73, 1262–1267. [Google Scholar] [CrossRef]
- Wopereis, S.; Wolvers, D.; van Erk, M.; Gribnau, M.; Kremer, B.; van Dorsten, F.A.; Boelsma, E.; Garczarek, U.; Cnubben, N.; Frenken, L.; et al. Assessment of inflammatory resilience in healthy subjects using dietary lipid and glucose challenges. BMC Med. Genom. 2013, 6, 44. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. (Bethesda Md.) 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Cong, X.; Tracy, M.; Edmunds, L.S.; Hosler, A.S.; Appleton, A.A. The relationship between inflammatory dietary pattern in childhood and depression in early adulthood. Brain Behav. Immun. Health 2020, 2, 100017. [Google Scholar] [CrossRef]
- Liu, Q.; Wen, Q.; Lv, J.; Shi, Z.; Guo, Y.; Pei, P.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; et al. The Prospective Associations of Lipid Metabolism-Related Dietary Patterns with the Risk of Diabetes in Chinese Adults. Nutrients 2022, 14, 980. [Google Scholar] [CrossRef]
- Sun, Q.; Wen, Q.; Lyu, J.; Sun, D.; Ma, Y.; Man, S.; Yin, J.; Jin, C.; Tong, M.; Wang, B.; et al. Dietary pattern derived by reduced-rank regression and cardiovascular disease: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 337–345. [Google Scholar] [CrossRef]
- Osei, T.B.; van Dijk, A.M.; Dingerink, S.; Chilunga, F.P.; Beune, E.; Meeks, K.A.C.; Bahendeka, S.; Schulze, M.B.; Agyemang, C.; Nicolaou, M.; et al. Reduced Rank Regression-Derived Dietary Patterns Related to the Fatty Liver Index and Associations with Type 2 Diabetes Mellitus among Ghanaian Populations under Transition: The RODAM Study. Nutrients 2021, 13, 3679. [Google Scholar] [CrossRef]
- Vermeulen, E.; Stronks, K.; Visser, M.; Brouwer, I.A.; Snijder, M.B.; Mocking, R.J.T.; Derks, E.M.; Schene, A.H.; Nicolaou, M. Dietary pattern derived by reduced rank regression and depressive symptoms in a multi-ethnic population: The HELIUS study. Eur. J. Clin. Nutr. 2017, 71, 987–994. [Google Scholar] [CrossRef]
- Sartorelli, D.S.; Zuccolotto, D.C.C.; Crivellenti, L.C.; Franco, L.J. Dietary patterns during pregnancy derived by reduced-rank regression and their association with gestational diabetes mellitus. Nutrition 2019, 60, 191–196. [Google Scholar] [CrossRef]
- Barker, A.; Lauria, A.; Schloot, N.; Hosszufalusi, N.; Ludvigsson, J.; Mathieu, C.; Mauricio, D.; Nordwall, M.; Van der Schueren, B.; Mandrup-Poulsen, T.; et al. Age-dependent decline of β-cell function in type 1 diabetes after diagnosis: A multi-centre longitudinal study. Diabetes Obes. Metab. 2014, 16, 262–267. [Google Scholar] [CrossRef]
- Rasoul, M.A.; Al-Mahdi, M.; Al-Kandari, H.; Dhaunsi, G.S.; Haider, M.Z. Low serum vitamin-D status is associated with high prevalence and early onset of type-1 diabetes mellitus in Kuwaiti children. BMC Pediatr. 2016, 16, 95. [Google Scholar] [CrossRef]
- Espino-Paisan, L.; de la Calle, H.; Fernández-Arquero, M.; Figueredo, M.A.; de la Concha, E.G.; Urcelay, E.; Santiago, J.L. Polymorphisms in chromosome region 12q13 and their influence on age at onset of type 1 diabetes. Diabetologia 2011, 54, 2033–2037. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, H.; Yang, S.; Kim, D.; Park, Y. STAT4 polymorphism is associated with early-onset type 1 diabetes, but not with late-onset type 1 diabetes. Ann. N. Y. Acad. Sci. 2008, 1150, 93–98. [Google Scholar] [CrossRef]
| Characteristic | T1D Cases (n = 69) | Controls (n = 69) | p-Value |
|---|---|---|---|
| Sex (female) | 33 (47.8) | 29 (42.0) | 0.4936 |
| Non-Hispanic White Ethnicity (yes) | 61 (88.4) | 63 (91.3) | 0.5728 |
| HLA–DR3/4 Genotype (yes) | 34 (49.3) | 13 (18.8) | 0.0002 |
| First Degree Relative with T1D (yes) | 45 (65.2) | 39 (56.5) | 0.2953 |
| Age at T1D Diagnosis | 9.7 ± 4.5 | n/a |
| Univariate Association Between the Nutrients and Genetically Adjusted Oxylipin PC1 | ||
|---|---|---|
| Nutrient | beta estimate | p-Value |
| Sodium | 0.113 | 0.0014 |
| Beta Cryptoxanthin | −0.106 | 0.0026 |
| Total Flavanone | −0.086 | 0.0148 |
| Total Anthocyanidins | 0.068 | 0.0605 |
| Linoleic Acid | 0.068 | 0.0630 |
| Lycopene | 0.060 | 0.0922 |
| Vitamin C | −0.058 | 0.1070 |
| Total Sugars | −0.056 | 0.1274 |
| Iron | −0.051 | 0.1526 |
| Alpha Linolenic Acid | 0.047 | 0.1933 |
| Univariate Association Between Nutrients and Genetically Adjusted Oxylipin PC2 | ||
| Nutrient | beta estimate | p-value |
| Potassium | −0.071 | 0.0534 |
| Magnesium | −0.061 | 0.0979 |
| Total Flavonols | −0.057 | 0.1094 |
| Vitamin B12 | −0.052 | 0.1477 |
| Linoleic Acid | −0.052 | 0.1581 |
| Vitamin C | −0.047 | 0.1948 |
| Nutrient Pattern | OR | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| NP1 | 0.442 | 0.233 | 0.840 | 0.0126 |
| NP2 | 0.560 | 0.294 | 1.181 | 0.1362 |
| Variable | Yes T1D (n = 81) | No T1D (n = 1852) | p-Value |
|---|---|---|---|
| Sex (female) | 40 (49.4) | 891 (48.1) | 0.8225 |
| Non-Hispanic White Ethnicity (yes) | 74 (91.4) | 1400 (75.6) | 0.0011 |
| HLA–DR3/4 Genotype (yes) | 34 (41.2) | 371 (20.0) | <0.0001 |
| First Degree Relative with T1D (yes) | 55 (67.9) | 925 (50.0) | 0.0016 |
| Age at T1D Diagnosis | 10.7 ± 5.4 | n/a |
| Shared Parameter | HR | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| Original NP1 (Average) | 0.54 | 0.22 | 1.333 | 0.1829 |
| Original NP1 (Cumulative) | 0.98 | 0.84 | 1.129 | 0.7310 |
| Simplified NP1 (Average) | 0.52 | 0.22 | 1.240 | 0.1402 |
| Simplified NP1 (Cumulative) | 0.99 | 0.85 | 1.152 | 0.9054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckner, T.; Johnson, R.K.; Vanderlinden, L.A.; Carry, P.M.; Romero, A.; Onengut-Gumuscu, S.; Chen, W.-M.; Fiehn, O.; Frohnert, B.I.; Crume, T.; et al. An Oxylipin-Related Nutrient Pattern and Risk of Type 1 Diabetes in the Diabetes Autoimmunity Study in the Young (DAISY). Nutrients 2023, 15, 945. https://doi.org/10.3390/nu15040945
Buckner T, Johnson RK, Vanderlinden LA, Carry PM, Romero A, Onengut-Gumuscu S, Chen W-M, Fiehn O, Frohnert BI, Crume T, et al. An Oxylipin-Related Nutrient Pattern and Risk of Type 1 Diabetes in the Diabetes Autoimmunity Study in the Young (DAISY). Nutrients. 2023; 15(4):945. https://doi.org/10.3390/nu15040945
Chicago/Turabian StyleBuckner, Teresa, Randi K. Johnson, Lauren A. Vanderlinden, Patrick M. Carry, Alex Romero, Suna Onengut-Gumuscu, Wei-Min Chen, Oliver Fiehn, Brigitte I. Frohnert, Tessa Crume, and et al. 2023. "An Oxylipin-Related Nutrient Pattern and Risk of Type 1 Diabetes in the Diabetes Autoimmunity Study in the Young (DAISY)" Nutrients 15, no. 4: 945. https://doi.org/10.3390/nu15040945
APA StyleBuckner, T., Johnson, R. K., Vanderlinden, L. A., Carry, P. M., Romero, A., Onengut-Gumuscu, S., Chen, W.-M., Fiehn, O., Frohnert, B. I., Crume, T., Perng, W., Kechris, K., Rewers, M., & Norris, J. M. (2023). An Oxylipin-Related Nutrient Pattern and Risk of Type 1 Diabetes in the Diabetes Autoimmunity Study in the Young (DAISY). Nutrients, 15(4), 945. https://doi.org/10.3390/nu15040945









