Association between Cognitive Impairment and Malnutrition in Hemodialysis Patients: Two Sides of the Same Coin
Abstract
1. Introduction
2. Research Design and Methods
2.1. Study Design and Data Collection
2.2. Nutritional Evaluation
2.3. Cognitive Evaluation
2.4. Laboratory Data
2.5. Statistical Analysis
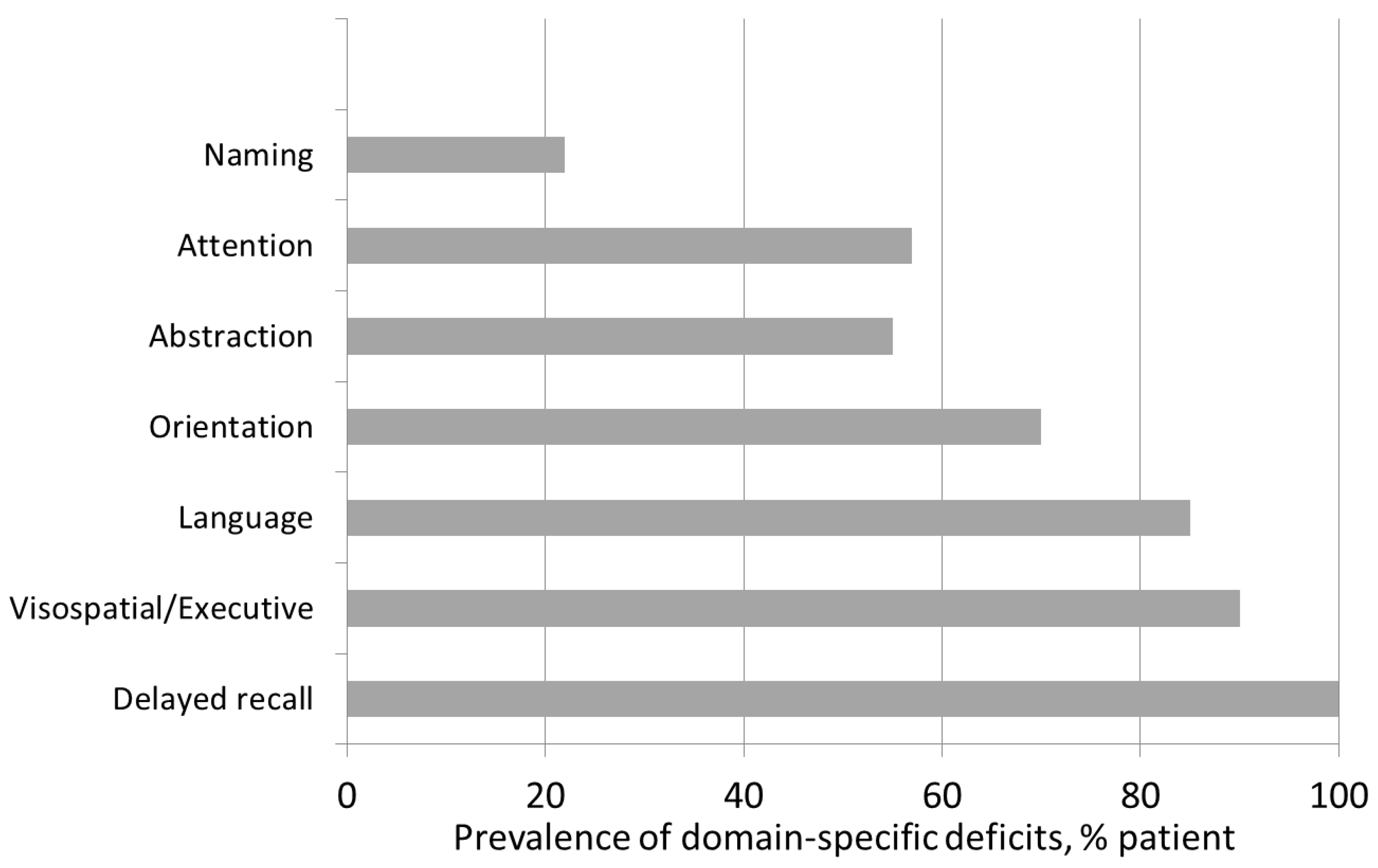
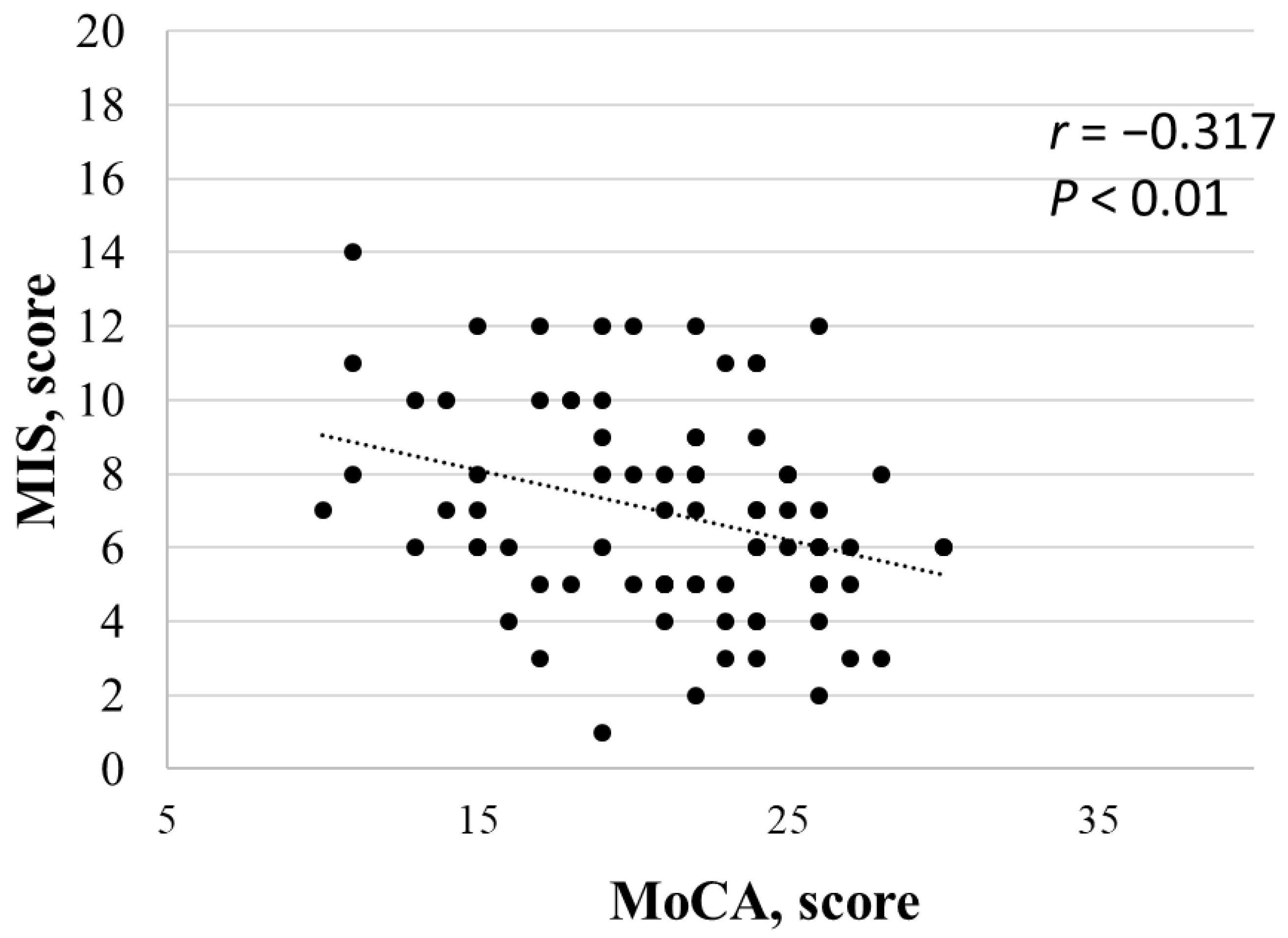
3. Results
4. Discussion
4.1. Nutritional Evaluation
4.2. Cognitive Evaluation
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipnicki, D.M.; Crawford, J.; Kochan, N.A.; Trollor, J.N.; Draper, B.; Reppermund, S.; Maston, K.; Mather, K.A.; Brodaty, H.; Sachdev, P.S.; et al. Risk factors for mild cognitive impairment, dementia and mortality: The Sydney Memory and Ageing Study. J. Am. Med. Dir. Assoc. 2017, 18, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Ackerson, L.; Tamura, M.K.; Manjula, K.T.; Kusek, J.W.; Sehgal, A.R.; Cohen, D.; Anderson, C.; Appel, L.; DeSalvo, K.; et al. Chronic Kidney Disease and Cognitive Function in Older Adults: Findings from the Chronic Renal Insufficiency Cohort Cognitive Study. J. Am. Geriatr. Soc. 2010, 58, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Sorensen, E.P.; Giang, L.M.; Drew, D.A.; Shaffi, K.; Strom, J.A.; Singh, A.K.; et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 2013, 80, 471–480. [Google Scholar] [CrossRef]
- van Zwieten, A.; Wong, G.; Ruospo, M.; Palmer, S.C.; Barulli, M.R.; Iurillo, A.; Saglimbene, V.; Natale, P.; Gargano, L.; Murgo, M.; et al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: The COG-NITIVE-HD study. Nephrol. Dial. Transpl. 2018, 33, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Pepin, M.; Franssen, C.F.; Viggiano, D.; Carriazo, S.; Gansevoort, R.T.; Gesualdo, L.; Hafez, G.; Malyszko, J.; Mayer, C.; et al. Chronic kidney disease and neurological disorders: Are uraemic toxins the missing piece of the puzzle? Nephrol. Dial. Transpl. 2021, 37, ii33–ii44. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Weiner, D.E.; Sarnak, M.J. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am. J. Kidney Dis. 2019, 74, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef]
- Fujisaki, K.; Tsuruya, K.; Yamato, M.; Toyonaga, J.; Noguchi, H.; Nakano, T.; Taniguchi, M.; Tokumoto, M.; Hirakata, H.; Kitazono, T. Cerebral oxidative stress induces spatial working memory dysfunction in uremic mice:neuroprotective effect of tempol. Nephrol. Dial. Transpl. 2014, 29, 529–538. [Google Scholar] [CrossRef]
- Guenzani, D.; Buoli, M.; Caldiroli, L.; Carnevali, G.S.; Serati, M.; Vezza, C.; Armelloni, S.; Messa, P.; Vettoretti, S. Malnutrition and inflammation are associated with severity of depressive and cognitive symptoms of old patients affected by chronic kidney disease. J. Psychosom. Res. 2019, 124, 109783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wang, H.; Hou, B.; You, H.; Yuan, J.; Luo, K.; Chen, L.; Li, M.; Xu, Q.; Zhu, Y.; et al. Malnutrition-inflammation is a risk factor for cerebral small vessel diseases and cognitive decline in peritoneal dialysis patients: A cross-sectional observational study. BMC Nephrol. 2017, 18, 366. [Google Scholar] [CrossRef] [PubMed]
- Abdulan, I.M.; Onofriescu, M.; Stefaniu, R.; Mastaleru, A.; Mocanu, V.; Alexa, I.D.; Covic, A. The predictive value of malnutrition for functional and cognitive status in elderly he-modialysis patients. Int. Urol. Nephrol. 2019, 51, 155–162. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Chen, X.; Han, P.; Song, P.; Zhao, Y.; Zhang, H.; Niu, J.; Yu, C.; Ding, W.; Zhao, J.; Zhang, L.; et al. Mediating Effects of Malnutrition on the Relationship between Depressive Symptoms Clusters and Muscle Function Rather than Muscle Mass in Older Hemodialysis Patients. J. Nutr. Health Aging 2022, 26, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Barello, S.; Anderson, G.; Acampora, M.; Bosio, C.; Guida, E.; Irace, V.; Guastoni, C.M.; Bertani, B.; Graffigna, G. The effect of psychosocial interventions on depression, anxiety, and quality of life in hemodialysis patients: A systematic review and a meta-analysis. Int. Urol. Nephrol. 2022. [published online ahead of print, 2022 Oct 1]. [Google Scholar] [CrossRef]
- Notaras, S.; Lambert, K.; Perz, J.; Makris, A. Diet in the management of non-dialysis dependent chronic kidney disease: Perceptions and practices of health professionals. BMC Nephrol. 2022, 23, 158. [Google Scholar] [CrossRef]
- Yang, Y.; Da, J.; Li, Q.; Long, Y.; Yuan, J.; Zha, Y. The Impact of Malnutrition, Inflammation on Cognitive Impairment in Hemodialysis Patients: A Multicenter Study. Kidney Blood Press. Res. 2022, 47, 711–721. [Google Scholar] [CrossRef]
- Avesani, C.M.; Sabatino, A.; Guerra, A.; Rodrigues, J.; Carrero, J.J.; Rossi, G.M.; Garibotto, G.; Stenvinkel, P.; Fiaccadori, E.; Lindholm, B. A Comparative Analysis of Nutritional Assessment Using Global Leadership Initiative on Malnutrition Versus Subjective Global Assessment and Malnutrition Inflammation Score in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2022, 32, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Santin, F.; Brito, F.D.S.B.; Lindholm, B.; Stenvinkel, P.; Avesani, C.M. Nutritional status of older patients on hemodialysis: Which nutritional markers can best predict clinical outcomes? Nutrition 2019, 65, 113–119. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Gurreebun, F.; Hartley, G.H.; Brown, A.L.; Ward, M.C.; Goodship, T.H. Nutritional screening in patients on hemodialysis: Is subjective global assessment an appropriate tool? J. Ren. Nutr. 2007, 17, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Friedman, A.N.; Fadem, S.Z. Reassessment of Albumin as a Nutritional Marker in Kidney Disease. J. Am. Soc. Nephrol. 2010, 21, 223–230. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, J.; Chen, J.; Liu, X.; Cai, Q.; Liu, P.; Huang, H. MICS, an easily ignored contributor to arterial calcification in CKD patients. Am. J. Physiol. Physiol. 2016, 311, F663–F670. [Google Scholar] [CrossRef]
- Dukkipati, R.; Kovesdy, C.P.; Colman, S.; Budoff, M.J.; Nissenson, A.R.; Sprague, S.M.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Relatively Low Serum Parathyroid Hormone With Malnutrition-Inflammation Complex and Survival in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2010, 20, 243–254. [Google Scholar] [CrossRef]
- Carlstedt, E.; Ridefelt, P.; Lind, L.; Rastad, J. Interleukin-6 Induced Suppression of Bovine Parathyroid Hormone Secretion. Biosci. Rep. 1999, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, L.; de Carvalho, A.B.; Avesani, C.M.; Kamimura, M.A.; Tilde]O, R.A.A.R.d.S.L.; Draibe, S.A.A.A. Increased Resting Energy Expenditure in Hemodialysis Patients with Severe Hyperparathyroidism. J. Am. Soc. Nephrol. 2004, 15, 2933–2939. [Google Scholar] [CrossRef]
- Kir, S.; Komaba, H.; Garcia, A.P.; Economopoulos, K.P.; Liu, W.; Lanske, B.; Hodin, R.A.; Spiegelman, B.M. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016, 23, 315–323. [Google Scholar] [CrossRef]
- Disthabanchong, S.; Vantanasiri, K.; Khunapornphairote, S.; Chansomboon, P.; Buachum, N.; Saeseow, S. Severe hyperparathyroidism is associated with nutritional impairment in maintenance hemodialysis patients. Front. Nutr. 2022, 9, 933918. [Google Scholar] [CrossRef]
- Kocyigit, S.E.; Bulut, E.A.; Aydin, A.E.; Isik, A.T. Improvement of nutritional status enhances cognitive and physical functions in older adults with orthostatic hypotension. Nutrition 2021, 90, 111261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-E.; Cheng, B.; Wang, Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: A systematic review and meta-analysis. Geriatr. Nurs. 2016, 37, 385–392. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Murray, A.M.; Guo, Y.-D.; Tian, R.; Ye, P.-P.; Li, X.; Li, G.-G.; Lu, F.-P.; Ma, Y.-C.; Sun, Y.; et al. Cognitive impairment and associated risk factors in older adult hemodialysis patients: A cross-sectional survey. Sci. Rep. 2020, 10, 12542. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.D.; Dawson, J.; Dickie, D.A.; Forbes, K.P.; McGlynn, D.; Quinn, T.; Mark, P.B. Investigating the relationship between cerebral blood flow and cognitive function in hemo-dialysis patients. J. Am. Soc. Nephrol. 2019, 30, 147–158. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Vargas-Vazquez, A.; Avila-Funes, J.A.; Aguilar-Salinas, C.A. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr. Diabetes Rev. 2019, 15, 456–470. [Google Scholar] [CrossRef]
- Weiner, D.E.; Scott, T.M.; Giang, L.M.; Agganis, B.T.; Sorensen, E.P.; Tighiouart, H.; Sarnak, M.J. Cardiovascular Disease and Cognitive Function in Maintenance Hemodialysis Patients. Am. J. Kidney Dis. 2011, 58, 773–781. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Huerta, M.; A González-Usigli, H.; Torres-Sánchez, E.D.; Delgado-Lara, D.L.; Pacheco-Moisés, F.P.; A Mireles-Ramírez, M.; Torres-Mendoza, B.M.; I Moreno-Cih, R.; E Velázquez-Brizuela, I. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes 2022, 13, 319–337. [Google Scholar] [CrossRef]
- Jones, D.J.; Harris, J.P.; Vaux, E.; Hadid, R.; Kean, R.; Butler, L.T. The nature of impairments of memory in patients with end-stage renal disease (ESRD). Physiol. Behav. 2015, 147, 324–333. [Google Scholar] [CrossRef]
- Ward, E.V.; Shanks, D.R. Implicit Memory and Cognitive Aging in Oxford. Res. Encycl. Psychol. 2018. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Paul, R.; Bhattacharya, P.; Borah, A. Neurological sequel of chronic kidney disease: From diminished Acetylcholinesterase activity to mitochondrial dysfunctions, oxidative stress and inflammation in mice brain. Sci. Rep. 2019, 9, 3097. [Google Scholar] [CrossRef]
- Mizumasa, T.; Hirakata, H.; Yoshimitsu, T.; Hirakata, E.; Kubo, M.; Kashiwagi, M.; Tanaka, H.; Kanai, H.; Fujimi, S.; Iida, M. Dialysis-Related Hypotension as a Cause of Progressive Frontal Lobe Atrophy in Chronic Hemodialysis Patients: A 3-Year Prospective Study. Nephron Clin. Pr. 2004, 97, c23–c30. [Google Scholar] [CrossRef]
- Verghese, J.; Lipton, R.B.; Hall, C.B.; Kuslansky, G.; Katz, M.J. Low blood pressure and the risk of dementia in very old individuals. Neurology 2003, 61, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Selvaskandan, H.; Hull, K.L.; Adenwalla, S.; Ahmed, S.; Cusu, M.C.; Graham-Brown, M.; Gray, L.; Hall, M.; Hamer, R.; Kanbar, A.; et al. Risk factors associated with COVID-19 severity among patients on maintenance hae-modialysis: A retrospective multicentre cross-sectional study in the UK. BMJ Open. 2022, 12, e054869. [Google Scholar] [CrossRef]
- Guidotti, R.; Pruijm, M.; Ambühl, P.M. COVID-19 Pandemic in Dialysis Patients: The Swiss Experience. Front. Public Health 2022, 10, 795701. [Google Scholar] [CrossRef] [PubMed]
- Beaumier, M.; Ficheux, M.; Couchoud, C.; Lassalle, M.; Launay, L.; Courivaud, C.; Tiple, A.; Lobbedez, T.; Chatelet, V. Is there sex disparity in vascular access at dialysis initiation in France? A mediation analysis using data from the Renal Epidemiology and Information Network registry. Clin. Kidney J. 2022, 15, 2144–2153. [Google Scholar] [CrossRef]
- Rusconi, M.L.; Suardi, A.; Zanetti, M.; Rozzini, L. Spatial navigation in elderly healthy subjects, amnestic and non amnestic MCI patients. J. Neurol. Sci. 2015, 359, 430–437. [Google Scholar] [CrossRef]
- Caffò, A.O.; Spano, G.; Tinella, L.; Lopez, A.; Ricciardi, E.; Stasolla, F.; Bosco, A. The Prevalence of Amnestic and Non-Amnestic Mild Cognitive Impairment and Its Association with Different Lifestyle Factors in a South Italian Elderly Population. Int. J. Environ. Res. Public Health 2022, 19, 3097. [Google Scholar] [CrossRef]
- Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef]
| n | 84 |
| Age, years | 75.8 (63.5–82.7) |
| Sex, M/F (%) | 44/40 (52/48) |
| BMI, Kg/m2 | 25.9 ± 6.4 |
| Dialysis vintage, months | 46.0 (22.1–66.9) |
Vascular access, n (%)
| 22 (26) 62 (74) |
Level of education, n (%)
| 8 (9.5) 47 (55.5) 29 (34.5) |
Comorbidities, n (%)
| 72 (86) 22 (26) 35 (42) |
| Pre-HD SBP, mmHg | 134.3 ± 17.8 |
| Post-HD SBP, mmHg | 133.1 ± 16.2 |
| Pre-HD DBP, mmHg | 71.5 ± 11.6 |
| Post-HD DBP, mmHg | 71.8 ± 10.4 |
| Calcium, mg/dL | 9.01 ± 0.59 |
| Phosphate, mg/dL | 5.16 ± 1.30 |
| Magnesium, meq/L | 1.91 ± 0.85 |
| 25D, ng/mL | 20.3 ± 7.0 |
| Uric acid, mg/dL | 5.9 ± 1.0 |
| PTH, pg/mL | 325.0 (194.5–496.7) |
| Hb, gr/dL | 10.7 ± 1.1 |
| CRP, mg/dL | 0.98 ± 1.86 |
| KT/V | 1.43 ± 0.24 |
| Pre-HD bicarbonate, mmol/L | 21.1 ± 2.8 |
| MoCA, score | 21.1 ± 2.8 |
| MCI, n (%) | 67 (80) |
| Malnourishment, n (%) | 34 (40) |
| Protein intake, gr/Kg/day | 1.04 ± 0.24 |
| Energy intake, Kcal/Kg/day | 24.9 ± 5.24 |
| Malnourished (n = 34) | Well-Nourished (n = 50) | p-Value | |
|---|---|---|---|
| Age, years | 79.8 (71.2–84.6) | 61.5 (47.4–70.0) | 0.011 |
| Sex, M/F (%) | 15/19 (44.1/55.9) | 29/21 (58.0/42.0) | 0.304 |
| Dialysis vintage, months | 56.0 (39.0–76.9) | 33.0 (20.0–59.0) | 0.018 |
Vascular access, n (%)
| 9 (26.5) 25 (73.5) | 13 (26.0) 37 (74.0) | 0.999 |
Levels of education, n (%)
| 19 (55.9) 15 (44.1) | 13 (26.0) 37 (74.0) | 0.006 |
Diabetes mellitus, n (%)
| 22 (64.7) 12 (35.3) | 40 (80.0) 10 (20.0) | 0.189 |
Arterial hypertension, n (%)
| 5 (14.7) 29 (85.3) | 7 (14.0) 43 (86.0) | 0.999 |
Cardiovascular disease, n (%)
| 19 (55.9) 15 (44.1) | 30 (60.0) 20 (40.0) | 0.880 |
| Pre-HD SBP, mmHg | 135.6 ± 19.3 | 134.5 ± 16.4 | 0.773 |
| Post-HD SBP, mmHg | 133.8 ± 17.3 | 132.9 ± 15.5 | 0.705 |
| Pre-HD DBP, mmHg | 70.2 ± 11.6 | 73.6 ± 10.4 | 0.072 |
| Post-HD DBP, mmHg | 69.1 ± 9.5 | 74.7 ± 9.8 | 0.023 |
| Calcium, mg/dL | 9.0 ± 0.6 | 9.0 ± 0.6 | 0.910 |
| Phosphate, mg/dL | 5.3 ± 1.5 | 5.0 ± 1.2 | 0.407 |
| Magnesium, meq/L | 2.01 ± 0.61 | 1.90 ± 0.91 | 0.970 |
| 25D, ng/mL | 21.5 ± 6.2 | 19.6 ± 8.2 | 0.072 |
| Uric acid, mg/dL | 5.8 ± 1.0 | 6.0 ± 1.1 | 0.556 |
| PTH, pg/mL | 361.0 (302.3–607.3) | 245.5 (177.3–407.8) | 0.014 |
| Hb, gr/dL | 10.9 ± 1.0 | 10.6 ± 1.1 | 0.260 |
| CRP, mg/dL | 1.68 ± 2.89 | 0.55 ± 0.38 | 0.002 |
| KT/V | 1.48 ± 0.22 | 1.49 ± 0.26 | 0.133 |
| Pre-HD bicarbonate, mmol/L | 21.8 ± 2.8 | 20.5 ± 2.8 | 0.065 |
| Albumin, gr/dL | 3.6 ± 0.3 | 3.8 ± 0.3 | 0.049 |
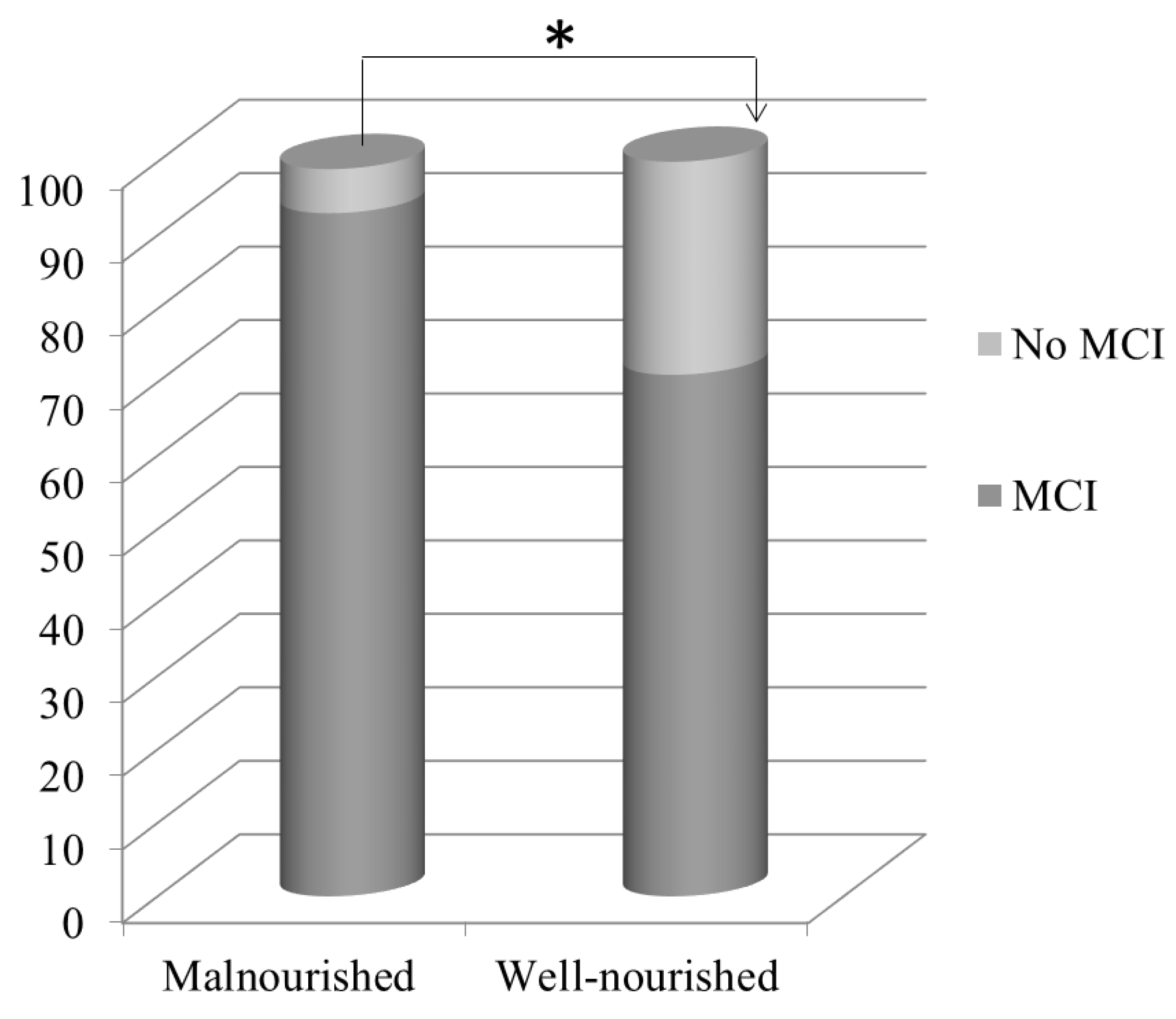
| MCI, n (%) | 29 (85) | 35 (70) | 0.014 |
| MoCA, score | 19.5 ± 4.6 | 22.1 ± 4.6 | 0.046 |
| MCI (n = 67) | No MCI (n = 17) | p-Value | |
|---|---|---|---|
| Age, years | 78.7 (72.1–84.5) | 61.5 (47.4–70.0) | <0.0001 |
| Sex, M/F (%) | 33/34; (49.2/50.8) | 10/7 (58.8/41.2) | 0.664 |
| Dialysis vintage, months | 46.0 (24.3–69.0) | 46.0 (19.0–59.0) | 0.470 |
Vascular access, n (%)
| 16 (23.9) 51 (76.1) | 7 (41.2) 10(58.8) | 0.261 |
Level of education, n (%)
| 31 (46.3) 36 (53.7) | 2 (11.7) 15 (88.3) | 0.011 |
Diabetes mellitus, n (%)
| 46 (68.7) 21 (31.3) | 16 (94.1) 1 (5.9) | 0.034 |
Arterial hypertension, n (%)
| 11 (16.4) 56 (83.5) | 1 (5.9) 16 (94.1) | 0.275 |
Cardiovascular disease, n (%)
| 40 (59.7) 27 (40.3) | 11 (64.7) 6 (35.3) | 0.920 |
| Pre-HD SBP, mmHg | 134.1 ± 18.2 | 135.7 ± 12.3 | 0.873 |
| Post-HD SBP, mmHg | 133.4 ± 15.9 | 132.2 ± 17.5 | 0.876 |
| Pre-HD DBP, mmHg | 69.6 ± 10.3 | 78.0 ± 8.1 | 0.006 |
| Post-HD DBP, mmHg | 70.8 ± 10.0 | 75.9 ± 9.8 | 0.069 |
| Calcium, mg/dL | 9.1 ± 0.6 | 8.7 ± 0.7 | 0.060 |
| Phosphate, mg/dL | 5.2 ± 1.4 | 5.3 ± 1.2 | 0.691 |
| Magnesium, meq/L | 1.92 ± 0.6 | 1.91 ± 0.91 | 0.930 |
| Uric acid, mg/dL | 5.9 ± 0.9 | 6.0 ± 1.5 | 0.839 |
| PTH, pg/mL | 325.0 (195.7–508.2) | 350.0 (213.0–413.0) | 0.930 |
| 25D, ng/mL | 20.6 ± 6.4 | 20.1 ± 7.5 | 0.332 |
| Hb, gr/dL | 10.7 ± 1.1 | 10.5 ± 1.3 | 0.839 |
| CRP, mg/dL | 0.79 ± 1.02 | 0.51 ± 0.29 | 0.361 |
| KT/V | 1.45 ± 0.21 | 1.42 ± 0.34 | 0.552 |
| Pre-HD bicarbonate, mmol/L | 21.0 ± 2.7 | 21.0 ± 3.3 | 0.548 |
| Albumin, gr/dL | 3.7 ± 0.4 | 3.8 ± 0.3 | 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotondi, S.; Tartaglione, L.; Pasquali, M.; Ceravolo, M.J.; Mitterhofer, A.P.; Noce, A.; Tavilla, M.; Lai, S.; Tinti, F.; Muci, M.L.; et al. Association between Cognitive Impairment and Malnutrition in Hemodialysis Patients: Two Sides of the Same Coin. Nutrients 2023, 15, 813. https://doi.org/10.3390/nu15040813
Rotondi S, Tartaglione L, Pasquali M, Ceravolo MJ, Mitterhofer AP, Noce A, Tavilla M, Lai S, Tinti F, Muci ML, et al. Association between Cognitive Impairment and Malnutrition in Hemodialysis Patients: Two Sides of the Same Coin. Nutrients. 2023; 15(4):813. https://doi.org/10.3390/nu15040813
Chicago/Turabian StyleRotondi, Silverio, Lida Tartaglione, Marzia Pasquali, Maria Josè Ceravolo, Anna Paola Mitterhofer, Annalisa Noce, Monica Tavilla, Silvia Lai, Francesca Tinti, Maria Luisa Muci, and et al. 2023. "Association between Cognitive Impairment and Malnutrition in Hemodialysis Patients: Two Sides of the Same Coin" Nutrients 15, no. 4: 813. https://doi.org/10.3390/nu15040813
APA StyleRotondi, S., Tartaglione, L., Pasquali, M., Ceravolo, M. J., Mitterhofer, A. P., Noce, A., Tavilla, M., Lai, S., Tinti, F., Muci, M. L., Farcomeni, A., & Mazzaferro, S. (2023). Association between Cognitive Impairment and Malnutrition in Hemodialysis Patients: Two Sides of the Same Coin. Nutrients, 15(4), 813. https://doi.org/10.3390/nu15040813






