Abstract
Previous studies have linked elevated plasma trimethylamine N-oxide (TMAO) levels to poor renal function. The relationship between TMAO and chronic kidney disease (CKD) in type 2 diabetes (T2D) is still unclear. We investigated the association between plasma TMAO levels and CKD in patients with T2D. A cross-sectional study of 133 patients with T2D with or without CKD has been conducted. Blood biomarkers of kidney function, diabetes, and inflammation were assessed in the study participants. Plasma TMAO levels were quantified using UPLC-MS/MS. People with T2D and CKD exhibited significantly higher plasma TMAO levels [10.16 (5.86–17.45) µmol/L] than those without CKD [4.69 (2.62–7.76) µmol/L] (p = 0.002). Participants in the highest quartile of TMAO levels (>8.38 µmol/L) presented relatively elevated serum creatinine levels and a higher number of people with CKD than those in the lower quartiles. TMAO levels were significantly correlated with kidney function biomarkers, including estimated glomerular filtration rate and urinary albumin to creatinine ratio. The association between TMAO and CKD was evident (p < 0.0001) and remained significant after adjusting for risk factors of kidney disease, including age, gender, body mass index, duration of diabetes, and smoking. These findings suggest the association between plasma TMAO and CKD in patients with T2D.
1. Introduction
Chronic kidney disease (CKD) has been recognised as a major public health issue, with a high morbidity and mortality burden worldwide [1]. Diabetes mellitus, hypertension, aging, and obesity have all been linked to the progression of CKD. Previous studies have reported that the global prevalence of CKD among patients with type 2 diabetes (T2D) is 42.3%, primarily identified at early stages [2]. Diabetic nephropathy accounts for the majority of CKD cases in diabetic patients [3], with albuminuria and a low glomerular filtration rate (GFR) being the key predictors of diabetic kidney disease [4]. Elevated cardiovascular risk has been associated with CKD and T2D, leading to microvascular degradation and disease progression [5,6,7].
Recently, the gut microbiota has been identified as playing a critical role in a number of chronic diseases [8]. TMAO is produced in the human body by the gut microbiota degradation of choline-containing compounds, L-carnitine, and betaine into trimethylamine (TMA), followed by oxidation by the flavin mono-oxygenase (FMO3) enzyme in the liver [9]. Trimethylamine N-oxide (TMAO) has been identified as a novel risk factor for cardiovascular diseases [9] and metabolic disorders, including T2D [10,11,12,13,14,15] and CKD [16]. However, the mechanism by which TMAO promotes its atherogenic effect in these diseases is not entirely understood. TMAO cannot be metabolised in the human body and is eliminated primarily through the kidneys via urine [17]. Therefore, patients with impaired renal excretion rate are at a high risk of accumulating TMAO in the circulation [16].
Cross-sectional, case-control, and longitudinal studies have consistently demonstrated the significant contribution of high blood TMAO levels to CKD progression [16,18,19,20,21,22]. A strong inverse correlation has also been observed between TMAO and GFR [14,16,18,19,20,23,24,25,26,27,28,29]. Impaired renal function has drastically affected TMAO levels in circulation and was associated with increased mortality risk [16]. Furthermore, TMAO levels have been found to be elevated in patients with end-stage renal disease and patients on haemodialysis compared to individuals with normal kidney function [19,20,30]. On the other hand, animal studies have reported that long-term exposure to elevated TMAO levels has contributed to collagen deposition and progressive tubulointerstitial fibrosis [16]. Systemic inflammation and inflammatory cytokines such as C-reactive protein (CPR) have also been correlated with TMAO in CKD patients [20,29]. Whether TMAO could be used as a biomarker for evaluating renal function remains unknown.
Diabetic patients are more likely to develop microvascular complications such as nephropathy, which can worsen blood and urinary biomarkers of kidney function [31]. Most studies published to date on the relationship between TMAO and CKD were focused on individuals with established CKD, either mild to moderate or end-stage, or on dialysis compared to healthy individuals. However, only limited studies investigated this association in patients with T2D [29,32]. This study examined the association between plasma TMAO levels and CKD in people with T2D.
2. Materials and Methods
2.1. Study Design and Population
People with T2D (n = 133) were recruited in a prospective case-control study as previously described [33]. The study was approved by the University of Newcastle Human Research Ethics Committee (H-2018-0138) and the King Saud University Institutional Review Board (E-18-3073). All procedures were performed in accordance with the Declaration of Helsinki. Patients with acute illnesses or infections, currently on any antimicrobials or probiotics within three months of enrolment, and on haemodialysis or peritoneal dialysis were excluded.
Out of n = 133 participants with T2D, n = 12 (9%) had clinically confirmed diagnosis of CKD based on the criteria of estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 and/or the presence of albuminuria (urinary albumin to creatinine ratio (UACR) >300 mg/gm). This study was adequately powered to detect a significant difference in TMAO levels among those with T2D, with or without CKD. After providing informed consent, blood samples and clinical data were obtained from all patients. Patient interviews and medical records were used to assess demographic information and comorbidities.
2.2. Markers of Diabetes, Renal Function, and Systemic Inflammation
Fasting plasma glucose (FPG) and glycosylated haemoglobin (HbA1c) levels were measured in all patients with T2D. Blood and urinary markers of kidney function were also measured, including serum creatinine, blood urea nitrogen (BUN), and electrolytes such as sodium (Na), potassium (K), calcium (Ca), phosphorus, urine creatinine, and UACR. The eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [34,35]. Other markers associated with CKD include haemoglobin, albumin, alkaline phosphatase (ALP), total bilirubin, gamma-glutamyl transferase (GGT), and inflammatory markers such as high sensitivity C-reactive protein (hs-CRP) have also been assessed. All biochemical parameters were analysed according to certified standard protocols at King Saud University Medical City (KSUMC) central laboratory using the Dimension Vista® 1500 Intelligent Lab System- version 3.10.2, DV311404 (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA).
2.3. Quantification of Trimethylamine N-Oxide (TMAO) Levels in Plasma
Plasma TMAO levels were quantified using stable isotope dilution ultra-high-performance liquid chromatography with electrospray ionisation tandem mass spectrometry (UPLC/MS/MS) by (Waters Corporation, Milford, MA, USA) with d9-(trimethyl)-labelled as internal standard, and Acquity UPLC BEH HILIC column [100 mm × 2.1 mm, 1.7 µm particle size] (Waters Corporation, Milford, MA, USA), as previously described [33,36]. The mobile gradient phase was composed of acetonitrile and 15 mmol/L ammonium formate (pH 3.5) at a flow rate of 0.4 mL/min. The multiple reaction monitoring (MRM) was at m/z 76.1→58.2 for TMAO and 85.04→66.04 for the internal standard d9- TMAO.
2.4. Statistical Analysis
All data analyses were conducted using SPSS (version 27, SPSS Inc., Chicago, IL, USA) and graphs were created using GraphPad Prism (version 9). Participants’ characteristics were summarised according to T2D cases with or without CKD. Laboratory parameters and biomarker values were described using percentages for categorical variables, mean ± standard deviation (SD) or medians (IQR), and 25th to 75th quartiles range for continuous variables. For normally distributed data, the unpaired t-test and one-way ANOVA test evaluated the differences between study groups. Nonparametric tests were used for the measurements of non-normally distrusted data. The Mann–Whitney U test was used to compare data between the two patient groups, while the Kruskal–Wallis test compared patient data between plasma TMAO quartiles. Comparisons between categorical variables were analyzed by Pearson’s chi-squared test or Fisher’s exact test. Spearman’s rank correlation coefficient was applied to assess the association between TMAO and biomarkers of kidney disease. Multiple linear regression analysis was performed to examine the association between TMAO and CKD, adjusting for demographic variables (e.g., age, gender, BMI, duration of T2D, and smoking) as well as biomarkers of kidney function (e.g., UACR and eGFR) using a backward stepwise approach. The variables included in Model 2 were incorporated in the nomogram to predict CKD risk using “rms” package in R software version 4.2.2. A significance level of 0.05 was used for all analyses.
3. Results
3.1. Subject Characteristics and and Metabolic Parameters
Characteristics and metabolic parameters of study participants (T2D with CKD, n = 12, and without CKD, n = 121) are displayed in Table 1. Subjects with T2D and CKD were older compared to those without CKD (63 vs. 55 years, p = 0.02), had T2D for a median of 20 years, were more likely to be smokers (25% vs. 7.4%, p = 0.043), and were more likely to have macroalbuminuria (33.3% vs. 4.13%, p = 0.004). No significant differences were observed between study participants with and without CKD in terms of BMI, gender, FPG, ALP, calcium, phosphorus, total bilirubin, UACR, urine creatinine, and hs-CRP. HbA1c, haemoglobin, serum creatinine, BUN, eGFR, albumin, K, Na, and GGT levels were significantly higher in CKD participants than in those without CKD.

Table 1.
Participant characteristics and metabolic parameters of patients with T2D, with or without CKD.
3.2. Plasma TMAO Levels and Markers of Kidney Disease
Patients with CKD exhibited significantly higher plasma TMAO levels [10.16 (5.86–17.45) µmol/L] than patients without CKD [4.69 (2.62–7.76) µmol/L] (p = 0.002) as presented in Table 1. The total study population was stratified into quartiles based on plasma TMAO distribution to assess the changes in characteristics and laboratory markers associated with TMAO levels, as indicated in Table 2. TMAO levels were less than 2.82 µmol/L in the lowest quartile (Q1) and greater than 8.38 µmol/L in the highest quartile (Q4). Notably, patients in the highest quartiles of TMAO levels had a greater proportion of CKD, higher levels of serum creatinine and BUN, and lower levels of eGFR (Table 2 and Figure 1). Other variables such as gender, BMI, laboratory markers (e.g., kidney function tests and electrolytes), and hs-CRP were not statistically significantly different across TMAO quartiles.

Table 2.
Participant characteristics by quartiles of TMAO levels.
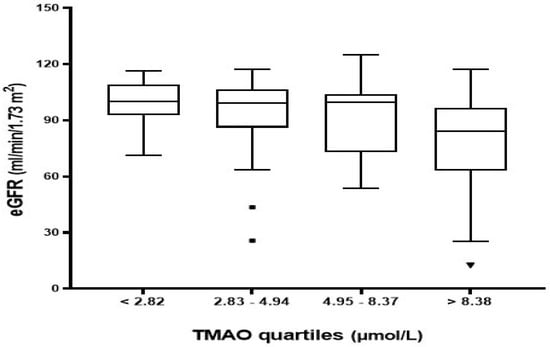
Figure 1.
Estimated GFR distribution across plasma TMAO quartiles. Q1 (<2.82), Q2 (2.83–4.94), Q3 (4.95–8.37), and Q4 (>8.38).
3.2.1. Correlation between Plasma TMAO Levels and Biomarkers of Kidney Disease
Plasma TMAO levels were positively correlated with age (rs = 0.198, p = 0.023), serum creatinine (rs = 0.266, p = 0.002), BUN (rs = 0.327, p < 0.0001), and UACR (rs = 0.183, p = 0.046) (Table 3). However, a significant inverse correlation was observed with eGFR (rs = −0.337, p < 0.0001) as shown in Figure 2.

Table 3.
Spearman’s rank correlation coefficient to assess the relationships between TMAO and markers of kidney function.
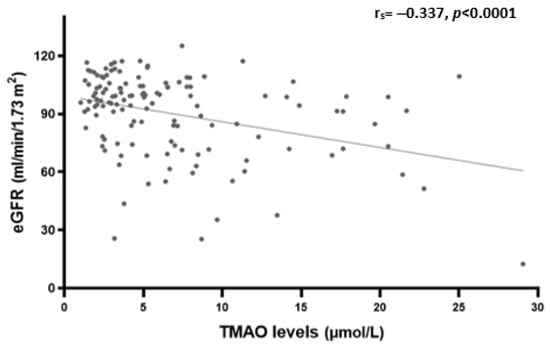
Figure 2.
Spearman’s rank correlation analysis between plasma TMAO levels and eGFR.
3.2.2. Association between Plasma TMAO Levels and CKD
Plasma TMAO levels were significantly associated with CKD in the non-adjusted Model 1 (β = 0.015, 95% CI [0.007, 0.024], p < 0.0001) as well as in Model 2 which adjusted for risk factors of CKD, including age, gender, BMI, duration of diabetes and smoking (β = 0.014, 95% CI [0.005, 0.022], p = 0.001) (Table 4). However, when the association was further adjusted for biomarkers of kidney disease including UACR and eGFR in models 3 and 4, respectively, plasma TMAO levels were no longer significantly associated with CKD as in Model 3 (β = 0.008, 95% CI [0.000, 0.016], p = 0.063) and Model 4 (β = 0.001, 95% CI [−0.005, 0.008], p = 0.706)].

Table 4.
Association between plasma TMAO levels and CKD using multiple linear regression models.
3.2.3. CKD Risk Prediction Nomogram
A nomogram was created to optimise the statistical predictive models into a single numerical probability estimate of CKD in the form of a graph. The nomogram was based on five parameters that were significant in multivariable analysis (Model 2) to predict the risk of CKD in T2D patients, including age, gender, BMI, diabetes duration, and smoking. The total point, which ranges from zero to two hundred and twenty, was computed by summing the points from each variable to determine CKD probability. The risk of CKD by total points was shown in the nomogram. The risk of CKD was lower than 10% for those below 90 points, and higher than 50% for those with over 200 points. The nomogram is shown in Figure 3.
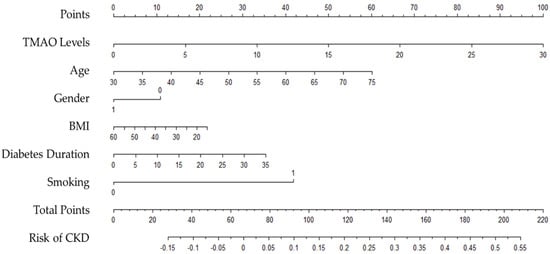
Figure 3.
Nomogram to predict the risk of CKD in people with type 2 diabetes.
Patients with more than 100 total points, for example, may have a 15% to 50% probability of developing CKD: TMAO levels greater than 7 µmol/L, age over 40 years, female gender, morbid obesity, BMI greater than 35 kg/m2, diabetes duration of more than 20 years, and smokers.
4. Discussion
This study explored the association between plasma TMAO levels and CKD in patients with T2D. Patients with CKD demonstrated higher levels of plasma TMAO than non-CKD patients. Plasma TMAO levels were positively correlated with biomarkers of renal function, including serum creatinine, BUN, and UACR, and were inversely correlated with eGFR. No significant relationship was found between plasma TMAO and other markers, including urine creatinine, serum electrolytes, ALP, total bilirubin, GGT, and hs-CRP. Elevated plasma TMAO levels were associated with CKD in the unadjusted model as well as when risk factors of CKD were adjusted for. However, the significant association was lost after further adjustment was made for CKD biomarkers, including UACR and eGFR. A simple monogram, based on the five variables that were significant in the multivariable analysis in Model 2 including TMAO level, age, BMI, gender, diabetes duration, and smoking demonstrated that TMAO levels contribute to the identification of T2D patients at high risk of developing CKD.
TMAO has been shown to aggravate kidney function decline and tubular interstitial injury, activate the inflammatory pathway via increasing p38 phosphorylation and human antigen R (HuR) level, upregulate NADPH oxidase 4 (NOX4), promote oxidative stress and nod-like receptor family pyrin domain containing three (NLRP3) inflammasome activation [37]. It has been speculated that TMAO can promote renal macrophage recruitment to induce tubular epithelial cell injury via the enhanced release of inflammatory cytokines [37]. CKD is also known to cause an imbalance in the gut microflora leading to a reduction in probiotics and a concurrent increase in toxigenic flora [38]; their toxic products may enter the host circulatory system and lead to sustained systemic inflammation.
Uncontrolled hyperglycaemia, particularly diabetes mellitus, remains a critical risk factor for CKD [39]. We [33] and others [13] have recently shown that TMAO levels are elevated in people with diabetes mellitus. In this study, we provide evidence that TMAO levels are further aggravated in those diagnosed with CKD. A recent study by Winther et al. showed that TMAO levels are elevated in individuals with T2D and albuminuria, placing them at a high risk of developing renal and cardiovascular disease [31], while the study by Al-Obaide et al. demonstrated higher TMAO levels in patients with T2D and advanced CKD [29]. In the current study, we report that TMAO levels are much higher in people with T2D who have progressed to develop CKD. Whether further increase in TMAO levels is the cause or consequence of progressing from T2D to CKD patients remains unknown. Higher plasma TMAO levels have been associated with an increased abundance of TMAO-producing bacteria in the intestinal microbiota of T2D patients with CKD [29]. In addition, TMAO is almost exclusively excreted by the kidneys [17], therefore, circulating levels can be expected to build up in CKD patients or those with renal impairment.
Accumulating evidence has demonstrated a significant association between TMAO and CKD [16,19,20,21,22], regardless of the patient’s diabetic status. However, the exact mechanism underlying the potential relationship between TMAO and CKD is still unclear. A linear incremental relationship has been noted between plasma TMAO levels and biomarkers of kidney histopathologic and functional impairment [16]. TMAO pathway has been found to contribute to kidney disease progression by dietary exposure to a choline-rich diet or TMAO directly, resulting in the development of tubule-interstitial fibrosis and dysfunction [16]. The study findings of an inverse correlation between TMAO and GFR substantiate previous findings suggesting the elimination of TMAO from the circulation to be mostly dependent on urinary excretion [22,40].
The study observations are in line with several previous studies in patients with cardiovascular diseases [23,24,25,26,27] and CKD [16,19,20,28]. Pelletier et al., on the other hand, observed only a modest correlation between TMAO and eGFR, which could be attributed to the study use of a different GFR formula than eGFR [22]. TMAO levels were also found to be positively correlated with serum creatinine and UACR as an early indicator of vascular injury in T2D, supporting earlier study findings [22,24,28,30]. In contrast to previous observations [20,30], this study found a nonsignificant correlation between TMAO levels and hs-CRP. The exact reason for the discrepancies in the findings between these studies and ours is unknown; however, these could be attributed to the study participants’ active disease and inflammatory state that may impact the outcome. TMAO metabolism is affected by the degree of CKD and haemodialysis status, as reported by other studies [20,30].
The association between plasma TMAO and CKD has been confirmed in the unadjusted model as well as after adjusting for CKD risk factors, including age, gender, BMI, diabetes duration, and smoking. However, when the model further adjusted for biomarkers of kidney disease such as UACR and eGFR, the association between TMAO and CKD was attenuated. This finding proposed a potential interaction between TMAO and biomarkers of kidney disease, suggesting TMAO as a surrogate marker for GFR and urine albumin as a predictor of poor outcomes in CKD patients [16]. Furthermore, our nomogram demonstrated that TMAO levels are one of the significant predictors of CKD progression in T2D patients. Increased TMAO levels have been shown to increase the likelihood of CKD development by up to 55% when combined with other T2D risk factors. This finding further supports the proposed link between TMAO and CKD in people with T2D.
The present study is the first in the Middle East to investigate the association between TMAO and blood markers of kidney disease in patients with T2D. We were able to understand the renal implications of TMAO and its correlation with poor renal outcomes in T2D patients. In this cross-sectional study, we have included only patients with a clinically confirmed diagnosis of T2D to control for any potential confounding produced by differences in diabetes diagnosis. The observational nature of the present study prohibits any inferences on the causality of TMAO and CKD in T2D patients. Patients from only one medical centre in the capital city of Saudi Arabia were included, limiting the generalizability of the findings. Further studies with a larger number of patients are warranted to verify the association between circulating TMAO levels and CKD with T2D.
In conclusion, our study has confirmed the association between circulating TMAO levels and CKD in a Saudi Arabian population where the dietary habits are different from the countries where the association has been previously established. Plasma TMAO levels are significantly associated with CKD, aggravated further in people with T2D, and correlated with biomarkers of renal function such as serum creatinine, eGFR, BUN, and UACR. Longitudinal and randomised controlled studies are warranted to establish if the progressive increase in TMAO and CKD is the cause or consequence of renal impairment in people with T2D. Identification of dietary and other lifestyle factors that can influence circulating TMAO levels may further enhance investigations into the prevention of T2D and CKD.
Author Contributions
Conceptualization, N.A.K., M.L.G. and K.A.A.; methodology, N.A.K., M.L.G. and K.A.A.; investigation, N.A.K.; data curation, N.A.K.; Statistical analysis, E.S., N.A.K. and R.N.T.; writing—original draft preparation, N.A.K.; writing—review and editing, M.L.G. and R.N.T.; supervision, M.L.G. and K.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the University of Newcastle Human Research Ethics Committee ((protocol code H-2018-0138, 5 July 2018) and the King Saud University Institutional Review Board (E-18-3073, 6 May 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available by contacting the corresponding author upon request.
Acknowledgments
The authors are grateful for the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for supporting the research and to the Central Laboratory at the College of Pharmacy, King Saud University for facilitating the lab analysis process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Bell, K.; Stanford, A.; Kern, D.M.; Tunceli, O.; Vupputuri, S.; Kalsekar, I.; Willey, V. Understanding CKD among patients with T2DM: Prevalence, temporal trends, and treatment patterns—NHANES 2007–2012. BMJ Open Diabetes Res. Care 2016, 4, e000154. [Google Scholar] [CrossRef]
- Soldatos, G.; Cooper, M.E. Diabetic nephropathy: Important pathophysiologic mechanisms. Diabetes Res. Clin. Pract. 2008, 82, S75–S79. [Google Scholar] [CrossRef]
- Ninomiya, T.; Perkovic, V.; de Galan, B.E.; Zoungas, S.; Pillai, A.; Jardine, M.; Patel, A.; Cass, A.; Neal, B.; Poulter, N.; et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. JASN 2009, 20, 1813–1821. [Google Scholar] [CrossRef]
- Pálsson, R.; Patel, U.D. Cardiovascular complications of diabetic kidney disease. Adv. Chronic. Kidney Dis. 2014, 21, 273–280. [Google Scholar] [CrossRef]
- Duran-Salgado, M.B.; Rubio-Guerra, A.F. Diabetic nephropathy and inflammation. World J. Diabetes 2014, 5, 393–398. [Google Scholar] [CrossRef]
- Shikata, K.; Makino, H. Microinflammation in the pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2013, 4, 142–149. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alcoholado, L.; Castellano-Castillo, D.; Jordán-Martínez, L.; Moreno-Indias, I.; Cardila-Cruz, P.; Elena, D.; Muñoz-Garcia, A.J.; Queipo-Ortuño, M.I.; Jimenez-Navarro, M. Role of gut microbiota on cardio-metabolic parameters and immunity in coronary artery disease patients with and without type-2 diabetes mellitus. Front. Microbiol. 2017, 8, 1936. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp. Clin. Endocrinol. Diabetes 2016, 124, 251–256. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: An observational study. PloS ONE 2014, 9, e114969. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Awwad, H.M.; Rabagny, Y.; Graeber, S.; Herrmann, W.; Geisel, J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016, 103, 703–711. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, B.; Vinge, E.; Skerfving, S. Pharmacokinetics of triethylamine and triethylamine-N-oxide in man. Toxicol. Appl. Pharmacol. 1989, 100, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, M.; Fang, X.; Teng, F.; Tan, X.; Li, X.; Wang, M.; Long, Y.; Xu, Y. Gut microbiota-derived trimethylamine N-oxide and kidney function: A systematic review and meta-Analysis. Adv. Nutr. 2021, 12, 1286–1304. [Google Scholar] [CrossRef]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. JASN 2016, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, C.; Hällqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PloS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef]
- Xu, K.-Y.; Xia, G.-H.; Lu, J.-Q.; Chen, M.-X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.-W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef]
- Pelletier, C.C.; Croyal, M.; Ene, L.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; Lemoine, S.; Guebre-Egziabher, F.; Juillard, L.; Soulage, C.O. Elevation of trimethylamine-N-oxide in chronic kidney disease: Contribution of decreased glomerular filtration rate. Toxins 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.M.; Allenspach, M.; Othman, A.; Saely, C.H.; Muendlein, A.; Vonbank, A.; Drexel, H.; von Eckardstein, A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015, 243, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Heaney, L.M.; Bhandari, S.S.; Jones, D.J.; Ng, L.L. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016, 102, 841–848. [Google Scholar] [CrossRef]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Rodriguez, J.A.; Fernández-Alonso, S. Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci. Rep. 2019, 9, 15580. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Mafune, A.; Iwamoto, T.; Tsutsumi, Y.; Nakashima, A.; Yamamoto, I.; Yokoyama, K.; Yokoo, T.; Urashima, M. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: A cross-sectional study. Clin. Exp. Nephrol. 2016, 20, 731–739. [Google Scholar] [CrossRef]
- Kim, R.B.; Morse, B.L.; Djurdjev, O.; Tang, M.; Muirhead, N.; Barrett, B.; Holmes, D.T.; Madore, F.; Clase, C.M.; Rigatto, C. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016, 89, 1144–1152. [Google Scholar] [CrossRef]
- Al-Obaide, M.A.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef]
- Winther, S.A.; Øllgaard, J.C.; Hansen, T.W.; von Scholten, B.J.; Reinhard, H.; Ahluwalia, T.S.; Wang, Z.; Gæde, P.; Parving, H.-H.; Hazen, S.; et al. Plasma trimethylamine N-oxide and its metabolic precursors and risk of mortality, cardiovascular and renal disease in individuals with type 2-diabetes and albuminuria. PLoS ONE 2021, 16, e0244402. [Google Scholar] [CrossRef] [PubMed]
- Kalagi, N.A.; Thota, R.N.; Stojanovski, E.; Alburikan, K.A.; Garg, M.L. Association between plasma trimethylamine N-oxide levels and type 2 diabetes: A case control study. Nutrients 2022, 14, 2093. [Google Scholar] [CrossRef]
- Al-Wakeel Jamal, S. Accuracy and precision of the CKD-EPI and MDRD predictive equations compared with glomerular filtration rate measured by inulin clearance in a Saudi population. Ann. Saudi Med. 2016, 36, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Zhang, Y.L.; Beck, G.J.; Froissart, M.; Hamm, L.L.; Lewis, J.B.; Mauer, M.; Navis, G.J.; et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am. J. Kidney Dis. 2010, 56, 486–495. [Google Scholar] [CrossRef]
- Awwad, H.M.; Geisel, J.; Obeid, R. Determination of trimethylamine, trimethylamine N-oxide, and taurine in human plasma and urine by UHPLC–MS/MS technique. J. Chromatogr. B 2016, 1038, 12–18. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, H.; Zhang, X.; Zhou, Z.; Zhou, M.; Hu, Z.; Zhu, F.; Zhang, L.; Nie, J. Trimethylamine-N-Oxide Aggravates Kidney Injury via Activation of p38/MAPK Signaling and Upregulation of HuR. Kidney Blood Press. Res. 2022, 47, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Brusasco, I.; Cabassi, A.; Morabito, S.; Fiaccadori, E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cai, R.; Sun, J.; Dong, X.; Huang, R.; Tian, S.; Wang, S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 2017, 55, 66–76. [Google Scholar] [CrossRef]
- Hai, X.; Landeras, V.; Dobre, M.A.; DeOreo, P.; Meyer, T.W.; Hostetter, T.H. Mechanism of prominent trimethylamine oxide (TMAO) accumulation in hemodialysis patients. PLoS ONE 2015, 10, e0143731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).