Chondroprotective Effects of Grapefruit (Citrus paradisi Macfad.) Juice in a Complete Freund’s Adjuvant Rat Model of Knee Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatments
2.3. Sample Collection
2.4. Biochemical Assay of SYF and Serum
2.5. Histopathological Examination
2.6. Statistical Analysis
3. Results
3.1. Effects on Body Weight and Knee Diameter
3.2. Effects on serum inflammatory biomarkers
3.3. Effects on serum MMP-1, cathepsin K, and osteocalcin
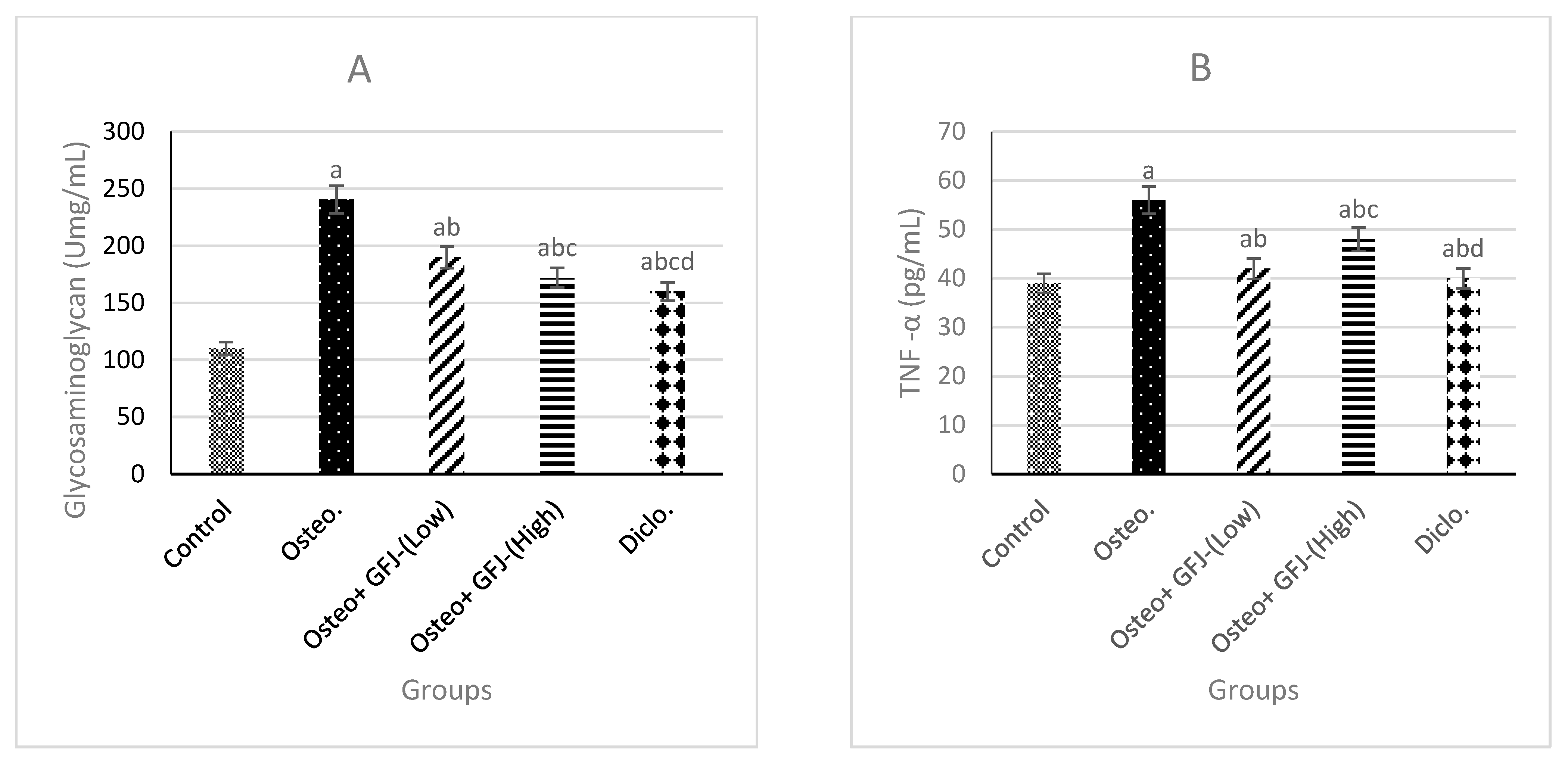
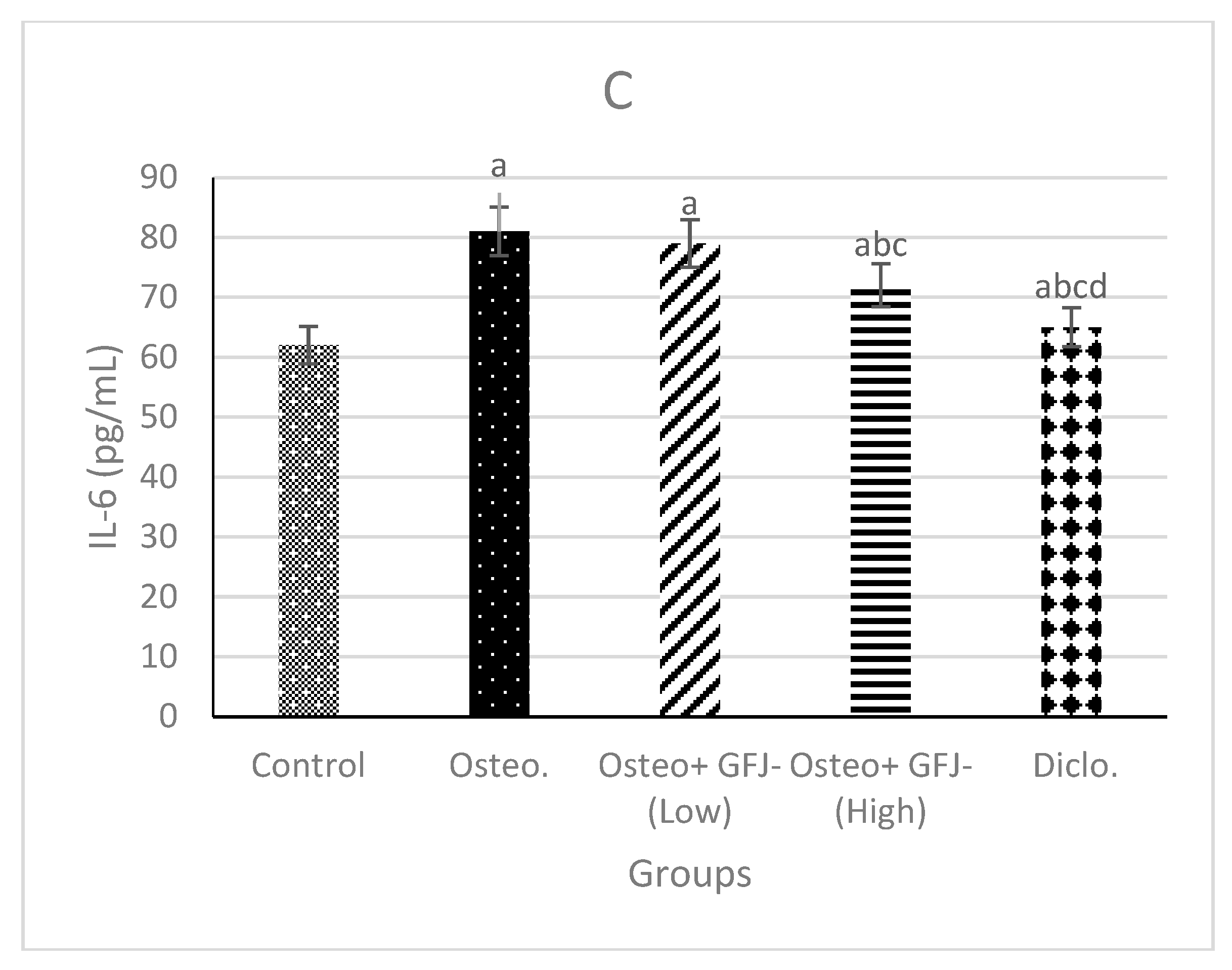
3.4. Effects on GAG, TNF-α, and IL-6 in SYF
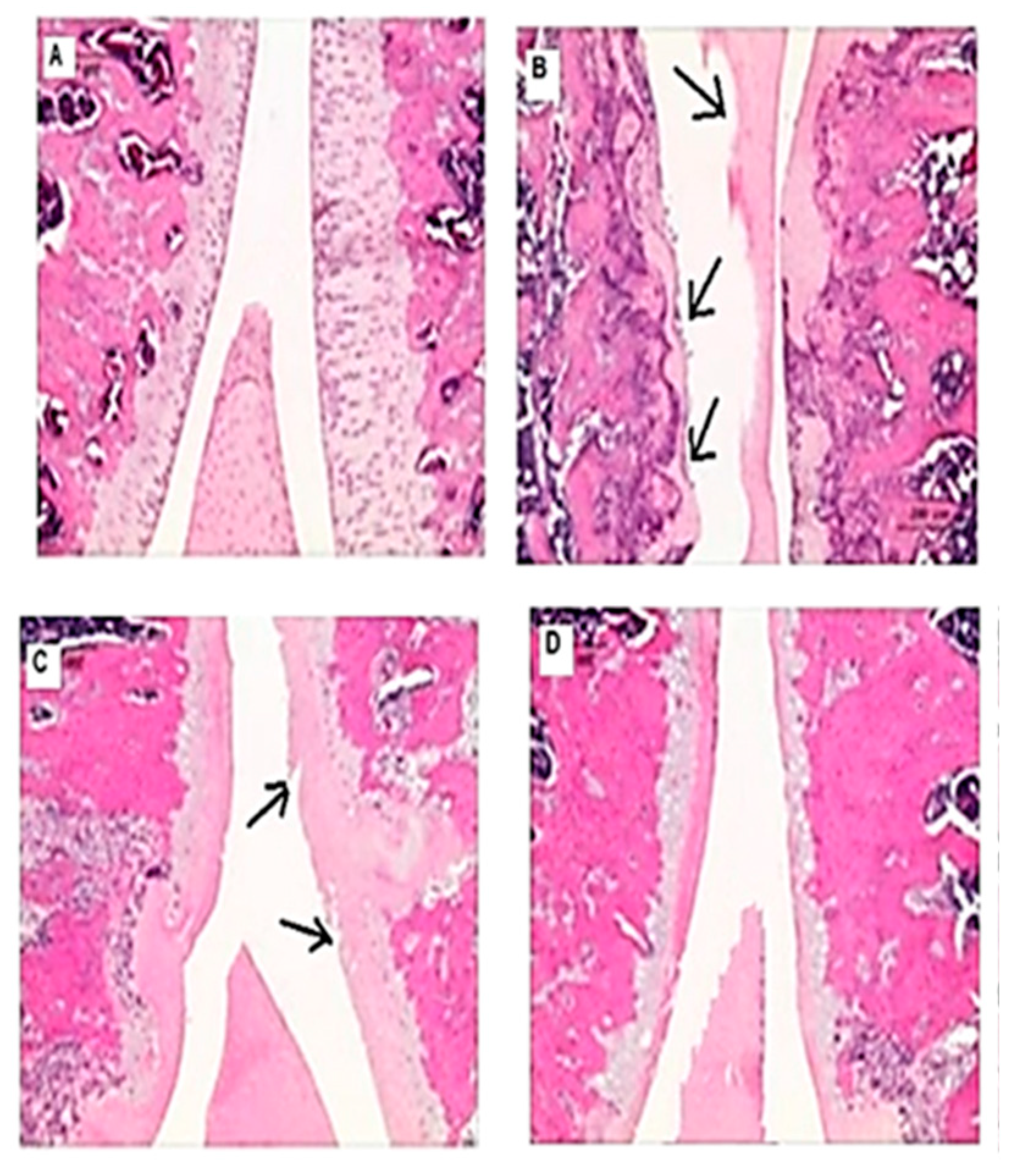
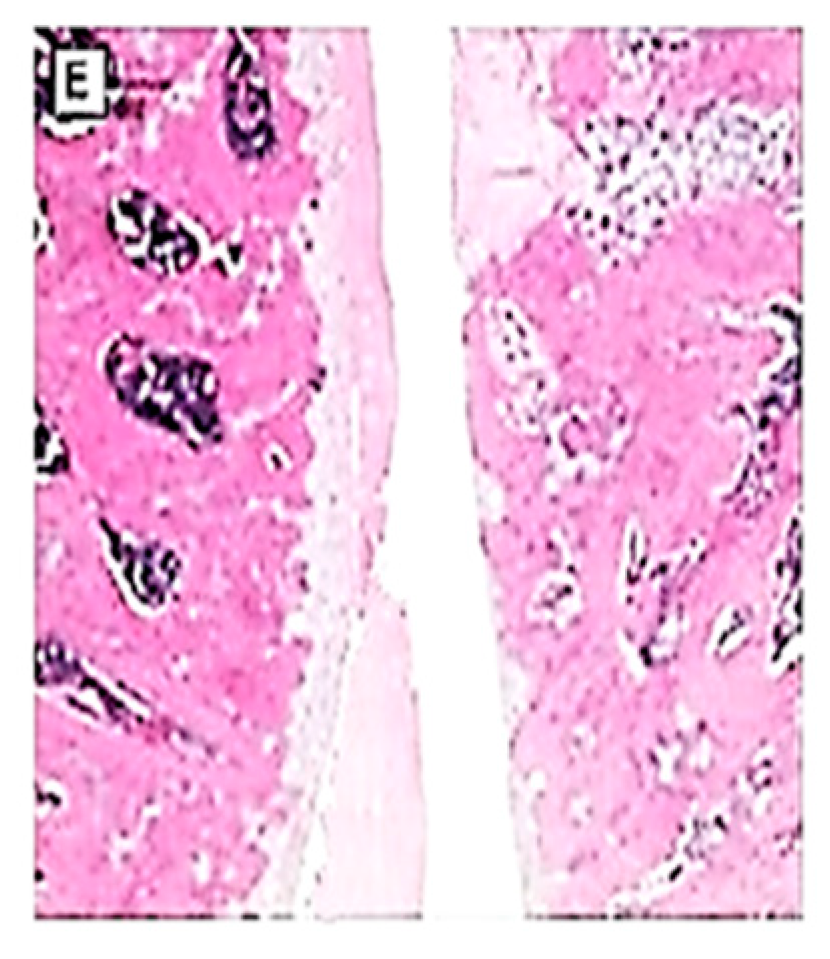
3.5. Effects on the macroscopic apperance of the articular cartilage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, H.; Xie, X.; Wen, Y.; Tan, Y.; Shangguan, Y.; Li, B.; Magdalou, J.; Wang, H.; Chen, L. Subchondral bone dysplasia partly participates in prenatal dexamethasone induced-osteoarthritis susceptibility in female offspring rats. Bone 2020, 133, 115245. [Google Scholar] [CrossRef] [PubMed]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; El-Ganainy, S.O. Thermoresponsive Hyalomer intra-articular hydrogels improve monoiodoacetate-induced osteoarthritis in rats. Int. J. Pharm. 2020, 573, 118859. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New insight on its pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Barreto, R.B.; Santana, B.H.d.; Martins, B.M.; Porto, E.S.; Severino, P.; Cardoso, J.C.; Souto, E.B.; Albuquerque-Júnior, R.L.d. Application of Formononetin for the Treatment of Knee Osteoarthritis Induced by Medial Meniscectomy in a Rodent Model. Appl. Sci. 2022, 12, 8591. [Google Scholar] [CrossRef]
- Maudens, P.; Jordan, O.; Allémann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, N.; Alnahdi, H.; Ayas, N.; Mohamed, A. Amelioration of cardiotoxic impacts of diclofenac sodium by vitamin B complex. Eur. Rev. Med. Pharm. Sci. 2015, 19, 671–681. [Google Scholar]
- Orabi, S.H.; Abd Eldaium, D.; Hassan, A.; El Sabagh, H.S.; Abd Eldaim, M.A. Allicin modulates diclofenac sodium induced hepatonephro toxicity in rats via reducing oxidative stress and caspase 3 protein expression. Environ. Toxicol. Pharmacol. 2020, 74, 103306. [Google Scholar] [CrossRef]
- Duarte, F.C.; Hurtig, M.; Clark, A.; Simpson, J.; Srbely, J.Z. Association between naturally occurring spine osteoarthritis in geriatric rats and neurogenic inflammation within neurosegmentally linked skeletal muscle. Exp. Gerontol. 2019, 118, 31–38. [Google Scholar] [CrossRef]
- Hamed, W.M.A.; Abid, K.Y.; Al-Amin, S. Hypoglycemic and hypolipidemic effects of grapefruit juice in diabetic rats. Tikrit J. Pure Sci. 2008, 13, 129–131. [Google Scholar]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Chudnovskiy, R.; Thompson, A.; Tharp, K.; Hellerstein, M.; Napoli, J.L.; Stahl, A. Consumption of clarified grapefruit juice ameliorates high-fat diet induced insulin resistance and weight gain in mice. PLoS ONE 2014, 9, e108408. [Google Scholar] [CrossRef]
- Bar-Yehuda, S.; Rath-Wolfson, L.; Del Valle, L.; Ochaion, A.; Cohen, S.; Patoka, R.; Zozulya, G.; Barer, F.; Atar, E.; Piña-Oviedo, S. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 3061–3071. [Google Scholar] [CrossRef]
- Levy, A.S.; Simon, O.; Shelly, J.; Gardener, M. 6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund’s adjuvant. BMC Pharmacol. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Cuciureanu, M.; Tuchilus, C.; Poiata, A.; Nechifor, M.; Cuciureanu, R. P1369 Modification of cephalexin serum concentration by grapefruit juice co-administration in rats. Int. J. Antimicrob. Agents 2007, 29, S380. [Google Scholar] [CrossRef]
- Sakr, S.A.; Zoil, M.E.-s.; El-Shafey, S.S. Ameliorative effect of grapefruit juice on amiodarone–induced cytogenetic and testicular damage in albino rats. Asian Pac. J. Trop. Biomed. 2013, 3, 573–579. [Google Scholar] [CrossRef]
- Juaira, T.; Begum, N.; Ali, T. Antinociceptive and Anti-inflammatory Effects of Combined Administration of a-tocopherol and Diclofenac. J. Bangladesh Soc. Physiol. 2015, 10, 30–35. [Google Scholar] [CrossRef]
- Freemont, A. Microscopic analysis of synovial fluid--the perfect diagnostic test? Ann. Rheum. Dis. 1996, 55, 695. [Google Scholar] [CrossRef]
- Akhter, S.; Irfan, H.M.; Ullah, A.; Jahan, S.; Roman, M.; Latif, M.B.; Mustafa, Z.; Almutairi, F.M.; Althobaiti, Y.S. Noscapine hydrochloride (benzyl-isoquinoline alkaloid) effectively prevents protein denaturation through reduction of IL-6, NF-kB, COX-2, Prostaglandin-E2 in rheumatic rats. Saudi Pharm. J. 2022, 30, 1791–1801. [Google Scholar] [CrossRef]
- Kobayashi-Miura, M.; Osago, H.; Hamasaki, Y.; Takano, I.; Akiho, M.; Hiyoshi, M.; Hara, N. Decrease in Glycosaminoglycan with Aging in Normal Rat Articular Cartilage Is Greater in Females than in Males. Cartilage 2022, 13, 19476035221102566. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Atik, O.Ş.; Erdoğan, D.; Seymen, C.M.; Bozkurt, H.H.; Kaplanoğlu, G.T. Is there crosstalk between subchondral bone, cartilage, and meniscus in the pathogenesis of osteoarthritis? Jt. Dis. Relat. Surg. 2016, 27, 062–067. [Google Scholar] [CrossRef]
- Findlay, D.M.; Kuliwaba, J.S. Bone–cartilage crosstalk: A conversation for understanding osteoarthritis. Bone Res. 2016, 4, 16028. [Google Scholar] [CrossRef]
- Suresh, P.; Kavitha, C.N.; Babu, S.M.; Reddy, V.P.; Latha, A.K. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund’s adjuvant-induced arthritis in albino rats. Inflammation 2012, 35, 1314–1321. [Google Scholar] [CrossRef]
- Hong, Y.H.; Song, C.; Shin, K.K.; Choi, E.; Hwang, S.-H.; Jang, Y.-J.; Taamalli, A.; Yum, J.; Kim, J.-H.; Kim, E. Tunisian Olea europaea L. leaf extract suppresses Freund’s complete adjuvant-induced rheumatoid arthritis and lipopolysaccharide-induced inflammatory responses. J. Ethnopharmacol. 2021, 268, 113602. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.H. Diclofenac in the treatment of osteoarthritis. Int. J. Clin. Rheumatol. 2013, 8, 185. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Saville, D.J.; Coville, P.F.; Wanwimolruk, S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm. Acta Helv. 2000, 74, 379–385. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Albanese, L.; Nuzzo, D.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Presentato, A.; Pagliaro, M.; Avellone, G. Flavonoids in lemon and grapefruit IntegroPectin. ChemistryOpen 2021, 10, 1055–1058. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. The major flavonoid of grapefruit: Naringin. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–44. [Google Scholar]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Nabavi, S.M.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Alam, M.A.; Kauter, K.; Brown, L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.A.; Going, S.B.; Chow, H.-H.S.; Patil, B.S.; Thomson, C.A. The effects of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metabolism 2012, 61, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Bello, F.O.; Ademosun, A.O. Hypocholesterolemic properties of grapefruit (Citrus paradisii) and shaddock (Citrus maxima) juices and inhibition of angiotensin-1-converting enzyme activity. J. Food Drug Anal. 2014, 22, 477–484. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef]
- Bei, M.-J.; Tian, F.-M.; Xiao, Y.-P.; Cao, X.-H.; Liu, N.; Zheng, Z.-Y.; Dai, M.-W.; Wang, W.-Y.; Song, H.-P.; Zhang, L. Raloxifene retards cartilage degradation and improves subchondral bone micro-architecture in ovariectomized rats with patella baja-induced-patellofemoral joint osteoarthritis. Osteoarthr. Cartil. 2020, 28, 344–355. [Google Scholar] [CrossRef]
- Aravinthan, A.; Hossain, M.A.; Kim, B.; Kang, C.-W.; Kim, N.S.; Hwang, K.-C.; Kim, J.-H. Ginsenoside Rb1 inhibits monoiodoacetate-induced osteoarthritis in postmenopausal rats through prevention of cartilage degradation. J. Ginseng Res. 2021, 45, 287–294. [Google Scholar] [CrossRef]
- Aiyalu, R.; Subramaniam, I.; Govindarajan, A.; Ramasamy, A. Formulation and Evaluation of Novel Herbal Aerosol for Arthritis. J. Rheumatol. Arthritic Dis. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Zhang, J. Meta-analysis of serum C-reactive protein and cartilage oligomeric matrix protein levels as biomarkers for clinical knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.; Leeming, D.; Dam, E.; Henriksen, K.; Alexandersen, P.; Pastoureau, P.; Altman, R.; Christiansen, C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr. Cartil. 2008, 16, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Deshpande, S.; Koppikar, S.; Patil, S.; Ingale, D.; Harsulkar, A. Glycosaminoglycan measured from synovial fluid serves as a useful indicator for progression of Osteoarthritis and complements Kellgren–Lawrence Score. BBA Clin. 2016, 6, 1–4. [Google Scholar] [CrossRef]
- Nirmal, P.S.; Jagtap, S.D.; Narkhede, A.N.; Nagarkar, B.E.; Harsulkar, A.M. New herbal composition (OA-F2) protects cartilage degeneration in a rat model of collagenase induced osteoarthritis. BMC Complement. Altern. Med. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- de la Garza, A.L.; Etxeberria, U.; Haslberger, A.; Aumueller, E.; Martínez, J.A.; Milagro, F.I. Helichrysum and grapefruit extracts boost weight loss in overweight rats reducing inflammation. J. Med. Food 2015, 18, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Zaidun, N.H.; Thent, Z.C.; Abd Latiff, A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Saenz, J.; Santa-María, C.; Reyes-Quiroz, M.E.; Geniz, I.; Jimenez, J.; Sobrino, F.; Alba, G. Grapefruit flavonoid Naringenin regulates the expression of LXRα in THP-1 macrophages by modulating AMP-activated protein kinase. Mol. Pharm. 2017, 15, 1735–1745. [Google Scholar] [CrossRef]
- Ren, X.; Shi, Y.; Zhao, D.; Xu, M.; Li, X.; Dang, Y.; Ye, X. Naringin protects ultraviolet B-induced skin damage by regulating p38 MAPK signal pathway. J. Dermatol. Sci. 2016, 82, 106–114. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.-f.; Sun, W.-x. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3746. [Google Scholar] [CrossRef]
- Souza, J.M., Jr.; Tuin, S.A.; Robinson, A.G.; Souza, J.G.O.; Bianchini, M.A.; Miguez, P.A. Effect of Flavonoid Supplementation on Alveolar Bone Healing-A Randomized Pilot Trial. Dent. J. 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal-Luna, J.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G. Grapefruit and its biomedical, antigenotoxic and chemopreventive properties. Food Chem. Toxicol. 2018, 112, 224–234. [Google Scholar] [CrossRef]
- Hirabara, S.; Kojima, T.; Takahashi, N.; Hanabayashi, M.; Ishiguro, N. Hyaluronan inhibits TLR-4 dependent cathepsin K and matrix metalloproteinase 1 expression in human fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 519–522. [Google Scholar] [CrossRef]
- Askari, A.; Ravansalar, S.A.; Naghizadeh, M.M.; Mosavat, S.H.; Khodadoost, M.; Jazani, A.M.; Hashempur, M.H. The efficacy of topical sesame oil in patients with knee osteoarthritis: A randomized double-blinded active-controlled non-inferiority clinical trial. Complement. Ther. Med. 2019, 47, 102183. [Google Scholar] [CrossRef]
- Bullon, P.; Chandler, L.; Segura Egea, J.J.; Perez Cano, R.; Martinez Sahuquillo, A. Osteocalcin in serum, saliva and gingival crevicular fluid: Their relation with periodontal treatment outcome in postmenopausal women. Med. Oral Patol. Oral Cir. Bucal 2007, 12, E193–E197. [Google Scholar]
- Deyhim, F.; Garica, K.; Lopez, E.; Gonzalez, J.; Ino, S.; Garcia, M.; Patil, B.S. Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 2006, 22, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Uehara, M.; Wu, J.; Wang, X.; Masuyama, R.; Suzuki, K.; Kanazawa, K.; Ishimi, Y. Hesperidin, a Citrus Flavonoid, Inhibits Bone Loss and Decreases Serum and Hepatic Lipids in Ovariectomized Mice. J. Nutr. 2003, 133, 1892–1897. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant Activity of Citrus Limonoids, Flavonoids, and Coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef]
- Xu, Z.; Li, N.; Wooley, P.H.; Yang, S.-Y.; Jiang, Y. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J. Orthop. Sci. 2013, 18, 478–485. [Google Scholar] [CrossRef]
- Wang, C.C.; Guo, L.; Tian, F.D.; An, N.; Luo, L.; Hao, R.H.; Wang, B.; Zhou, Z.H. Naringenin regulates production of matrix metalloproteinases in the knee-joint and primary cultured articular chondrocytes and alleviates pain in rat osteoarthritis model. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. Biol. 2017, 50, e5714. [Google Scholar] [CrossRef]
- Hasan, U.H.; Uttra, A.M.; Qasim, S.; Ikram, J.; Saleem, M.; Niazi, Z.R. Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine 2020, 66, 153134. [Google Scholar] [CrossRef]
- Mobasheri, A. Intersection of inflammation and herbal medicine in the treatment of osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 604–616. [Google Scholar] [CrossRef]
- Yao, H.; Xu, J.K.; Zheng, N.Y.; Wang, J.L.; Mok, S.W.; Lee, Y.W.; Shi, L.; Wang, J.Y.; Yue, J.; Yung, S.H.; et al. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthr. Cartil. 2019, 27, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.W.; Kang, J.W.; Kim, Y.J.; Lee, S.Y.; Shin, J.; Lee, S.; Lee, S.M. Effect of GCSB-5, a Herbal Formulation, on Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Evid. -Based Complement. Altern. Med. 2012, 2012, 730907. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, H.-J.; Kim, M.-K.; Kim, Y.-I.; Kim, H.J.; Bae, S.-K.; Nör, J.E.; Bae, M.-K. Naringenin stimulates osteogenic/odontogenic differentiation and migration of human dental pulp stem cells. J. Dent. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Larsson, S.; Englund, M.; Struglics, A.; Lohmander, L.S. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr. Cartil. 2015, 23, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef] [PubMed]
- Aborehab, N.M.; El Bishbishy, M.H.; Refaiy, A.; Waly, N.E. A putative Chondroprotective role for IL-1β and MPO in herbal treatment of experimental osteoarthritis. BMC Complement. Altern. Med. 2017, 17, 495. [Google Scholar] [CrossRef]
- Ye, C.; Chen, J.; Qu, Y.; Qi, H.; Wang, Q.; Yang, Z.; Wu, A.; Wang, F.; Li, P. Naringin in the repair of knee cartilage injury via the TGF-β/ALK5/Smad2/3 signal transduction pathway combined with an acellular dermal matrix. J. Orthop. Transl. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Cunha, J.E.; Barbosa, G.M.; Castro, P.A.T.d.S.; Luiz, B.L.F.; Silva, A.C.A.; Russo, T.L.; Vasilceac, F.A.; Cunha, T.M.; Cunha, F.Q.; Salvini, T.F. Knee osteoarthritis induces atrophy and neuromuscular junction remodeling in the quadriceps and tibialis anterior muscles of rats. Sci. Rep. 2019, 9, 6366. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, W.J.; Olayaki, L.A. Diabetes escalates knee osteoarthritis in rats: Evidence of adaptive mechanism. Environ. Toxicol. Pharmacol. 2018, 61, 1–7. [Google Scholar] [CrossRef]
| Groups | Body Weight Gain (g) | Knee Diameter (mm) |
|---|---|---|
| Control | 84.4 ± 2.47 | 1.6 |
| Osteo. | 31.5 ± 3.28 a | 2.9 a |
| Osteo+GFJ (low) | 52.5 ± 1.66 ab | 2.5 ab |
| Osteo+GFJ (high) | 39.6 ± 2.63 abc | 2.2 abc |
| Osteo+Diclo | 58.7 ± 2.01 abcd | 2.0 abcd |
| Groups | CRP (pg/mL) | IL-1β (pg/mL) | PGE2 (pg/mL) |
|---|---|---|---|
| Control | 20.60 ± 1.44 | 71.87 ± 1.95 | 329.78 ± 8.3 |
| Osteo. | 42.65 ± 3.28 a | 267.44 ± 3.68 a | 789.66 ± 11.6 a |
| Osteo+GFJ (low) | 32.54 ± 3.65 ab | 210.92 ± 4.28 ab | 537.53 ± 15.2 ab |
| Osteo+GFJ (High) | 27.76 ± 2.78 abc | 170.48 ± 3.68 abc | 490.89 ± 11.1 abc |
| Osteo+Diclo | 24.87 ± 2.26 abcd | 101.55 ± 4.68 abcd | 395.61 ± 12.4 abcd |
| Groups | Osteocalcin (pg/mL) | MMP-1 (pg/mL) | Cathepsin K (pg/mL) |
|---|---|---|---|
| Control | 13.60 ± 1.44 | 76.87 ± 1.95 | 48.78 ± 8.3 |
| Osteo. | 4.55 ± 1.87 a | 163.76 ± 8.43 a | 142.66 ± 11.6 a |
| Osteo+GFJ (low) | 10.56 ± 1.88 ab | 132.85 ± 5.88 ab | 114.53 ± 15.2 ab |
| Osteo+GFJ (high) | 7.12 ± 1.83 abc | 92.54 ± 3.98 abc | 89.89 ± 11.1 abc |
| Osteo+Diclo | 8.87 ± 1.65 abcd | 120.64 ± 6.96 abcd | 72.61 ± 12.4 abcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alazragi, R.S.; Baeissa, H.M. Chondroprotective Effects of Grapefruit (Citrus paradisi Macfad.) Juice in a Complete Freund’s Adjuvant Rat Model of Knee Osteoarthritis. Nutrients 2023, 15, 798. https://doi.org/10.3390/nu15040798
Alazragi RS, Baeissa HM. Chondroprotective Effects of Grapefruit (Citrus paradisi Macfad.) Juice in a Complete Freund’s Adjuvant Rat Model of Knee Osteoarthritis. Nutrients. 2023; 15(4):798. https://doi.org/10.3390/nu15040798
Chicago/Turabian StyleAlazragi, Reem S., and Hanadi M. Baeissa. 2023. "Chondroprotective Effects of Grapefruit (Citrus paradisi Macfad.) Juice in a Complete Freund’s Adjuvant Rat Model of Knee Osteoarthritis" Nutrients 15, no. 4: 798. https://doi.org/10.3390/nu15040798
APA StyleAlazragi, R. S., & Baeissa, H. M. (2023). Chondroprotective Effects of Grapefruit (Citrus paradisi Macfad.) Juice in a Complete Freund’s Adjuvant Rat Model of Knee Osteoarthritis. Nutrients, 15(4), 798. https://doi.org/10.3390/nu15040798





