Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Recommendation for Protein Restriction in International Guidelines for CKD
3. RCTs of the LPD and sVLPD for Patients with CKD
4. The Protein Sources in the Modern Diet
5. Does “Vegetarian sVLPD” Improve Kidney Outcomes?
6. A Plant-Based Diet as an Alternative Dietary Practice for Patients with CKD
7. Does a Plant-Based Diet Increase the Risk of Hyperkalemia?
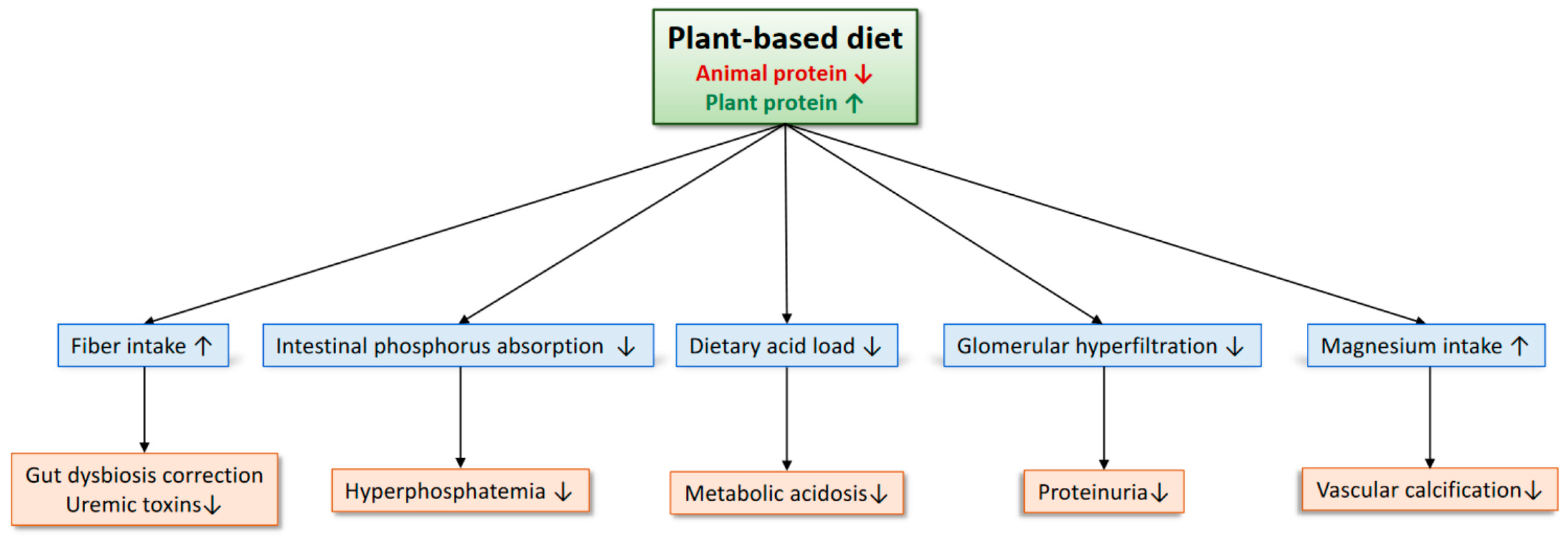
8. Benefits of a Plant-Based Diet for Patients with CKD
- (1)
- Uremic toxins are involved in the progression of CKD and cardiovascular events [1]. Patients with advanced CKD often develop metabolic acidosis with elevated anion gap due to the accumulation of uremic anions, which has recently been shown to be associated with a higher risk of CKD progression and cardiovascular events [43,44]. Reducing the production of uremic toxins, therefore, may be useful to improve clinical outcomes of patients with CKD. Many of the protein-bound uremic toxins, such as indoxyl sulphate and p-cresyl sulfate, are derived from the by-products of aromatic amino acid breakdown by the gut microbiome. A plant-based diet may reduce gut-derived uremic toxins by increasing fiber intake and modulating the intestinal microbiota. In an RCT of 40 patients undergoing hemodialysis, an increased intake of dietary fiber for 6 weeks led to a 29% reduction in a free plasma level of indoxyl sulfate [45]. A meta-analysis of RCTs also reported a significant reduction in various uremic solutes by dietary fiber, although evidence in non-dialysis patients with CKD is scarce [46].
- (2)
- Metabolic acidosis is a risk factor for the progression of CKD, especially when pH is low [47]. Acid load is detrimental to the kidney via activation of the angiotensin-aldosterone system, endothelin 1, and the complement pathway [48]. Although sodium bicarbonate has been used to treat metabolic acidosis, it may become a cause of sodium retention and an elevation in blood pressure. Increasing alkali-producing plant foods and decreasing acid-producing animal foods are another efficacious way in correcting metabolic acidosis. Goraya et al. reported that 3 years of dietary acid reduction with fruits and vegetables increased plasma total CO2 levels, reduced urinary angiotensinogen, and delayed the deterioration of GFR in patients with CKD stage G3 without increasing plasma potassium levels [49]. Fruits and vegetables may be preferable to sodium bicarbonate as an alkali therapy for patients with CKD because fruits and vegetables significantly reduce blood pressure levels compared to sodium bicarbonate, presumably by decreasing the sodium load [49]. Further evidence is needed to examine the feasibility of this dietary therapy in the real-world clinical setting of patients with CKD.
- (3)
- Elevated serum phosphate levels lead to vascular calcification and are associated with an increased risk of cardiovascular events and mortality in patients with CKD, although the benefits of phosphate-lowering therapies on clinical outcomes remain uncertain [50]. Because plant phosphorus is bound to phytates, which are difficult for humans to digest, it is much less bioavailable than animal phosphorus and inorganic phosphorus in food additives. Therefore, a plant-based diet can reduce the phosphorus burden. In fact, a 70% plant-protein-based diet for 4 weeks significantly decreases urinary phosphorus excretion and serum fibroblast growth factor 23 levels [41]. In contrast to phosphate binders, which may increase the occurrence of nausea and constipation, a plant-based diet would be helpful in reducing such adverse gastrointestinal effects by increasing the dietary intake of fiber.
- (4)
- Plant proteins are less likely to induce glomerular hyperfiltration compared to animal proteins. Kontessis et al. have reported that soy protein neither increased GFR nor renal plasma flow as animal protein did among healthy individuals [51]. Additionally, urinary albumin clearance was significantly lower after the ingestion of soy protein compared to that of animal protein. Therefore, plant proteins may be advantageous over animal proteins to prevent the elevation in glomerular pressure. Further studies should clarify whether this beneficial effect of soy protein can be observed in patients with CKD who already receive RAS inhibitors and SGLT2 inhibitors.
- (5)
- A plant-based diet can increase the intake of magnesium [52]. Experimental evidence shows that magnesium inhibits calcification of vascular smooth muscle cells induced by phosphate [53]. A high magnesium diet prevented aortic calcification in animal models of CKD [53]. In an RCT of patients with CKD stages G3 and G4, an oral magnesium supplementation significantly retarded the progression of coronary artery calcification [54]. Magnesium might also improve the prognosis of patients with CKD, as described below [55,56].
9. Magnesium and Clinical Outcomes in CKD
9.1. Prevalence and Causes of Hypomagnesemia in CKD
9.2. Hypomagnesemia and the Risk of CKD Progression
9.3. Magnesium as a Calcification Inhibitor
9.4. Magnesium and Clinical Outcomes in Hemodialysis Patients
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravid, J.D.; Kamel, M.H.; Chitalia, V.C. Uraemic solutes as therapeutic targets in CKD-associated cardiovascular disease. Nat. Rev. Nephrol. 2021, 17, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, T.H.; Meyer, T.W.; Rennke, H.G.; Brenner, B.M. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int. 1986, 30, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Palacio, C.; Tovar, A.R.; Torres, N.; Cruz, C.; Hernández-Pando, R.; Salas-Garrido, G.; Pedraza-Chaverri, J.; Correa-Rotter, R. Proinflammatory gene expression and renal lipogenesis are modulated by dietary protein content in obese Zucker fa/fa rats. Am. J. Physiol. Renal Physiol. 2011, 300, F263–F271. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Lipworth, L.; Cavanaugh, K.L.; Young, B.A.; Tucker, K.L.; Carithers, T.C.; Taylor, H.A.; Correa, A.; Kabagambe, E.K.; Ikizler, T.A. Protein Intake and Long-term Change in Glomerular Filtration Rate in the Jackson Heart Study. J. Ren. Nutr. 2018, 28, 245–250. [Google Scholar] [CrossRef]
- Esmeijer, K.; Geleijnse, J.M.; de Fijter, J.W.; Kromhout, D.; Hoogeveen, E.K. Dietary protein intake and kidney function decline after myocardial infarction: The Alpha Omega Cohort. Nephrol. Dial. Transplant. 2020, 35, 106–115. [Google Scholar] [CrossRef]
- CKD Work Group. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease; International Society of Nephrology: Brussels, Belgium, 2013; Volume 3, pp. 1–150. [Google Scholar]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; WHO Technical Report Series 935; WHO: Geneva, Switzerland, 2007; 265p. [Google Scholar]
- Fouque, D.; Laville, M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst. Rev. 2009, 3, CD001892. [Google Scholar] [CrossRef]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD001892. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef]
- Locatelli, F.; Alberti, D.; Graziani, G.; Buccianti, G.; Redaelli, B.; Giangrande, A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Northern Italian Cooperative Study Group. Lancet 1991, 337, 1299–1304. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Pota, A.; Bellizzi, V.; Di Giuseppe, D.; Di Micco, L.; Minutolo, R.; Pisani, A.; Sabbatini, M.; Ravani, P. Effect of a low- versus moderate-protein diet on progression of CKD: Follow-up of a randomized controlled trial. Am. J. Kidney Dis. 2009, 54, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.P.; Tauber-Lassen, E.; Jensen, B.R.; Parving, H.H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002, 62, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Mircescu, G.; Gârneaţă, L.; Stancu, S.H.; Căpuşă, C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D. The Role for Protein Restriction in Addition to Renin-Angiotensin-Aldosterone System Inhibitors in the Management of CKD. Am. J. Kidney Dis. 2019, 73, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef]
- Bellizzi, V.; Signoriello, S.; Minutolo, R.; Di Iorio, B.; Nazzaro, P.; Garofalo, C.; Calella, P.; Chiodini, P.; De Nicola, L.; ERIKA Study Group Investigators of the Italian Society of Nephrology-Conservative Therapy of CKD Work Group. No additional benefit of prescribing a very low-protein diet in patients with advanced chronic kidney disease under regular nephrology care: A pragmatic, randomized, controlled trial. Am. J. Clin. Nutr. 2022, 115, 1404–1417. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L., 3rd. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007-2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef]
- Lin, Y.; Bolca, S.; Vandevijvere, S.; De Vriese, S.; Mouratidou, T.; De Neve, M.; Polet, A.; Van Oyen, H.; Van Camp, J.; De Backer, G.; et al. Plant and animal protein intake and its association with overweight and obesity among the Belgian population. Br. J. Nutr. 2011, 105, 1106–1116. [Google Scholar] [CrossRef]
- Budhathoki, S.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Goto, A.; Kotemori, A.; Ishihara, J.; Takachi, R.; Charvat, H.; Mizoue, T.; et al. Association of Animal and Plant Protein Intake with All-Cause and Cause-Specific Mortality in a Japanese Cohort. JAMA Intern. Med. 2019, 179, 1509–1518. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Chen, H.; Shen, J.; Xuan, J.; Zhu, A.; Ji, J.S.; Liu, X.; Cao, Y.; Zong, G.; Zeng, Y.; Wang, X.; et al. Plant-based dietary patterns in relation to mortality among older adults in China. Nat. Aging 2022, 2, 224–230. [Google Scholar] [CrossRef]
- Chen, Z.; Drouin-Chartier, J.P.; Li, Y.; Baden, M.Y.; Manson, J.E.; Willett, W.C.; Voortman, T.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Indices and Subsequent Risk of Type 2 Diabetes in Women and Men: Three U.S. Prospective Cohorts. Diabetes Care 2021, 44, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Bahadoran, Z.; Mirmiran, P.; Sadeghian-Sharif, S.; Azizi, F. Dietary phytochemical index is inversely associated with the occurrence of hypertension in adults: A 3-year follow-up (the Tehran Lipid and Glucose Study). Eur. J. Clin. Nutr. 2015, 69, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Dybvik, J.S.; Svendsen, M.; Aune, D. Vegetarian and vegan diets and the risk of cardiovascular disease, ischemic heart disease and stroke: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Nutr. 2022, 62, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef]
- Jhee, J.H.; Kee, Y.K.; Park, J.T.; Chang, T.I.; Kang, E.W.; Yoo, T.H.; Kang, S.W.; Han, S.H. A Diet Rich in Vegetables and Fruit and Incident CKD: A Community-Based Prospective Cohort Study. Am. J. Kidney Dis. 2019, 74, 491–500. [Google Scholar] [CrossRef]
- Banerjee, T.; Carrero, J.J.; McCulloch, C.; Burrows, N.R.; Siegel, K.R.; Morgenstern, H.; Saran, R.; Powe, N.R. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Dietary Factors and Prevention: Risk of End-Stage Kidney Disease by Fruit and Vegetable Consumption. Am. J. Nephrol. 2021, 52, 356–367. [Google Scholar] [CrossRef]
- Lew, Q.J.; Jafar, T.H.; Koh, H.W.; Jin, A.; Chow, K.Y.; Yuan, J.M.; Koh, W.P. Red Meat Intake and Risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.M.; Joshi, S.; Brown-Tortorici, A.; Kramer, H.M. Medical nutrition therapy using plant-focused low-protein meal plans for management of chronic kidney disease in diabetes. Curr. Opin. Nephrol. Hypertens. 2022, 31, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Clase, C.M.; Carrero, J.J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; Dunkler, D.; Gao, P.; Teo, K.K.; Yusuf, S.; O’Donnell, M.J.; Mann, J.F.; Clase, C.M. ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014, 86, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Murali, S.B.; Bross, R.; Nissenson, A.R.; Kopple, J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 338–347. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef]
- Picard, K.; Griffiths, M.; Mager, D.R.; Richard, C. Handouts for Low-Potassium Diets Disproportionately Restrict Fruits and Vegetables. J. Ren. Nutr. 2021, 31, 210–214. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef]
- González-Ortiz, A.; Xu, H.; Ramos-Acevedo, S.; Avesani, C.M.; Lindholm, B.; Correa-Rotter, R.; Espinosa-Cuevas, Á.; Carrero, J.J. Nutritional status, hyperkalaemia and attainment of energy/protein intake targets in haemodialysis patients following plant-based diets: A longitudinal cohort study. Nephrol. Dial. Transplant. 2021, 36, 681–688. [Google Scholar] [CrossRef]
- Asahina, Y.; Sakaguchi, Y.; Kajimoto, S.; Hattori, K.; Doi, Y.; Oka, T.; Kaimori, J.Y.; Isaka, Y. Association of Time-Updated Anion Gap with Risk of Kidney Failure in Advanced CKD: A Cohort Study. Am. J. Kidney Dis. 2022, 79, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Sakaguchi, Y.; Kajimoto, S.; Hattori, K.; Doi, Y.; Oka, T.; Kaimori, J.Y.; Isaka, Y. Time-updated anion gap and cardiovascular events in advanced chronic kidney disease: A cohort study. Clin. Kidney J. 2021, 15, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Feng, P.; Xu, Y.; Hou, Y.Y.; Ojo, O.; Wang, X.H. The Role of Dietary Fiber Supplementation in Regulating Uremic Toxins in Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2021, 31, 438–447. [Google Scholar] [CrossRef]
- Kajimoto, S.; Sakaguchi, Y.; Asahina, Y.; Kaimori, J.Y.; Isaka, Y. Modulation of the Association of Hypobicarbonatemia and Incident Kidney Failure with Replacement Therapy by Venous pH: A Cohort Study. Am. J. Kidney Dis. 2021, 77, 35–43. [Google Scholar] [CrossRef]
- Wesson, D.E. The Continuum of Acid Stress. Clin. J. Am. Soc. Nephrol. 2021, 16, 1292–1299. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef]
- Lioufas, N.M.; Pascoe, E.M.; Hawley, C.M.; Elder, G.J.; Badve, S.V.; Block, G.A.; Johnson, D.W.; Toussaint, N.D. Systematic Review and Meta-Analyses of the Effects of Phosphate-Lowering Agents in Nondialysis CKD. J. Am. Soc. Nephrol. 2022, 33, 59–76. [Google Scholar] [CrossRef]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef]
- Farmer, B. Nutritional adequacy of plant-based diets for weight management: Observations from the NHANES. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 365S–368S. [Google Scholar] [CrossRef]
- Ter Braake, A.D.; Shanahan, C.M.; de Baaij, J.H.F. Magnesium Counteracts Vascular Calcification: Passive Interference or Active Modulation? Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1431–1445. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Obi, Y.; Monden, C.; Oka, T.; Yamaguchi, S.; Matsui, I.; Hashimoto, N.; Matsumoto, A.; Shimada, K.; et al. A Randomized Trial of Magnesium Oxide and Oral Carbon Adsorbent for Coronary Artery Calcification in Predialysis CKD. J. Am. Soc. Nephrol. 2019, 30, 1073–1085. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Isaka, Y. Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies. Nutrients 2017, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y. The emerging role of magnesium in CKD. Clin. Exp. Nephrol. 2022, 26, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hamano, T.; Sakaguchi, Y.; Yamaguchi, S.; Kubota, K.; Senda, M.; Yonemoto, S.; Shimada, K.; Matsumoto, A.; Hashimoto, N.; et al. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: A common electrolyte abnormality in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Takayanagi, K.; Iwashita, T.; Ikari, A.; Anzai, N.; Okazaki, S.; Hara, H.; Hatano, M.; Sano, T.; Ogawa, T.; et al. Down-regulation of magnesium transporting molecule, claudin-16, as a possible cause of hypermagnesiuria with the development of tubulo-interstitial nephropathy. Magnes. Res. 2018, 31, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Shoji, T.; Hayashi, T.; Suzuki, A.; Shimizu, M.; Mitsumoto, K.; Kawabata, H.; Niihata, K.; Okada, N.; Isaka, Y.; et al. Hypomagnesemia in type 2 diabetic nephropathy: A novel predictor of end-stage renal disease. Diabetes Care 2012, 35, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Matsui, I.; Oka, T.; Yamaguchi, S.; Kubota, K.; Shimada, K.; Matsumoto, A.; Hashimoto, N.; Isaka, Y. Low magnesium diet aggravates phosphate-induced kidney injury. Nephrol. Dial. Transplant. 2019, 34, 1310–1319. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Iwatani, H.; Hamano, T.; Tomida, K.; Kawabata, H.; Kusunoki, Y.; Shimomura, A.; Matsui, I.; Hayashi, T.; Tsubakihara, Y.; et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int. 2015, 88, 833–842. [Google Scholar] [CrossRef]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodríguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; Montes de Oca, A.; Vergara, N.; Steppan, S.; et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef]
- Ter Braake, A.D.; Smit, A.E.; Bos, C.; van Herwaarden, A.E.; Alkema, W.; van Essen, H.W.; Bravenboer, N.; Vervloet, M.G.; Hoenderop, J.G.J.; de Baaij, J.H.F. Magnesium prevents vascular calcification in Klotho deficiency. Kidney Int. 2020, 97, 487–501. [Google Scholar] [CrossRef]
- Tzanakis, I.P.; Stamataki, E.E.; Papadaki, A.N.; Giannakis, N.; Damianakis, N.E.; Oreopoulos, D.G. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: A pilot study. Int. Urol. Nephrol. 2014, 46, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Pasch, A.; Brandi, L. The Effect of Increasing Dialysate Magnesium on Serum Calcification Propensity in Subjects with End Stage Kidney Disease: A Randomized, Controlled Clinical Trial. Clin J Am Soc Nephrol. 2018, 13, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Kubota, K.; Oka, T.; Yamaguchi, S.; Matsumoto, A.; Hashimoto, N.; Mori, D.; Obi, Y.; Matsui, I.; et al. Anion Gap as a Determinant of Ionized Fraction of Divalent Cations in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2018, 13, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Hoshino, J.; Masakane, I. Magnesium and Risk of Hip Fracture among Patients Undergoing Hemodialysis. J. Am. Soc. Nephrol. 2018, 29, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Iseki, K.; Tsubakihara, Y.; Isaka, Y.; Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PLoS ONE 2014, 9, e116273. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, Y.; Kaimori, J.-Y.; Isaka, Y. Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease. Nutrients 2023, 15, 1002. https://doi.org/10.3390/nu15041002
Sakaguchi Y, Kaimori J-Y, Isaka Y. Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease. Nutrients. 2023; 15(4):1002. https://doi.org/10.3390/nu15041002
Chicago/Turabian StyleSakaguchi, Yusuke, Jun-Ya Kaimori, and Yoshitaka Isaka. 2023. "Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease" Nutrients 15, no. 4: 1002. https://doi.org/10.3390/nu15041002
APA StyleSakaguchi, Y., Kaimori, J.-Y., & Isaka, Y. (2023). Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease. Nutrients, 15(4), 1002. https://doi.org/10.3390/nu15041002





