Low Weight Polysaccharide of Hericium erinaceus Ameliorates Colitis via Inhibiting the NLRP3 Inflammasome Activation in Association with Gut Microbiota Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Purification of HEP10
2.3. Structural Characterization of HEP10
2.3.1. Sugar and Protein Contents
2.3.2. Monosaccharide Composition Analysis
2.3.3. Molecular Weight Analysis
2.3.4. Infrared (IR) Spectrometry
2.3.5. NMR Spectroscopy Analysis
2.4. Animals
2.4.1. Acute Colitis Model and HEP10 Treatment
2.4.2. Measurement of Nitric Oxide (NO) in Serum
2.4.3. Assay of Colonic Antioxidant Index and Inflammatory Cytokines
2.5. Cell Culture
2.5.1. Cell Viability
2.5.2. Measurement of NO/Nitrite and Cytokines in Culture Medium [16]
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blot Analysis
2.8. Gut Microbiota Analyses
2.9. Short Chain Fatty Acids (SCFAs) Analysis
2.10. Statistical Analysis
3. Results and Discussion
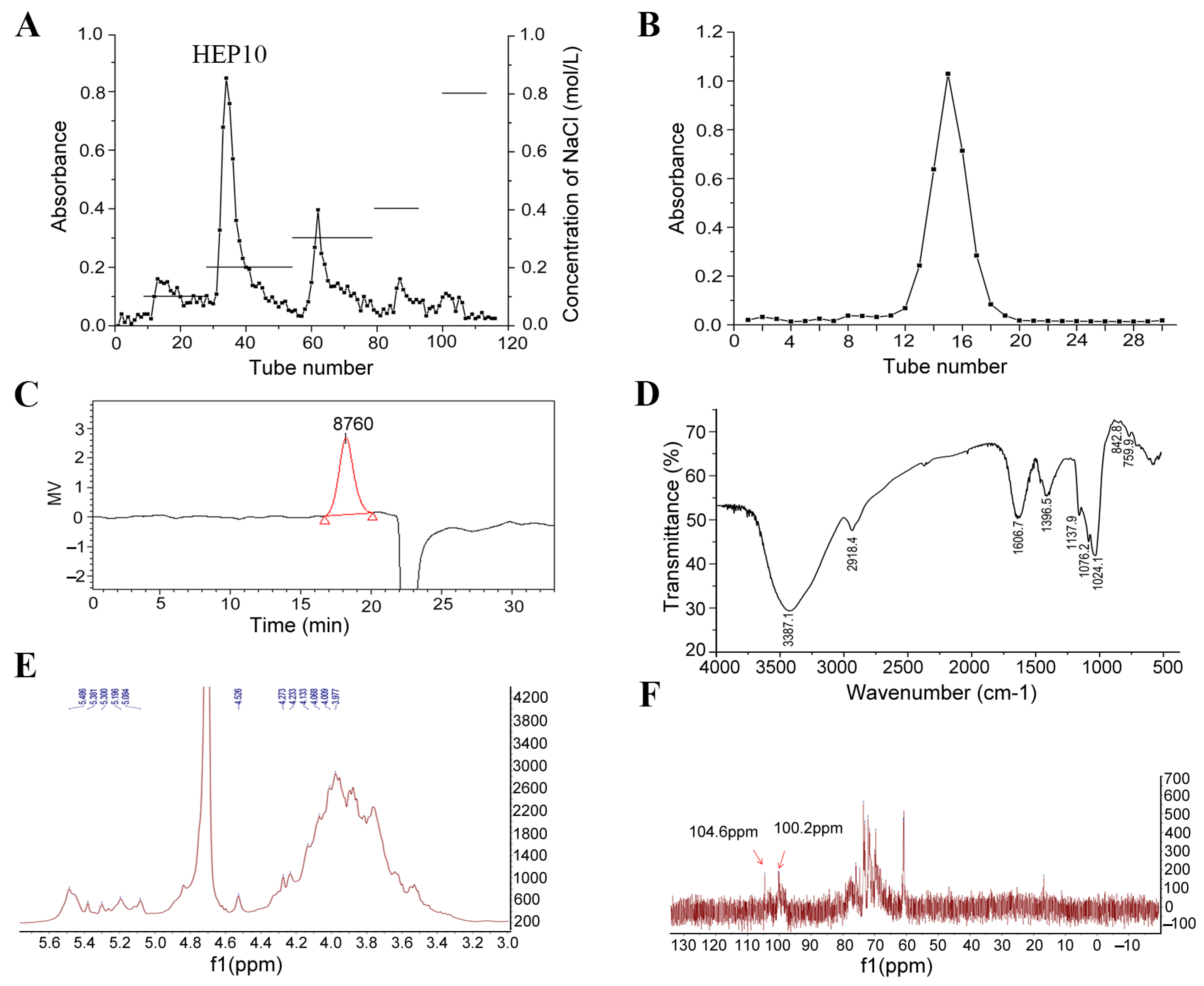
3.1. Purification of HEP10
3.2. Subsubsection Structural Characterization of HEP10
3.2.1. Monosaccharide Composition
3.2.2. FT-IR Spectrum
3.2.3. NMR Analysis of HEP10
3.3. HEP10 Inhibited LPS-Induced Inflammation in RAW264.7 Cells
3.3.1. Effect of HEP10 on Cell Viability and Cytokine Production In Vitro
3.3.2. Effect of HEP10 on LPS-Induced Inflammasome Activation In Vitro
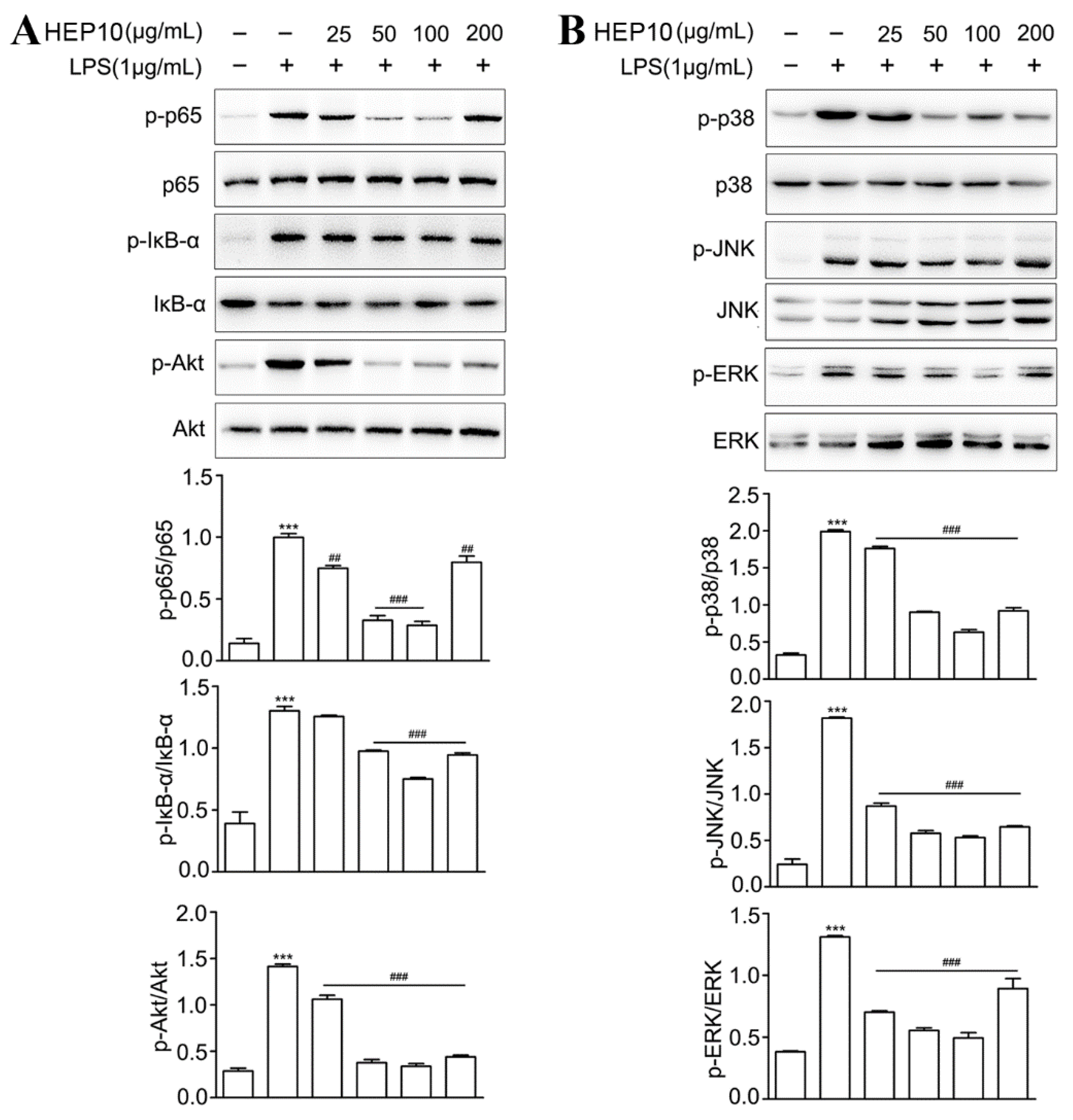
3.3.3. HEP10 Downregulated LPS-Induced NF-κB, AKT, and MAPK Signaling In Vitro
3.4. Anti-Inflammatory Effects of HEP10 in Mice with Ulcerative Colitis
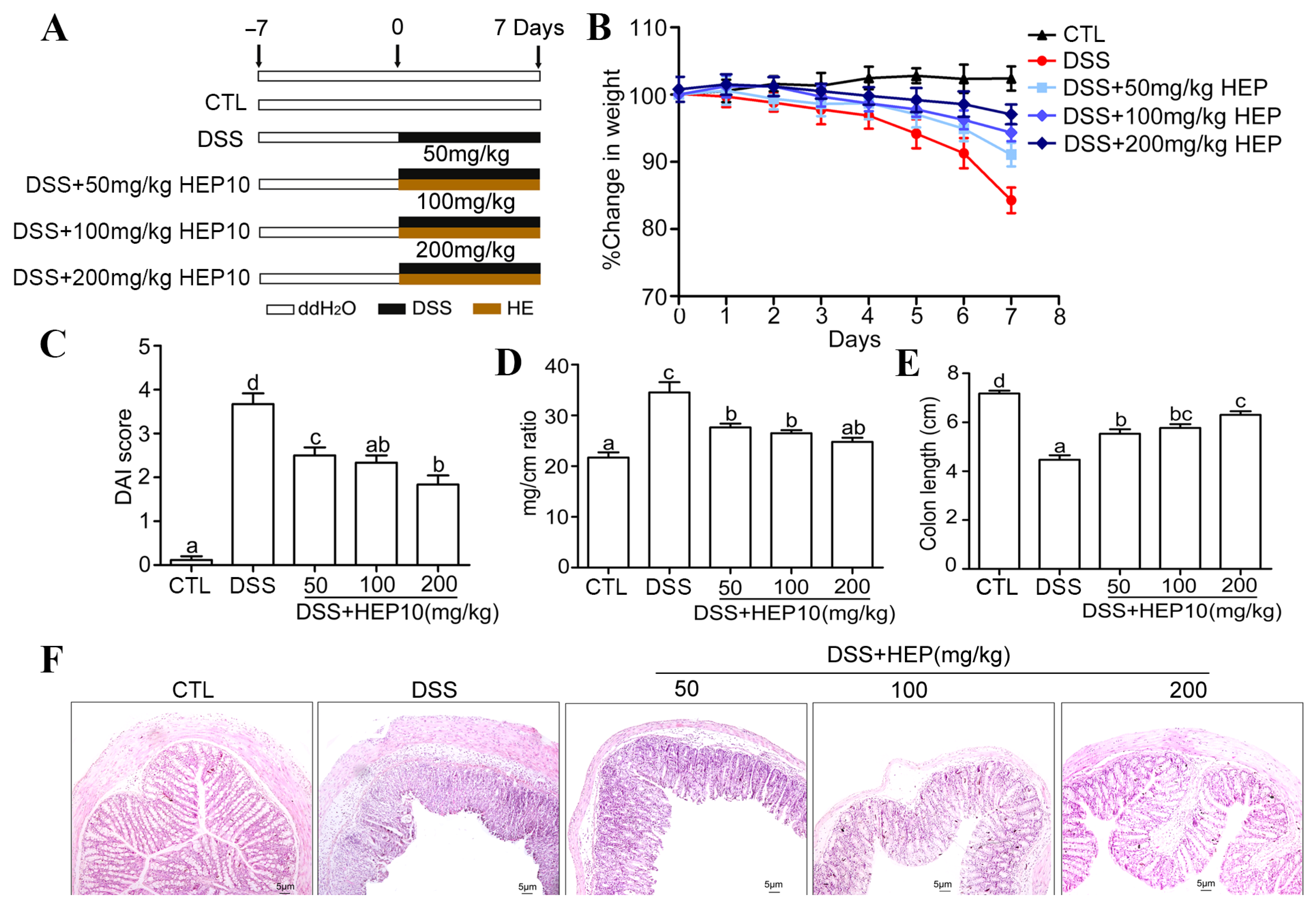
3.4.1. HEP10 Attenuated the Severity of Colitis in Mice Treated with DSS
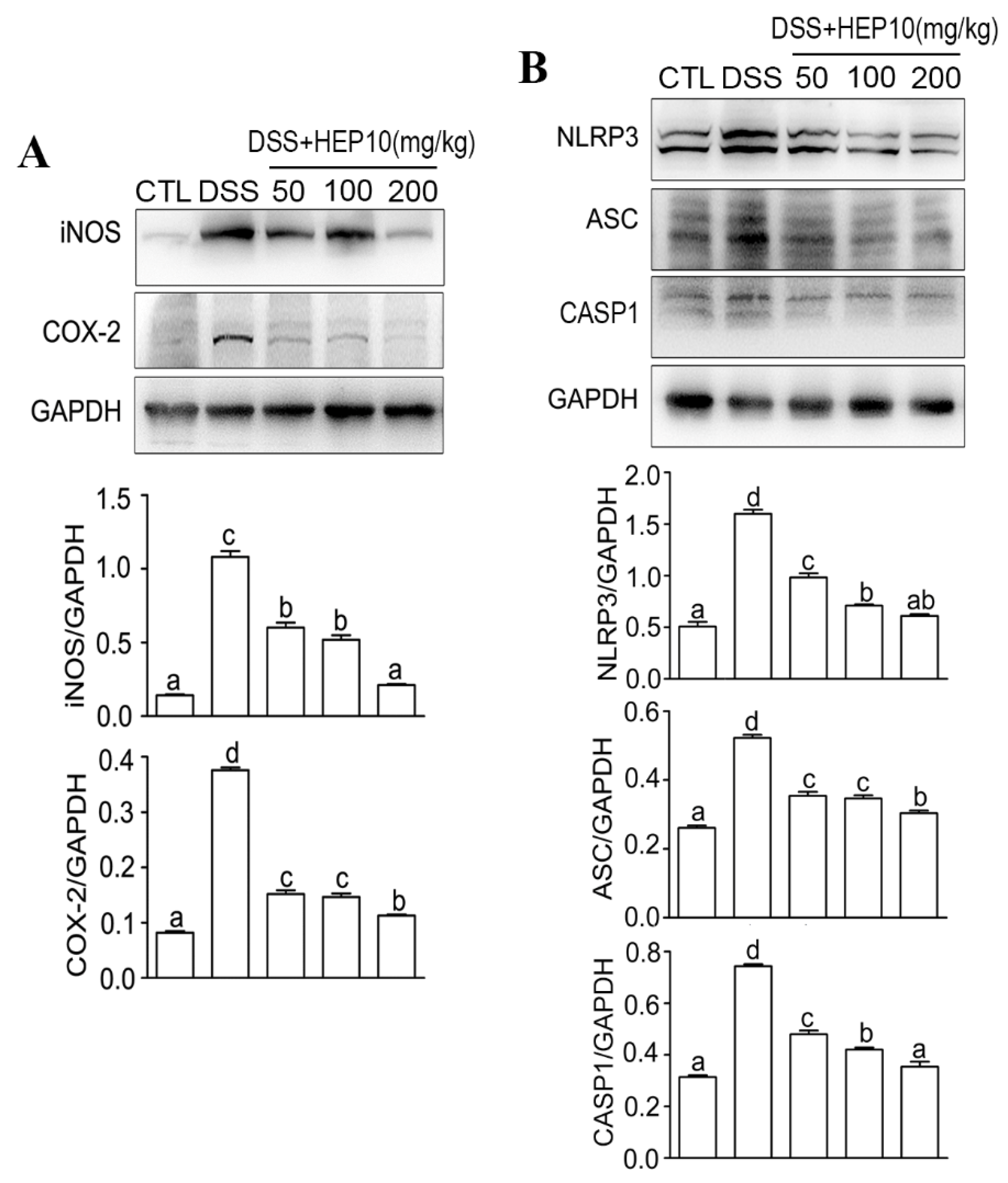
3.4.2. HEP10 Inhibited Oxidative Stress and Cytokine Production in Colon Tissues of Mice with DSS-Induced Colitis
3.4.3. HEP10 Blocked Activation of NLRP3 Inflammasome in Colon of Mice with DSS-Induced Colitis
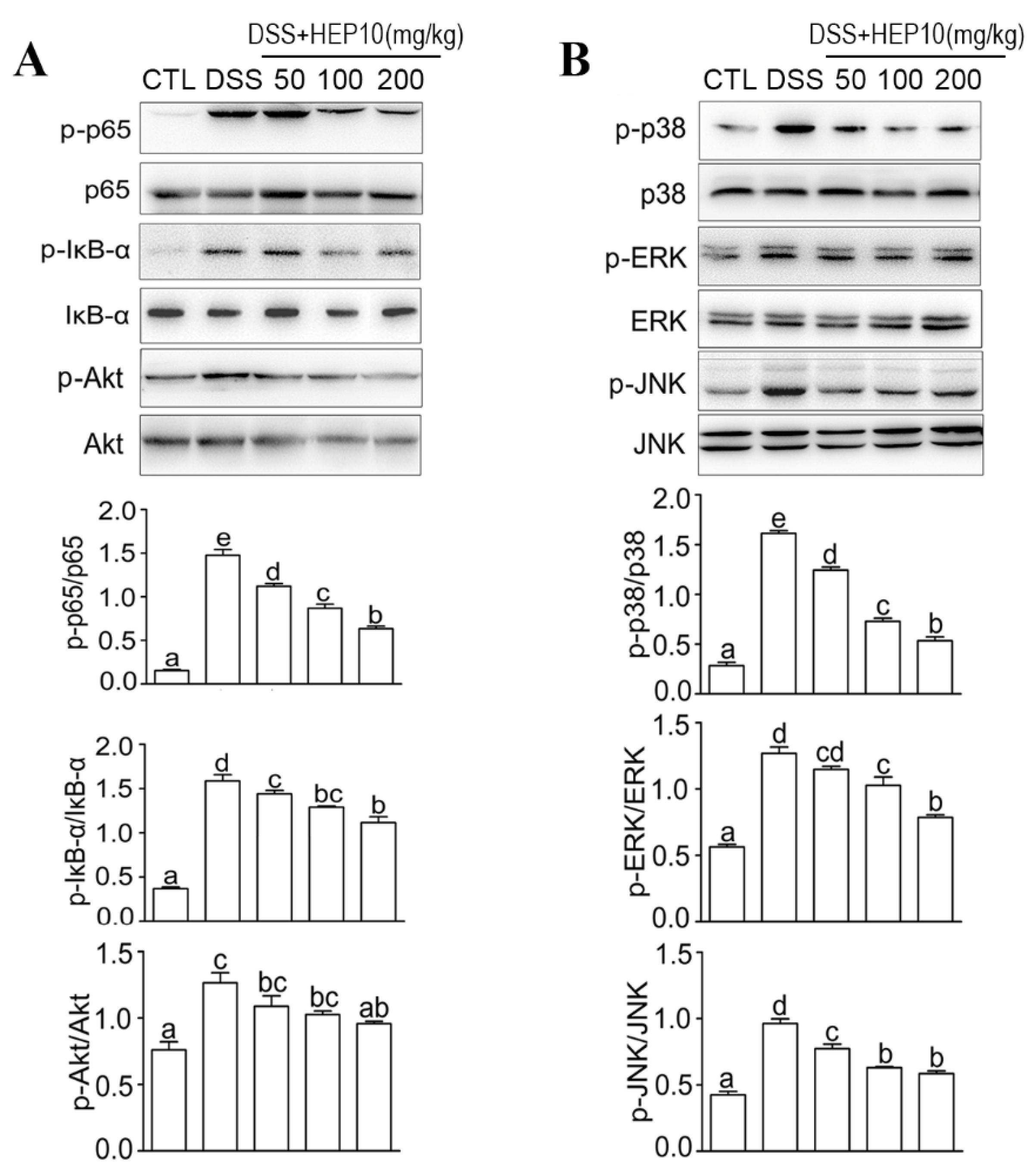
3.4.4. HEP10 Blocked NF-κB, AKT, and MAPK Signaling in Colon of Mice with DSS-Induced Colitis
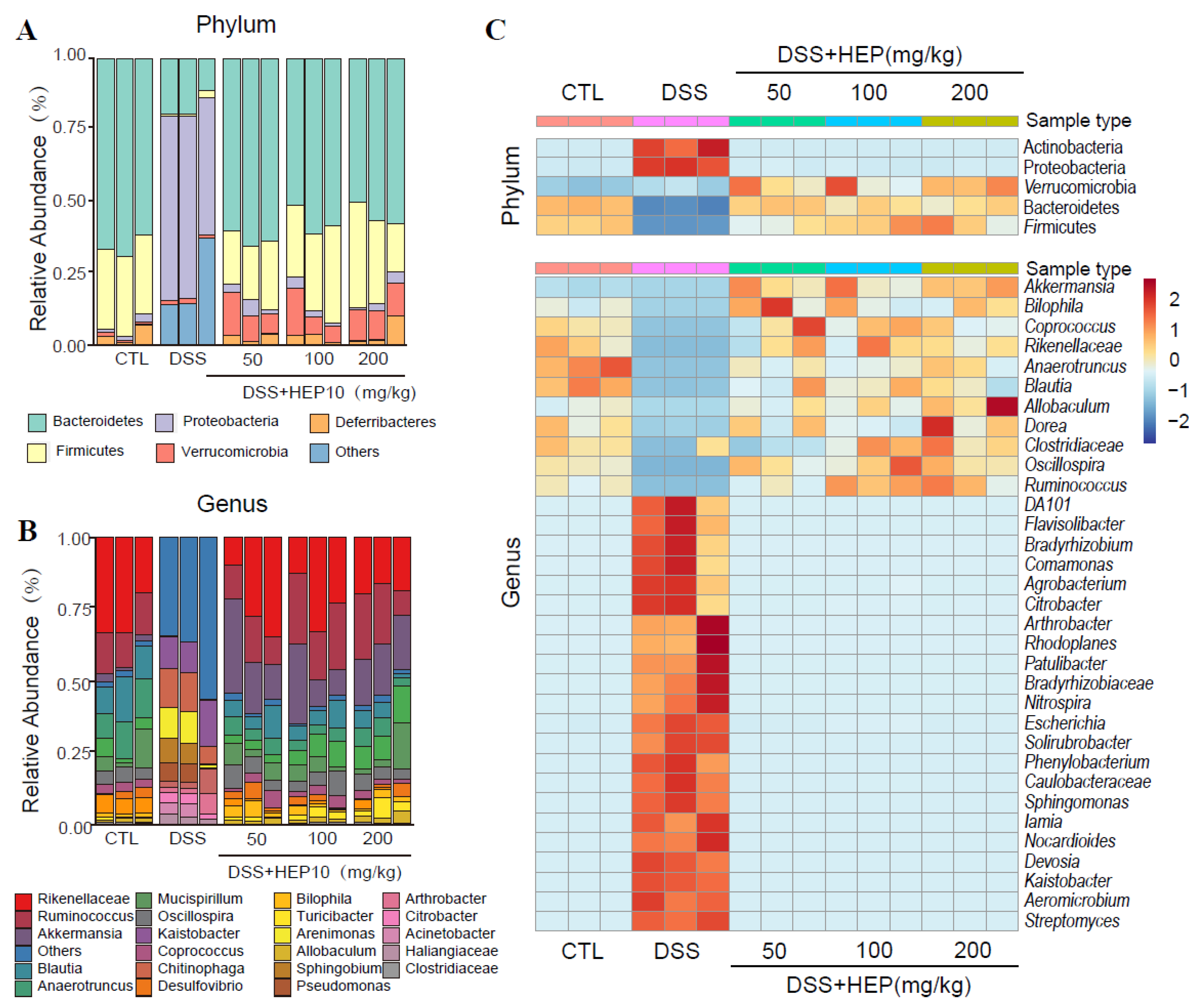
3.5. HEP10 Modulated the Structure and Metabolic Function of the Gut Microbiota in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Santilli, F.; Guerci, M.; Simeone, P.; Ardizzone, S.; Massari, A.; Giuffrida, P.; Tripaldi, R.; Malara, A.; Liani, R.; et al. Oxidative stress and thromboxane-dependent platelet activation in inflammatory bowel disease: Effects of anti-TNF-alpha treatment. Thromb. Haemost. 2016, 116, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Lemire, M.; Fortin, G.; Louis, E.; Silverberg, M.S.; Collette, C.; Baba, N.; Libioulle, C.; Belaiche, J.; Bitton, A.; et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 2009, 41, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef]
- Ulziijargal, E.; Mau, J.L. Nutrient compositions of culinary-medicinal mushroom fruiting bodies and mycelia. Int. J. Med. Mushrooms 2011, 13, 343–349. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Zhang, X.; Huang, H. Structure characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its immunomodulatory activities. Food Funct. 2018, 9, 294–306. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, E.K. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010, 297, 144–154. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Kim, J.H.; Nam, S.H.; Friedman, M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem. 2011, 59, 9861–9869. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Guo, X.-R.; Qin, Z.; Wang, X.-D.; Liu, H.-M.; Liu, Y.-L. Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment. Int. J. Biol. Macromol. 2020, 164, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. JoVE 2012, 60, e3678. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, S.; Cheng, P.; Lu, Z.M.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine 2017, 26, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, P.; Cheng, Y.L.; Guo, Y.H.; Hu, B.; Yao, W.R.; Zhu, X.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, Y.; Geng, Y.; Xu, Z. Analysis of short chain fatty acids by ultra-performance liquid chromatography. Chin. J. Bioprocess Eng. 2019, 17, 365–371. [Google Scholar] [CrossRef]
- Li, Q.Z.; Wu, D.; Zhou, S.; Liu, Y.F.; Li, Z.P.; Feng, J.; Yang, Y. Structure elucidation of a bioactive polysaccharide from fruiting bodies of Hericium erinaceus in different maturation stages. Carbohydr. Polym. 2016, 144, 196–204. [Google Scholar] [CrossRef]
- Jia, L.M.; Liu, L.; Dong, Q.; Fang, J.N. Structural investigation of a novel rhamnoglucogalactan isolated from the fruiting bodies of the fungus Hericium erinaceus. Carbohydr. Res. 2004, 339, 2667–2671. [Google Scholar] [CrossRef]
- Wang, Z.J.; Luo, D.H.; Liang, Z.Y. Structure of polysaccharides from the fruiting body of Hericium erinaceus Pers. Carbohydr. Polym. 2004, 57, 241–247. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Sun, P.L.; Zhang, J.S.; Tang, C.H.; Fan, J.M.; Shi, X.M.; Pan, Y.J. Structural investigation of a novel fucoglucogalactan isolated from the fruiting bodies of the fungus Hericium erinaceus. Food Chem. 2007, 104, 451–456. [Google Scholar] [CrossRef]
- Mitic, Z.; Nikolic, G.S.; Cakic, M.; Premovic, P.; Ilic, L. FTIR spectroscopic characterization of Cu(II) coordination compounds with exopolysaccharide pullulan and its derivatives. J. Mol. Struct. 2009, 924, 264–273. [Google Scholar] [CrossRef]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Jiang, J.G.; Li, M.Q.; Zheng, C.Y.; Zhu, W. Structural characterization and immunomodulatory activity of novel polysaccharides from Citrus aurantium Linn. variant amara Engl. J. Funct. Foods 2017, 35, 352–362. [Google Scholar] [CrossRef]
- Wang, W.J.; Ma, X.B.; Jiang, P.; Hu, L.L.; Zhi, Z.J.; Chen, J.L.; Ding, T.; Ye, X.Q.; Liu, D.H. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloid 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Li, H.; Mao, W.; Hou, Y.; Gao, Y.; Qi, X.; Zhao, C.; Chen, Y.; Chen, Y.; Li, N.; Wang, C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012, 114, 414–418. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yang, L.L.; Lee, T.J.F. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX 2 gene expression via suppression of nuclear factor-kappa B activation. Biochem. Pharmacol. 2000, 59, 1445–1457. [Google Scholar] [CrossRef]
- Pilar Utrilla, M.; Jesus Peinado, M.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Elena Rodriguez-Cabezas, M.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, Y.; Lin, L.; Sun, Y.; Zheng, C. Carcinoembryonic antigen related cellular adhesion molecule 1 alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Life Sci. 2016, 149, 120–128. [Google Scholar] [CrossRef]
- Schwanke, R.C.; Marcon, R.; Meotti, F.C.; Bento, A.F.; Dutra, R.C.; Pizzollatti, M.G.; Calixto, J.B. Oral administration of the flavonoid myricitrin prevents dextran sulfate sodium-induced experimental colitis in mice through modulation of PI3K/Akt signaling pathway. Mol. Nutr. Food Res. 2013, 57, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Shen, P.; Cao, Y.; Lu, X.; Gao, X.; Fu, Y.; Liu, B.; Zhang, N. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. Int. Immunopharmacol. 2016, 41, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Fu, Y.; Yang, Z. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS- induced acute colitis. Sci. Rep. 2016, 6, 28370. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef]
- Li, R.; Chen, Y.; Shi, M.; Xu, X.; Zhao, Y.; Wu, X.; Zhang, Y. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-kappa B signaling and enhancing antioxidant effect. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 1012–1020. [Google Scholar] [CrossRef]
- Katsanos, K.H.; Papadakis, K.A. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver 2017, 11, 455–463. [Google Scholar] [CrossRef]
- Harrison, L.; Stephan, K.; Friston, K. CHAPTER 38—Effective Connectivity. In Statistical Parametric Mapping; Friston, K., Ashburner, J., Kiebel, S., Nichols, T., Penny, W., Eds.; Academic Press: London, UK, 2007; pp. 508–521. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Sun, Q.; Gao, R.; Sheng, Y.; Guan, T.; Li, W.; Zhou, L.; Liu, C.; Li, H.; Lu, Z.; et al. Low Weight Polysaccharide of Hericium erinaceus Ameliorates Colitis via Inhibiting the NLRP3 Inflammasome Activation in Association with Gut Microbiota Modulation. Nutrients 2023, 15, 739. https://doi.org/10.3390/nu15030739
Ren Y, Sun Q, Gao R, Sheng Y, Guan T, Li W, Zhou L, Liu C, Li H, Lu Z, et al. Low Weight Polysaccharide of Hericium erinaceus Ameliorates Colitis via Inhibiting the NLRP3 Inflammasome Activation in Association with Gut Microbiota Modulation. Nutrients. 2023; 15(3):739. https://doi.org/10.3390/nu15030739
Chicago/Turabian StyleRen, Yilin, Qige Sun, Ruonan Gao, Yinyue Sheng, Tianyue Guan, Wang Li, Lingxi Zhou, Chang Liu, Huaxiang Li, Zhenming Lu, and et al. 2023. "Low Weight Polysaccharide of Hericium erinaceus Ameliorates Colitis via Inhibiting the NLRP3 Inflammasome Activation in Association with Gut Microbiota Modulation" Nutrients 15, no. 3: 739. https://doi.org/10.3390/nu15030739
APA StyleRen, Y., Sun, Q., Gao, R., Sheng, Y., Guan, T., Li, W., Zhou, L., Liu, C., Li, H., Lu, Z., Yu, L., Shi, J., Xu, Z., Xue, Y., & Geng, Y. (2023). Low Weight Polysaccharide of Hericium erinaceus Ameliorates Colitis via Inhibiting the NLRP3 Inflammasome Activation in Association with Gut Microbiota Modulation. Nutrients, 15(3), 739. https://doi.org/10.3390/nu15030739





