Aerobic Exercise Ameliorates Myocardial Fibrosis via Affecting Vitamin D Receptor and Transforming Growth Factor-β1 Signaling in Vitamin D-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Exercise Program
2.3. Body Weight and Body Composition
2.4. Echocardiography
2.5. Serum Biochemical Testing
2.6. Morphology
2.7. Quantitative Real-Time PCR
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
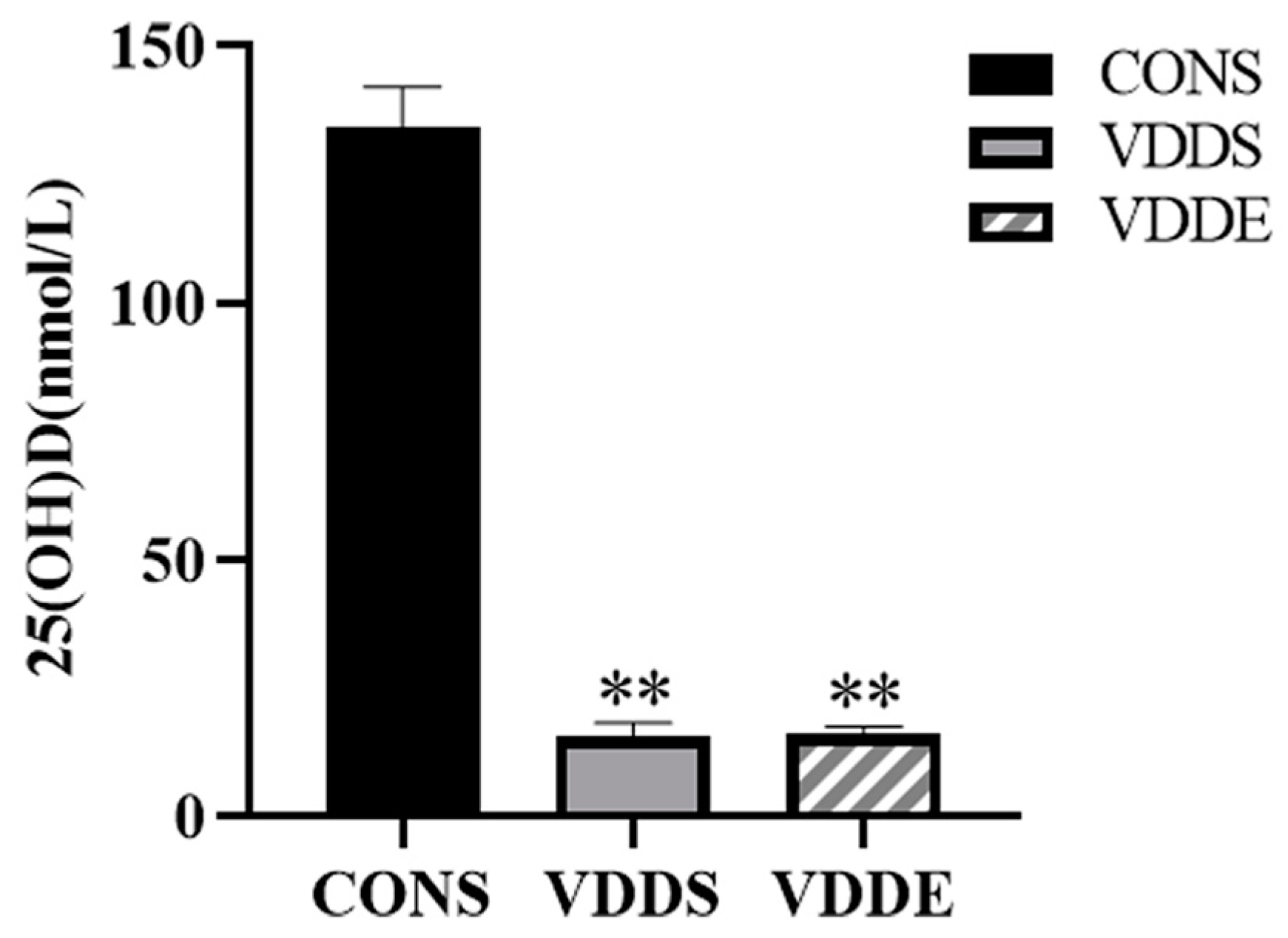
3.1. The Modeling of Vitamin D-Deficient Mice
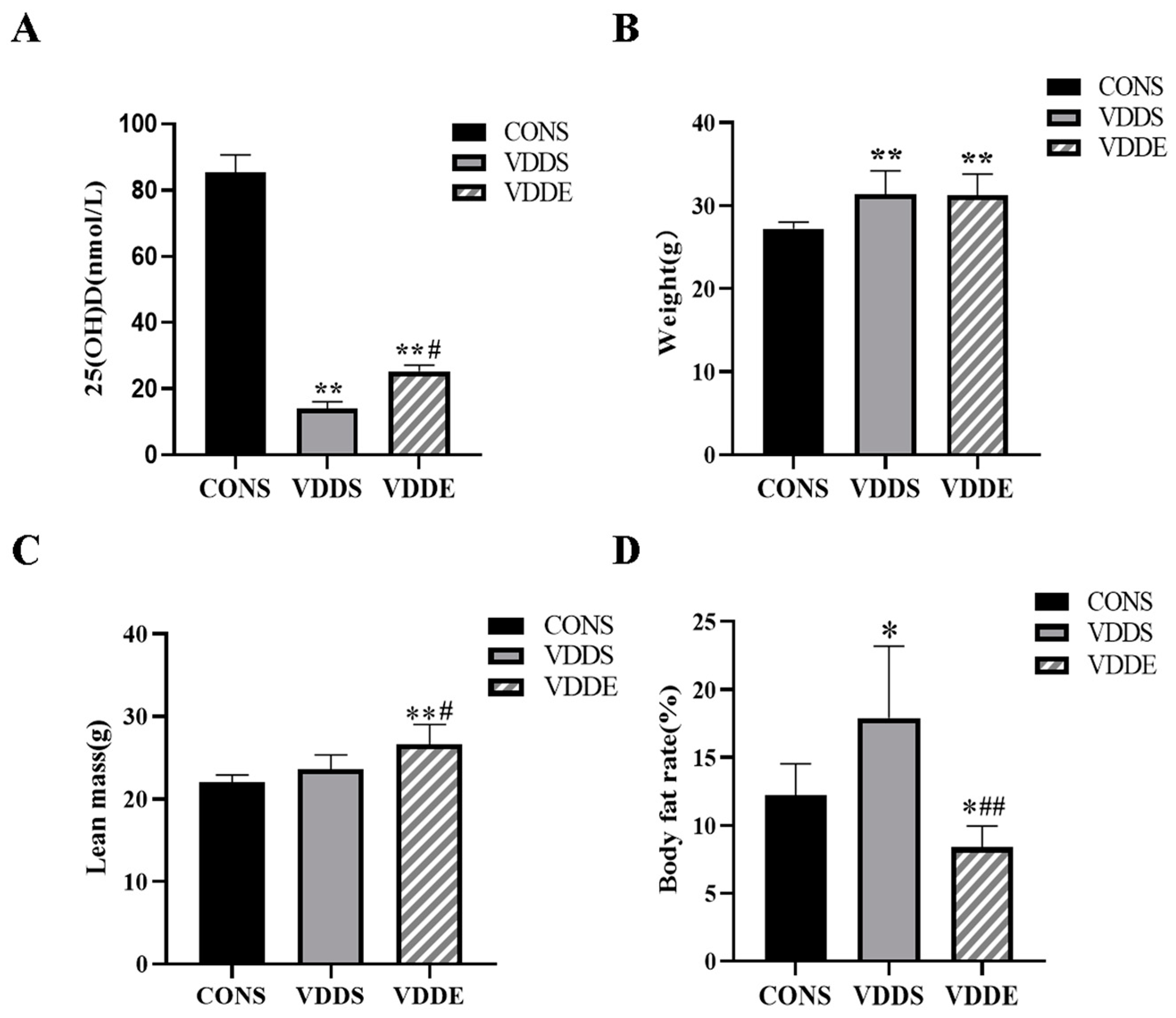
3.2. Effect of Aerobic Exercise Training on the Serum 25(OH)D Levels, Body Weight, Lean Mass and Body Fat Rate of Vitamin D-Deficient Mice
3.3. Aerobic Exercise Training Caused Myocardial Hypertrophy in Vitamin D-Deficient Mice
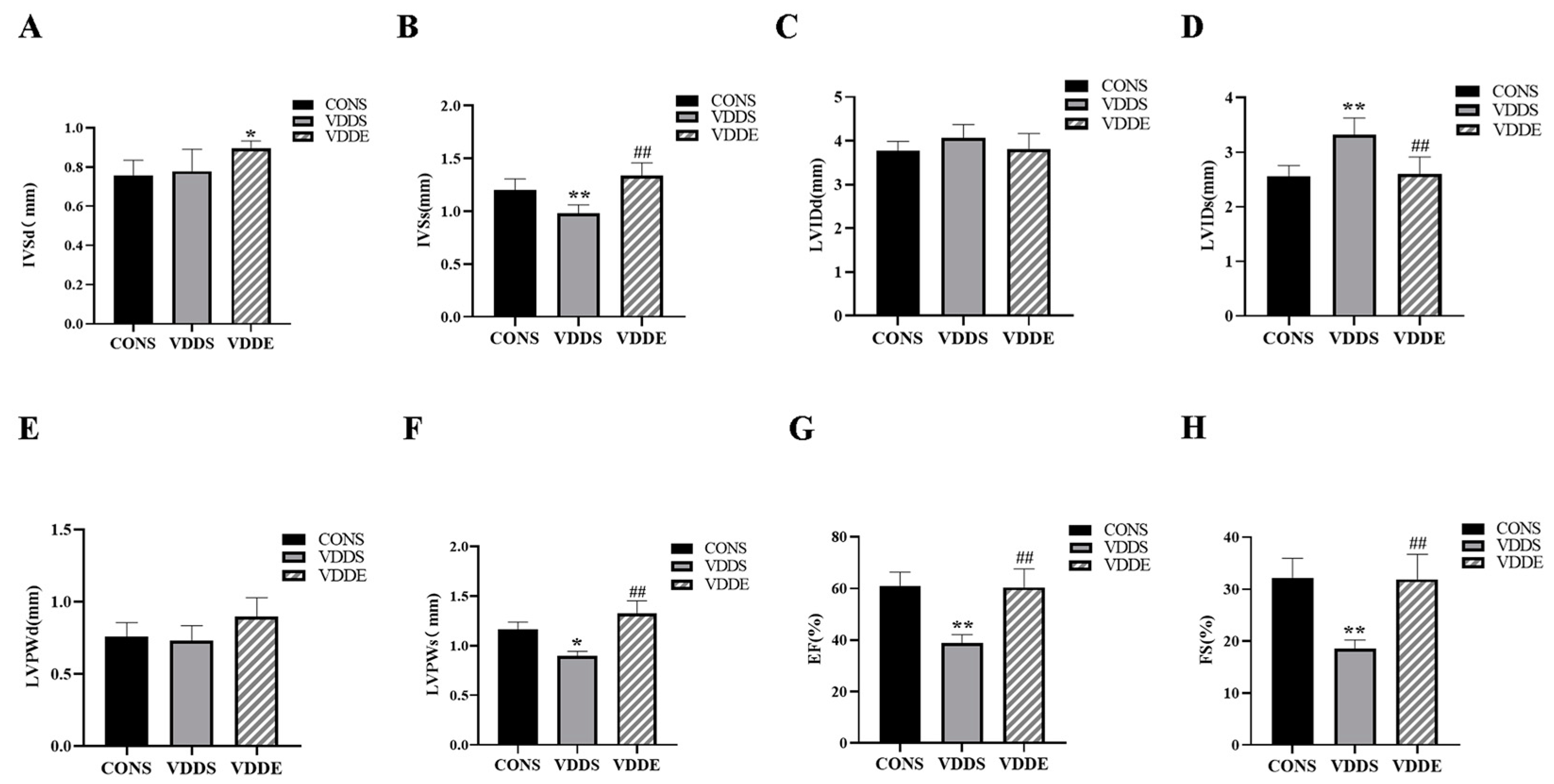
3.4. Aerobic Exercise Training Improved Cardiac Function in Vitamin D-Deficient Mice
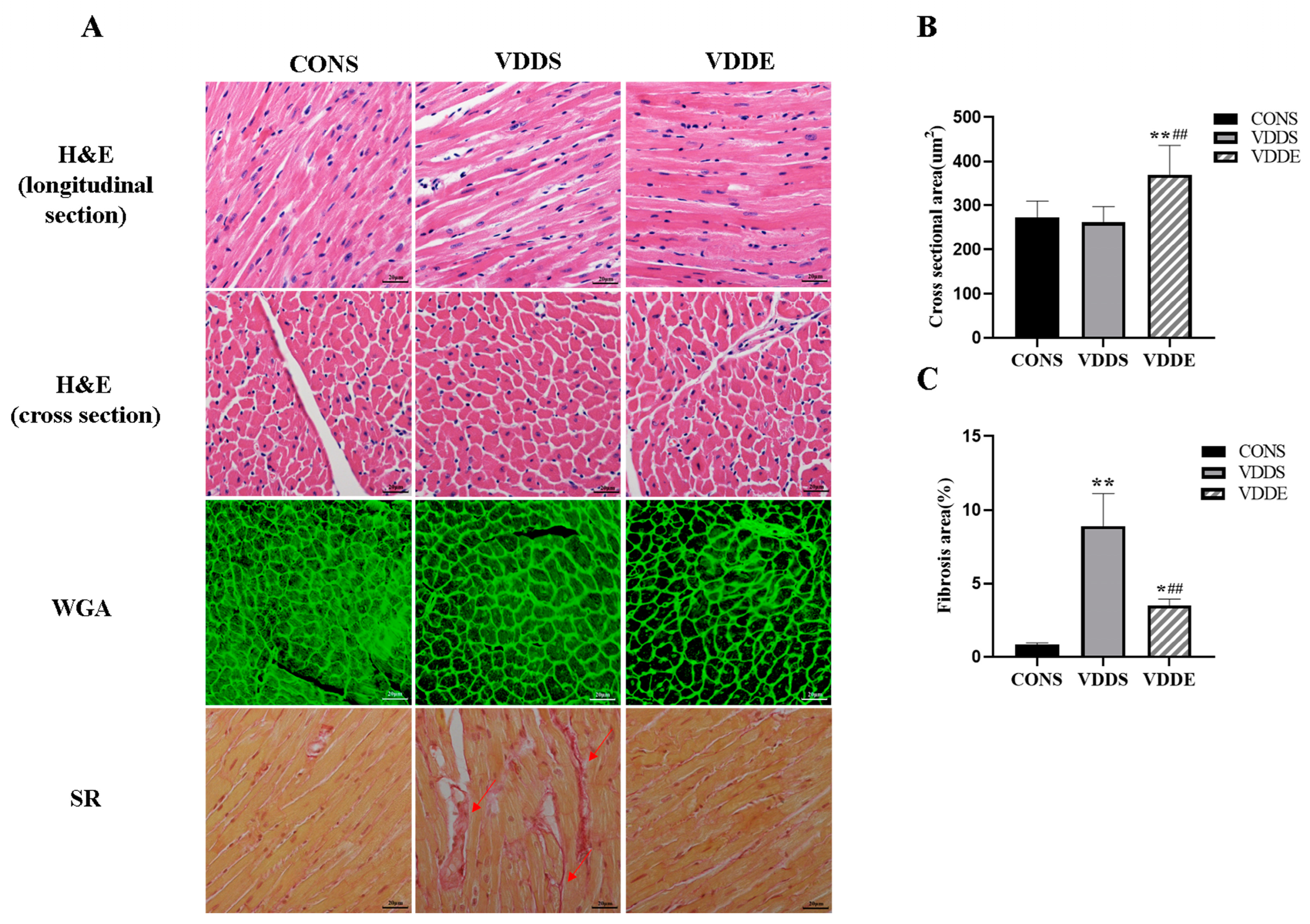
3.5. Effect of Aerobic Exercise Training on the Myocardial Morphology of Vitamin D-Deficient Mice
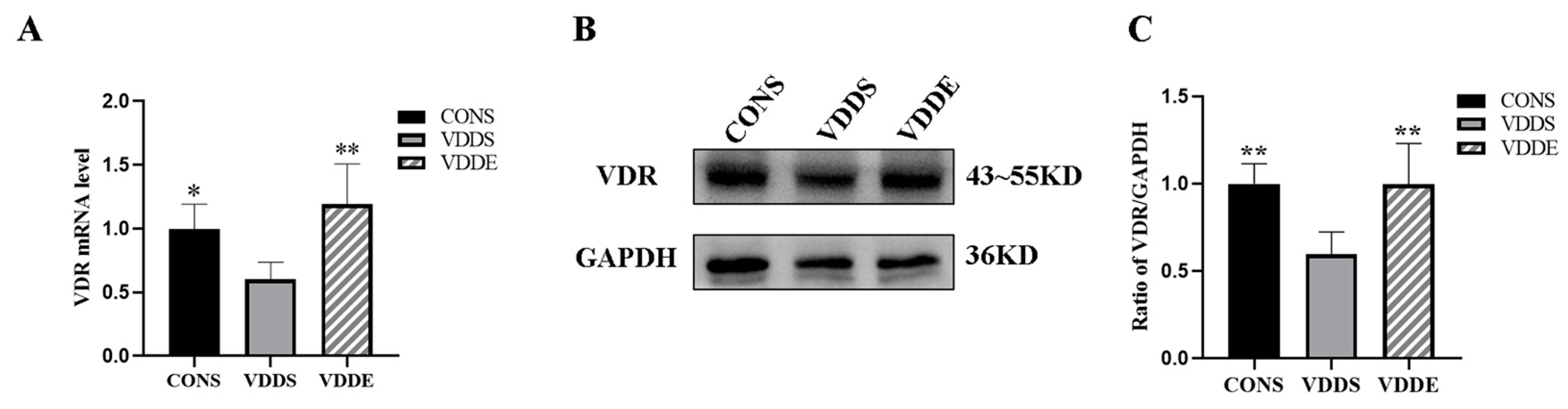
3.6. Aerobic Exercise Training Upregulated Myocardial VDR Expression in Vitamin D-Deficient Mice
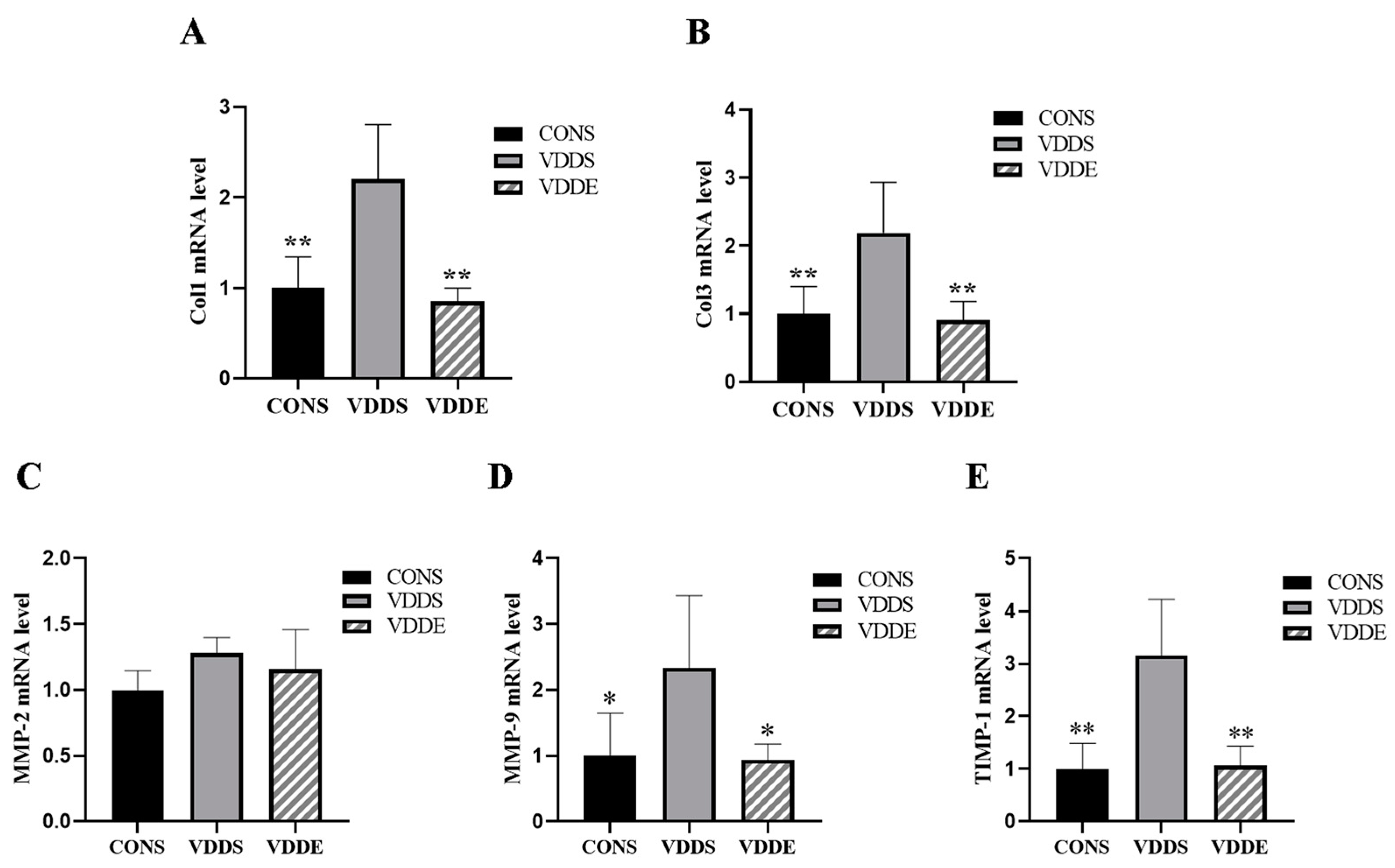
3.7. Aerobic Exercise Training Downregulated Myocardial Fibrotic Factors Expression in Vitamin D-Deficient Mice
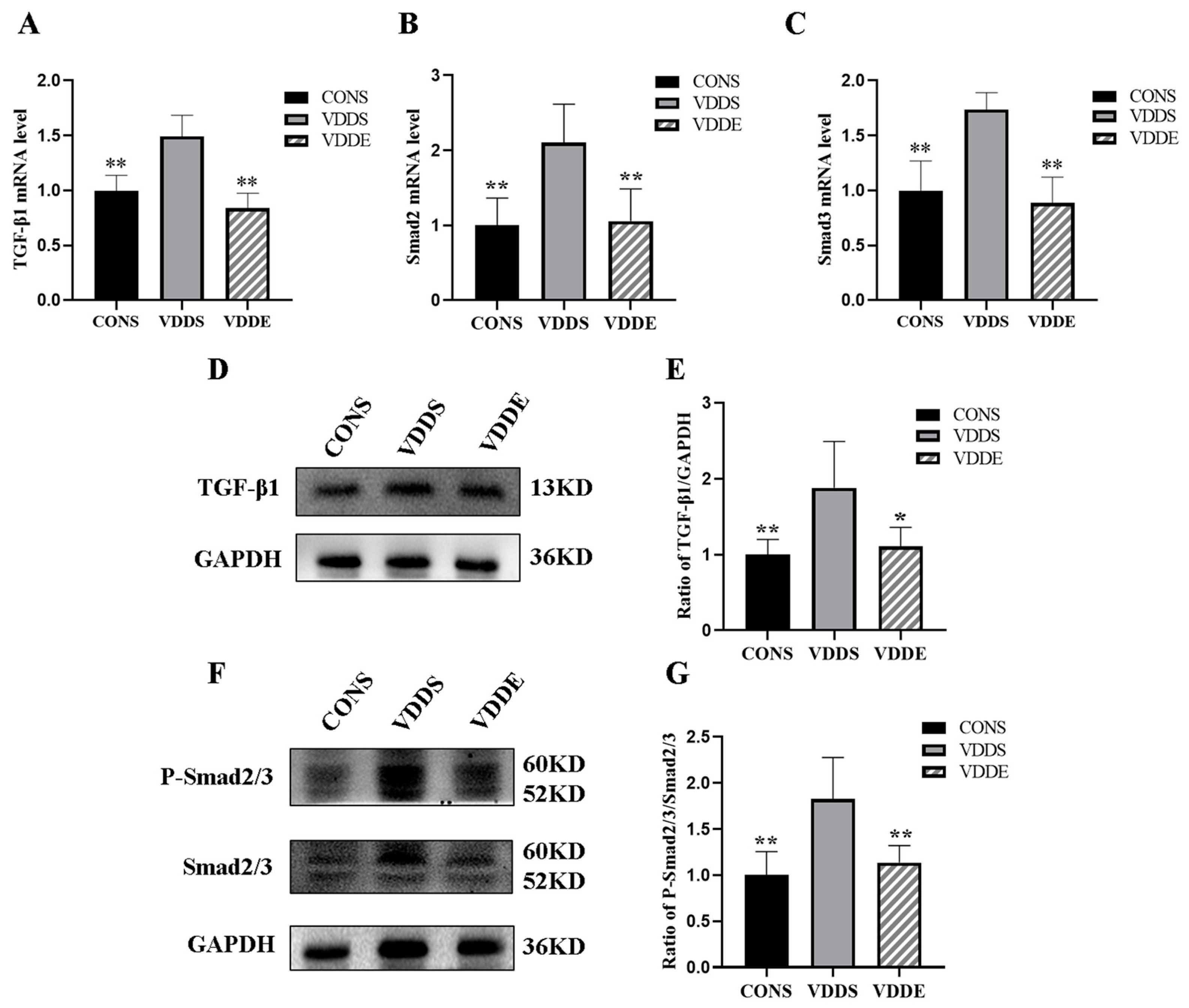
3.8. Aerobic Exercise Training Inhibited TGF-β1-Smad2/3 Pathway in Vitamin D-Deficient Mice
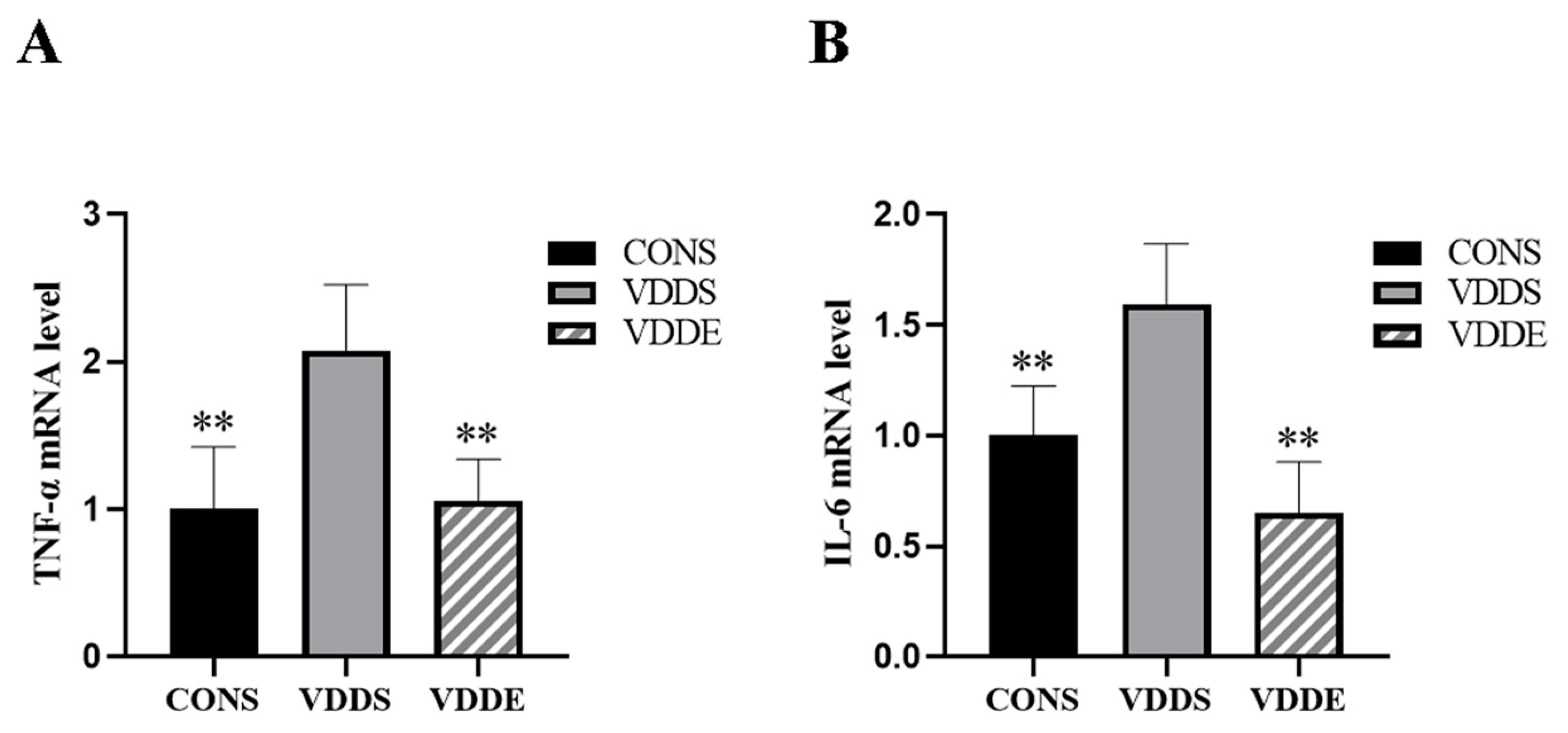
3.9. Aerobic Exercise Training Downregulated Inflammatory Factors Expression in Vitamin D-Deficient Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendes, M.M.; Charlton, K.; Thakur, S.; Ribeiro, H.; Lanham-New, S.A. Future perspectives in addressing the global issue of vitamin D deficiency. Proc. Nutr. Soc. 2020, 79, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; de Jongh, R.T.; van Schoor, N.M. Trends in Vitamin D Status Around the World. JBMR Plus 2021, 5, e10585. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Svobodova, J.; Imran, S.; Stanford, C.; Razzaque, M.S. Vitamin D deficiency: A single centre analysis of patients from 136 countries. J. Steroid Biochem. Mol. Biol. 2016, 164, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, D.B.; Xiao, G.B.; Ding, B.; Chen, E.Q. An epidemiology survey of vitamin D deficiency and its influencing factors. Med. Clin. 2020, 154, 7–12. [Google Scholar] [CrossRef]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Global Health 2020, 8, e134–e142. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Hill, T.R.; Aspray, T.J. The role of vitamin D in maintaining bone health in older people. Ther. Adv. Musculoskelet. Dis. 2017, 9, 89–95. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef]
- Javaheri, S.; Redline, S. Insomnia and Risk of Cardiovascular Disease. Chest 2017, 152, 435–444. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Gyongyosi, M.; Winkler, J.; Ramos, I.; Do, Q.T.; Firat, H.; McDonald, K.; Gonzalez, A.; Thum, T.; Diez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail 2017, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, M.; Hankins, S.R.; Nunez, A.E.; Watson, R.A.; Weinstock, P.J.; Newschaffer, C.J.; Eisen, H.J.; Drexel Cardiovascular Health Collaborative Education, Research, and Evaluation Group. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am. J. Cardiol. 2012, 110, 834–839. [Google Scholar] [CrossRef]
- Liu, L.; Cui, S.; Volpe, S.L.; May, N.S.; Sukumar, D.; DiMaria-Ghalili, R.A.; Eisen, H.J. Vitamin d deficiency and metabolic syndrome: The joint effect on cardiovascular and all-cause mortality in the United States adults. World J. Cardiol. 2022, 14, 411–426. [Google Scholar] [CrossRef]
- D’Amelio, P. Vitamin D Deficiency and Risk of Metabolic Syndrome in Aging Men. World J. Mens. Health 2021, 39, 291–301. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Rahman, A.; Fike, L.; Kavtaradze, N.; Uphoff, I.; Hooper, C.; Tangpricha, V.; et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J. Am. Coll. Cardiol. 2011, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yu, W.; Cao, H.; Li, J.; Liu, B.; Wang, J.; Shao, Y.; Fan, Y.; Yang, J.; Zhang, Q.; et al. Meta-analysis of circulating 25-hydroxyvitamin D levels and risk of cardiovascular and all-cause mortality in elderly population. Int. J. Cardiol. 2014, 176, 1025–1029. [Google Scholar] [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hypponen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, W.; Li, Y.; Wang, B.; Yu, J.; Xu, Z. Vitamin D Deficiency Harms Patients with Coronary Heart Disease by Enhancing Inflammation. Med. Sci. Monit. 2018, 24, 9376–9384. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Ziaie, N.; Saravi, M.; Norouzi, A.; Noei-Teymoordash, S.; Jokar-Darzi, F.; Norouzi, F.; Rajabi-Fumashi, M.; Zahedi-Tajrishi, F.; Norouzi, S. Association of vitamin D deficiency and premature coronary artery disease. Casp. J. Intern. Med. 2019, 10, 80–85. [Google Scholar]
- Perge, P.; Boros, A.M.; Geller, L.; Osztheimer, I.; Szilagyi, S.; Tahin, T.; Apor, A.; Nagy, K.V.; Zima, E.; Molnar, L.; et al. Vitamin D Deficiency Predicts Poor Clinical Outcomes in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. Dis. Markers 2019, 2019, 4145821. [Google Scholar] [CrossRef]
- Nikolova, M.; Nazifova-Tasinova, N.; Vankova, D.; Gerova, D.; Yotov, Y.; Atanasov, A.; Pasheva, M.; Kiselova-Kaneva, Y.; Galunska, B. Vitamin D Status in Patients with Atrial Fibrillation and Heart Failure—Is there a Link? Clin. Lab. 2021, 67, 1337–1348. [Google Scholar] [CrossRef]
- Patel, U.; Yousuf, S.; Lakhani, K.; Raval, P.; Kaur, N.; Okafor, T.; Shah, C.; Singh, H.; Martin, M.; Nwodika, C.; et al. Prevalence and Outcomes Associated with Vitamin D Deficiency among Indexed Hospitalizations with Cardiovascular Disease and Cerebrovascular Disorder-A Nationwide Study. Medicines 2020, 7, 72. [Google Scholar] [CrossRef]
- Nizami, H.L.; Katare, P.; Prabhakar, P.; Kumar, Y.; Arava, S.K.; Chakraborty, P.; Maulik, S.K.; Banerjee, S.K. Vitamin D Deficiency in Rats Causes Cardiac Dysfunction by Inducing Myocardial Insulin Resistance. Mol. Nutr. Food Res. 2019, 63, e1900109. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Kong, J.; Chen, S.; Cao, L.P.; Qiao, G.; Zheng, W.; Liu, W.; Li, X.; Gardner, D.G.; Li, Y.C. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E125–E132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hershey, S.; Ahmed, S.; Nibbelink, K.; Simpson, R.U. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J. Steroid Biochem. Mol. Biol. 2007, 103, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Meems, L.M.; Cannon, M.V.; Mahmud, H.; Voors, A.A.; van Gilst, W.H.; Sillje, H.H.; Ruifrok, W.P.; de Boer, R.A. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J. Steroid Biochem. Mol. Biol. 2012, 132, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Lin, K.; Wang, H.; Wei, H.; Ji, B.; Yang, Z.; Peng, C.; Xiao, X.; Deng, H. 1,25(OH)2D3 improves cardiac dysfunction, hypertrophy, and fibrosis through PARP1/SIRT1/mTOR-related mechanisms in type 1 diabetes. Mol. Nutr. Food Res. 2017, 61, 1600338. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Liu, J.; Wang, D.; Li, D.; Jiang, C.; Tang, Y.; Wang, J.; Zhang, T.; Li, Y.; et al. 1,25-Dihydroxyvitamin D attenuates diabetic cardiac autophagy and damage by vitamin D receptor-mediated suppression of FoxO1 translocation. J. Nutr. Biochem. 2020, 80, 108380. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Ruan, C.; Chen, M.; Cao, R.; Sheng, L.; Chang, N.; Xu, T.; Zhao, P.; Liu, X.; et al. Novel Insights into the Cardioprotective Effects of Calcitriol in Myocardial Infarction. Cells 2022, 11, 1676. [Google Scholar] [CrossRef]
- Xiong, M.; Gong, J.; Liu, Y.; Xiang, R.; Tan, X. Loss of vitamin D receptor in chronic kidney disease: A potential mechanism linking inflammation to epithelial-to-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2012, 303, F1107–F1115. [Google Scholar] [CrossRef]
- Walters, M.R. Newly identified actions of the vitamin D endocrine system. Endocr. Rev. 1992, 13, 719–764. [Google Scholar]
- Song, J.; Chen, X.; Cheng, L.; Rao, M.; Chen, K.; Zhang, N.; Meng, J.; Li, M.; Liu, Z.Q.; Yang, P.C. Vitamin D receptor restricts T helper 2-biased inflammation in the heart. Cardiovasc. Res. 2018, 114, 870–879. [Google Scholar] [CrossRef]
- Panizo, S.; Carrillo-Lopez, N.; Naves-Diaz, M.; Solache-Berrocal, G.; Martinez-Arias, L.; Rodrigues-Diez, R.R.; Fernandez-Vazquez, A.; Martinez-Salgado, C.; Ruiz-Ortega, M.; Dusso, A.; et al. Regulation of miR-29b and miR-30c by vitamin D receptor activators contributes to attenuate uraemia-induced cardiac fibrosis. Nephrol. Dial. Transplant. 2017, 32, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Wang, K.; Liang, X.; Wang, W.; Hu, X.; Huang, Z.; Wang, Y. Aerobic Exercise Ameliorates Myocardial Inflammation, Fibrosis and Apoptosis in High-Fat-Diet Rats by Inhibiting P2X7 Purinergic Receptors. Front. Physiol. 2019, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1alpha/PI3K/Akt signaling. J. Cell Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Cai, M.; Zhang, J.; Song, W.; Zhu, W.; Xi, L.; Tian, Z. Role of Muscle-Specific Histone Methyltransferase (Smyd1) in Exercise-Induced Cardioprotection against Pathological Remodeling after Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 7010. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, Y.; Bo, W.; Liang, Q.; Zhu, W.; Cai, M.; Tian, Z. Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-beta1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 12341. [Google Scholar] [CrossRef]
- Soori, R.; Amini, A.A.; Choobineh, S.; Eskandari, A.; Behjat, A.; Ghram, A.; Voltarelli, F.A. Exercise attenuates myocardial fibrosis and increases angiogenesis-related molecules in the myocardium of aged rats. Arch. Physiol. Biochem. 2022, 128, 1–6. [Google Scholar] [CrossRef]
- Xiao, L.; He, H.; Ma, L.; Da, M.; Cheng, S.; Duan, Y.; Wang, Q.; Wu, H.; Song, X.; Duan, W.; et al. Effects of miR-29a and miR-101a Expression on Myocardial Interstitial Collagen Generation After Aerobic Exercise in Myocardial-infarcted Rats. Arch. Med. Res. 2017, 48, 27–34. [Google Scholar] [CrossRef]
- Lijnen, P.J.; Petrov, V.V.; Fagard, R.H. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol. Genet. Metab. 2000, 71, 418–435. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J. Mol. Cell Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- De Metrio, M.; Milazzo, V.; Rubino, M.; Cabiati, A.; Moltrasio, M.; Marana, I.; Campodonico, J.; Cosentino, N.; Veglia, F.; Bonomi, A.; et al. Vitamin D plasma levels and in-hospital and 1-year outcomes in acute coronary syndromes: A prospective study. Medicine 2015, 94, e857. [Google Scholar] [CrossRef] [PubMed]
- Goleniewska, B.; Kacprzak, M.; Zielinska, M. Vitamin D level and extent of coronary stenotic lesions in patients with first acute myocardial infarction. Cardiol. J. 2014, 21, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Karur, S.; Veerappa, V.; Nanjappa, M.C. Study of vitamin D deficiency prevalence in acute myocardial infarction. Int. J. Cardiol. Heart Vessel 2014, 3, 57–59. [Google Scholar] [CrossRef]
- Lee, J.H.; Gadi, R.; Spertus, J.A.; Tang, F.; O’Keefe, J.H. Prevalence of vitamin D deficiency in patients with acute myocardial infarction. Am. J. Cardiol. 2011, 107, 1636–1638. [Google Scholar] [CrossRef]
- Tokarz, A.; Kusnierz-Cabala, B.; Kuzniewski, M.; Gacon, J.; Mazur-Laskowska, M.; Stepien, E.L. Seasonal effect of vitamin D deficiency in patients with acute myocardial infarction. Kardiol. Pol. 2016, 74, 786–792. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Ma, M.; Chen, W.; Hua, Y.; Jia, H.; Song, Y.; Wang, Y. Aerobic exercise ameliorates cardiac hypertrophy by regulating mitochondrial quality control and endoplasmic reticulum stress through M(2) AChR. J. Cell Physiol. 2021, 236, 6581–6596. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.S.V.R.; Stringuetta Belik, F.; Hueb, J.C.; de Souza Goncalves, R.; Costa Teixeira Caramori, J.; Perez Vogt, B.; Barretti, P.; Zanati Bazan, S.G.; De Stefano, G.; Martin, L.C.; et al. Aerobic Exercise Training and Nontraditional Cardiovascular Risk Factors in Hemodialysis Patients: Results from a Prospective Randomized Trial. Cardiorenal Med. 2019, 9, 391–399. [Google Scholar] [CrossRef]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxid. Med. Cell Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef]
- Hoseini, R.; Damirchi, A.; Babaei, P. Vitamin D increases PPARgamma expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition 2017, 36, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Aly, Y.E.; Abdou, A.S.; Rashad, M.M.; Nassef, M.M. Effect of exercise on serum vitamin D and tissue vitamin D receptors in experimentally induced type 2 Diabetes Mellitus. J. Adv. Res. 2016, 7, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.Q. Small mammalian animal models of heart disease. Am. J. Cardiovasc. Dis. 2016, 6, 70–80. [Google Scholar] [PubMed]
- Fernando, P.; Bonen, A.; Hoffman-Goetz, L. Predicting submaximal oxygen consumption during treadmill running in mice. Can. J. Physiol. Pharmacol. 1993, 71, 854–857. [Google Scholar] [CrossRef]
- Gogulothu, R.; Nagar, D.; Gopalakrishnan, S.; Garlapati, V.R.; Kallamadi, P.R.; Ismail, A. Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in Vitamin D deficient rats. J. Steroid Biochem. Mol. Biol. 2020, 197, 105525. [Google Scholar] [CrossRef]
- Yates, N.J.; Tesic, D.; Feindel, K.W.; Smith, J.T.; Clarke, M.W.; Wale, C.; Crew, R.C.; Wharfe, M.D.; Whitehouse, A.J.O.; Wyrwoll, C.S. Vitamin D is crucial for maternal care and offspring social behaviour in rats. J. Endocrinol. 2018, 237, 73–85. [Google Scholar] [CrossRef]
- Merino, O.; Sanchez, R.; Gregorio, B.M.; Sampaio, F.J.; Risopatron, J. Effects of Diet-Induced Obesity and Deficient in Vitamin D on Spermatozoa Function and DNA Integrity in Sprague-Dawley Rats. Biomed. Res. Int. 2018, 2018, 5479057. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Pin, L.; Zhu, S.; Zhong, X.; Zhang, Y.; Shun, M.; Liu, Y.; Hou, M. Plantamajoside attenuates isoproterenol-induced cardiac hypertrophy associated with the HDAC2 and AKT/ GSK-3beta signaling pathway. Chem. Biol. Interact. 2019, 307, 21–28. [Google Scholar] [CrossRef]
- Thomas, C.V.; Coker, M.L.; Zellner, J.L.; Handy, J.R.; Crumbley, A.J., 3rd; Spinale, F.G. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 1998, 97, 1708–1715. [Google Scholar] [CrossRef]
- Li, Y.Y.; Kadokami, T.; Wang, P.; McTiernan, C.F.; Feldman, A.M. MMP inhibition modulates TNF-alpha transgenic mouse phenotype early in the development of heart failure. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H983–H989. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feng, Y.Q.; Kadokami, T.; McTiernan, C.F.; Draviam, R.; Watkins, S.C.; Feldman, A.M. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 12746–12751. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc. Res. 2000, 46, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Wang, Y.; Li, H.; Li, Y.; Sahil, A.; Lv, J.; Liu, Y.; Yang, Z.; Dong, R.; Xue, H.; et al. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-beta1/Smads signaling in diabetic cardiomyopathy. FASEB J. 2020, 34, 5282–5298. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Hu, C.; Song, Q.; Li, Y.; Da, X.; Yu, Y.; Li, H.; Clark, I.M.; Chen, Q.; Wang, Q.K. ADAMTS16 activates latent TGF-beta, accentuating fibrosis and dysfunction of the pressure-overloaded heart. Cardiovasc. Res. 2020, 116, 956–969. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Taniguchi, H.; Kubo, T.; Higuchi, M. Effects of chronic endurance exercise training on serum 25(OH)D concentrations in elderly Japanese men. Endocrine 2018, 59, 330–337. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.-B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an Acute Bout of Endurance Exercise on Serum 25(OH)D Concentrations in Young Adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef]
- Luckey, S.W.; Haines, C.D.; Konhilas, J.P.; Luczak, E.D.; Messmer-Kratzsch, A.; Leinwand, L.A. Cyclin D2 is a critical mediator of exercise-induced cardiac hypertrophy. Exp. Biol. Med. 2017, 242, 1820–1830. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; Patterson, N.L.; McMullen, J.R. Understanding Key Mechanisms of Exercise-Induced Cardiac Protection to Mitigate Disease: Current Knowledge and Emerging Concepts. Physiol. Rev. 2018, 98, 419–475. [Google Scholar] [CrossRef]
- Weeks, K.L.; Tham, Y.K.; Yildiz, S.G.; Alexander, Y.; Donner, D.G.; Kiriazis, H.; Harmawan, C.A.; Hsu, A.; Bernardo, B.C.; Matsumoto, A.; et al. FoxO1 is required for physiological cardiac hypertrophy induced by exercise but not by constitutively active PI3K. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1470–H1485. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Mehdipoor, M.; Damirchi, A.; Razavi Tousi, S.M.T.; Babaei, P. Concurrent vitamin D supplementation and exercise training improve cardiac fibrosis via TGF-beta/Smad signaling in myocardial infarction model of rats. J. Physiol. Biochem. 2021, 77, 75–84. [Google Scholar] [CrossRef] [PubMed]
| CONS | VDDS | VDDE | |
|---|---|---|---|
| HW/TL (mg/mm) | 7.44 ± 0.61 | 7.65 ± 0.72 | 8.65 ± 0.85 *# |
| LVW/TL (mg/mm) | 2.87 ± 0.34 | 3.00 ± 0.19 | 3.48 ± 0.38 **# |
| CONS | VDDS | VDDE | |
|---|---|---|---|
| Ca (mmol/L) | 1.68 (1.63, 1.70) | 1.67 ± 0.04 | 1.66 (1.56, 1.69) |
| P (mmol/L) | 1.97 ± 0.46 | 2.15 ± 0.33 | 2.26 ± 0.32 |
| HDL-C (mmol/L) | 4.10 ± 0.47 | 3.74 ± 0.50 | 3.84 ± 0.21 |
| LDL-C (mmol/L) | 1.18 ± 0.44 | 0.87 ± 0.64 | 1.32 ± 0.43 |
| TG (mmol/L) | 1.12 ± 0.17 | 1.28 ± 0.17 | 1.14 ± 0.16 |
| TC (mmol/L) | 2.65 ± 0.42 | 2.71 ± 0.30 | 2.81 ± 0.33 |
| PTH (ng/L) | 210.52 ± 51.03 | 178.19 ± 50.39 | 225.74 ± 30.16 |
| INS (ng/L) | 22.40 ± 4.27 | 22.96 ± 7.37 | 21.32 ± 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Wang, K.; Zhang, J.; Cao, Z.-B. Aerobic Exercise Ameliorates Myocardial Fibrosis via Affecting Vitamin D Receptor and Transforming Growth Factor-β1 Signaling in Vitamin D-Deficient Mice. Nutrients 2023, 15, 741. https://doi.org/10.3390/nu15030741
Cui X, Wang K, Zhang J, Cao Z-B. Aerobic Exercise Ameliorates Myocardial Fibrosis via Affecting Vitamin D Receptor and Transforming Growth Factor-β1 Signaling in Vitamin D-Deficient Mice. Nutrients. 2023; 15(3):741. https://doi.org/10.3390/nu15030741
Chicago/Turabian StyleCui, Xiaoning, Ke Wang, Jinghua Zhang, and Zhen-Bo Cao. 2023. "Aerobic Exercise Ameliorates Myocardial Fibrosis via Affecting Vitamin D Receptor and Transforming Growth Factor-β1 Signaling in Vitamin D-Deficient Mice" Nutrients 15, no. 3: 741. https://doi.org/10.3390/nu15030741
APA StyleCui, X., Wang, K., Zhang, J., & Cao, Z.-B. (2023). Aerobic Exercise Ameliorates Myocardial Fibrosis via Affecting Vitamin D Receptor and Transforming Growth Factor-β1 Signaling in Vitamin D-Deficient Mice. Nutrients, 15(3), 741. https://doi.org/10.3390/nu15030741






