Abstract
(1) Background: German working adults are particularly at risk of non-alcoholic fatty-liver disease (NAFLD), which is connected to increased cardiovascular and overall morbidity and mortality. Dietary behavior (DB) and health knowledge are crucial factors in the conceptual NAFLD model, which can directly influence this disease. These two factors largely align with the concept of food literacy (FL), which deals with proficiency in food-related skills and knowledge to promote healthy DB and prevent NAFLD. However, the potential of FL for NAFLD prevention remains unknown, because FL has not been tested in connection with DB and NAFLD. Therefore, the current study examined the direct and indirect connections between FL, DB, and NAFLD in a mediation model. (2) Methods: A total of 372 working adults (38% female) participated in a cross-sectional study by completing self-report questionnaires on FL and DB. In addition, an independent physician assessed the fatty-liver index (FLI) as an indicator of NAFLD in an occupational health checkup. (3) Results: The mediation model revealed that FL had a direct moderate connection with DB (β = 0.25, p < 0.01), but no direct connection with the FLI (β = −0.05, p = 0.36). However, DB showed a small to moderate connection with the FLI (β = −0.14, p = 0.01), which could indicate the indirect-only mediation of the relationship between FL and NAFLD via DB. (4) Conclusion: These results confirm the value of DB for the prevention of NAFLD. In addition, FL might be a vital component for improving DB and thereby function as a resource in the prevention of NAFLD. However, future longitudinal research is needed to substantiate the value of FL with respect to NAFLD.
1. Introduction
Non-alcoholic fatty-liver disease (NAFLD) is the fatty degeneration of the liver with more than 5% hepatocytes without other identifiable causes, such as excessive alcohol consumption [1]. The global disease burden of NAFLD in the adult population has risen due to the increasing prevalence rates of obesity and diabetes mellitus type II, caused by drastic lifestyle changes during the last few decades, amongst other factors [2]. In 2022, the prevalence of NAFLD in adults reached more than 30% both globally and in Germany [3,4,5,6]. This places NAFLD as the leading risk factor for cirrhosis and hepatocellular carcinoma in adults. In addition, NAFLD is the hepatic manifestation of metabolic syndrome, being closely linked to other non-communicable diseases, such as diabetes mellitus [2], and increased cardiovascular and overall morbidity and mortality [7].
The conceptual NALFD model by Lazarus et al. [8] postulated the importance of the underlying influences in the development of this condition, with health systems and individual environments holding the potential to provide access to affordable quality NAFLD care for the population at the environmental level. At this level, similar to prevention strategies for other non-communicable diseases [9], occupational health and safety management could play a central role in mitigating the burden of NAFLD, due to the high proportion of adults’ waking hours spent at work [10,11]. In addition to the importance of the underlying environmental influences, the conceptual NAFLD model highlights the value of direct influences on NAFLD, such as dietary behavior, mental health, and health knowledge. These direct influences largely align with findings in working adults, which highlights the relevance of the workplace for NAFLD prevention by indicating an increased risk for NAFLD in this population due to unhealthy dietary behavior [3], occupational stress [12], and long working hours [13].
Focusing on DB might be particularly vital because of the strong association of DB with overweight and obesity as well as diabetes mellitus type II, which play a central part in the etiology of NAFLD [14,15]. To promote healthy DB and, consequently, to prevent NAFLD at the environmental level, global and national recommendations for DB offer guidelines such as eating 400 g of fruits and vegetables per day and deriving less than 30% of one’s daily energy supply from fats [16]. With respect to these guidelines, a national study at German worksites showed that most working adults in Germany do not reach these recommendations and fail to adhere to the guidelines. For example, the 24 h intake of energy by carbohydrates was insufficient, while the overall intake by fat was too high in this population [17]. In addition, less than 10% of German working adults met the guidelines for vegetable and fruit intake, while the overall meat intake in this population was too high [18]. In addition to this characterization of DB in German working adults, a systematic review by Houttu et al. [19] also showed an overall effect of dietary changes on NAFLD in working adults. However, the long-term effectiveness of dietary interventions in the workplace is limited [20], which stresses the need to explore the determinants of DB to effectively prevent NAFLD in this target group.
1.1. Introducing Food Literacy in Connection with the Food Choice Framework
These findings highlight the importance of healthy DB for NAFLD, and also emphasize the challenges to effectively explaining and improving DB in working adults. The food choice framework by Chen and Antonelli [21] can be used to explain DB. This model comprises food-internal factors (e.g., sensory features) and food-external factors (e.g., food information and environment) on the first level, which impact personal-state factors (e.g., habits and experiences) and cognitive factors (e.g., knowledge and skills) on the second level within an individual’s cultural, economic, and political context. These second-level factors consequentially determine an individual’s food choice and DB.
A cognitive factor in the food choice framework that has received increasing attention over the last two decades and shares substantial conceptual connections with DB and health is the concept of food literacy (FL) [22,23,24]. FL describes how to find, understand, assess, and apply information with respect to one’s ability to plan, prepare, and consume food that is suitable or needed to promote health [25,26,27]. Truman et al. [28] showed in a recent review that most studies adopt Nutbeam’s definition [29] of health literacy to describe the construct of FL. This definition comprises functional, interactive, and critical FL elements, which focus on understanding, communicating, and critically applying food-related information towards a healthy dietary lifestyle. Consequently, functional FL comprises the understanding of information about food and the acquisition of nutritional knowledge, while interactive FL covers aspects of sharing knowledge and opinions on food-related information, and critical FL describes the realization of the information and knowledge, such as choosing between healthy and unhealthy food items in the supermarket, as well as cooking skills [28].
FL shows numerous connections with the food choice framework, in addition to the conceptual integration as a cognitive factor, because this concept is positively associated with the personal-state factors of food experience and habits by shaping a healthy dietary lifestyle in working adults. From an environmental perspective, FL directly impacts food-external factors, such as the understanding of food information or the navigation of the individual food environment, for example, by communicating knowledge on healthy food choice at the workplace to facilitate healthy DB [30,31]. In addition, FL is sensitive towards social, economic, and cultural components, with current conceptualizations postulating that individual FL cannot be separated from contextual factors that influence personal DB [32].
1.2. Current State of Research on the Connections between Food Literacy, Dietary Behavior, and Indicators of Non-Alcoholic Fatty-Liver Disease
These findings are in line with the conceptual connection of FL with the food choice framework by Chen and Antonelli [21]. However, this integration of FL can also be extended with respect to the aforementioned conceptual NAFLD model proposed by Lazarus et al. [8]. DB and health knowledge are incorporated as direct influences on NAFLD, sharing substantial common ground with the food-related knowledge component of FL and representing the desired outcomes of this concept by aiming for healthy DB [27]. In summary, the theoretical background assumes a positive connection between FL and healthy DB and a lower risk for NAFLD and implies either a direct or indirect connection between FL and NAFLD, with DB serving as a potential mediator [8,21,33,34].
Empirical research on FL and DB supports this assumption, with a scoping review by Vettori et al. [35] demonstrating a positive relationship between FL and improved dietary behavior, such as healthier food choices and less use of sweetened drinks, across various adult samples. In addition, an intervention study in Australian adults confirmed these findings and displayed a positive effect of FL on DB [36]. With respect to the workplace setting, a longitudinal study in German office workers displayed a positive small-to-moderate influence of FL on working adults’ DB over the course of 18 months following a three-week workplace health promotion program (WHPP) focusing on FL and DB [37].
While these findings confirm the connection between FL and DB, the literature on the connection between FL and health and NAFLD, in particular, is scarce [38]. However, Cheng et al. [39] investigated a health-promoting association between the closely linked factors of health literacy and fatty-liver disease in a sample of Taiwanese adults, which might also imply a possible direct connection between FL and NAFLD. Furthermore, an observational study in nursing students in Australia found a potentially health-enhancing connection between FL with NAFLD-related lipid measurements [40,41]. Although these studies might imply a positive direct connection between FL and NAFLD, mediating factors such as DB and health behavior were not included, which leaves the question of whether the connection between FL and NAFLD in working adults is direct or indirect unanswered. A structural equation modeling approach in German working adults examined a direct positive connection between domain-specific health literacy with respect to physical activity, namely physical-activity-related health competence, and the objective health outcome of metabolic syndrome [42], which is closely linked to NALFD [43].
1.3. Aims of the Study
Considering the relevance of preventing NAFLD in German working adults and improving DB in this population, a focus on FL might be particularly crucial in the prevention and treatment of this disease. FL might play a vital role and promote healthy DB and thereby impact NAFLD in German working adults. Current theories and empirical findings confirm the connection between FL and DB but are conceptually inconsistent as to a direct or indirect connection between FL and NAFLD. In addition to this theoretical inconsistency, there seems to be a strong demand for empirical research to close this gap and address FL, DB, and NALFD in German working adults in particular [26,44]. Explaining the connection between FL and NAFLD might be particularly important, as FL might be relevant for practitioners in the development of WHPPs to improve DB and to mitigate the burden of NAFLD. To the knowledge of the authors, previous studies have not employed a mediation analysis to address the direct and indirect connections between FL and NAFLD potentially mediated by DB. Results from such an analysis might be of value for longitudinal studies on potential causal pathways. Therefore, this study investigated the connections between FL, DB, and NAFLD in German working adults via a mediation analysis. Thus, the following hypotheses regarding potential connections between FL and DB and NAFLD were derived:
H1:
FL has a direct positive connection with DB in working adults.
H2:
FL has a direct negative connection with indicators of NAFLD in working adults.
H3:
FL has an indirect negative connection with NAFLD mediated by dietary behavior in working adults.
2. Materials and Methods
2.1. Study Design
This study’s data originated from a research project evaluating aspects of occupational health management in a large German technology company aiming to refine their WHPPs. The data concerning this research question were collected at the start of a three-week in-person WHPP with multiple cohorts taking place from April 2019 to December 2019. Self-report measures as well as objective health measures were assessed in a quantitative, cross-sectional, and monocentric study. Participants received information via e-mail or by a brochure. They had to submit written consent before filling out the paper/pencil survey and take part in an occupational health check. The study fulfilled the company’s data privacy guidelines, in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of the School of Medicine of the Technical University of Munich (IRB number: 645/20 S-KH).
2.2. Measures
In addition to the self-report measures for the level of food literacy and DB, objective health parameters were assessed by an independent team of physicians via an occupational check at the beginning of the WHPP to calculate the FLI. In addition, subjective health, gender, age, relationship status, educational status, type of occupation, medical history, and medication intake were included as control variables.
2.2.1. Self-Report Measures
- The short food literacy questionnaire (SFLQ) developed by Gréa Krause et al. [26] was adapted for German subjects in order to analyze food literacy. The SFLQ consists of 12 items with responses on 4-, 5- and 6-point Likert scales ranging from very bad to very good, with total scores ranging from 7 to 52. It includes questions on functional, interactive, and critical literacy regarding dietary behavior (e.g., “How easy is it for you to evaluate if a specific food is relevant for a healthy diet?”). Higher scores indicate higher food literacy. With a Cronbach’s alpha of α = 0.82, the internal consistency was good [26].
- The working adults’ DB was evaluated by Winkler and Döring’s [45] food frequency list (FFL). The FFL examines an individual’s frequency of food intake in a total of 25 food groups (e.g., “How often do you eat the following foods? “cooked vegetables”) on a 6-point Likert scale (6 = nearly daily, 5 = several times per week, 4 = about once a week, 3 = several times per month, 2 = once a month, and 1 = never) [45,46]. In addition, the FFL provides a score for the individual’s DB based on an optimal intake of each food group derived from national DB recommendations [47], with total scores ranging from 0 to 30. The higher the total score on the FFL, the better the participant’s DB [47].
- Subjective health was determined as a control variable by using the Short Form 12 Version 2.0 (SF-12) in German, which has been validated in several studies, for example, in the German adult population [48,49]. The SF-12 captures the physical and mental components of an individual’s subjective health [48,50]. The SF-12 contains 12 questions, which aim to assess the following eight domains: general health, physical functioning, physical role, body pain, vitality, social functioning, emotional role, and mental health. Standardized component scores were calculated for both mental and physical health ranging from 0 to 100, with higher scores indicating better subjective health. This study combined the mental and the physical component scores to create an overall indicator of subjective health, as has been reported in previous studies [51].
- The Godin–Shepard Leisure-Time Physical Activity Questionnaire (GSLTPAQ) [52] examines individuals’ PA during leisure time at mild, moderate, and vigorous intensity (e.g., “Over the last 7 days (i.e., the last week), how many times on average did you do the following kinds of exercise for more than 30 min during your free time?”) as a control variable. This measure results in the cumulative weighted leisure score index (LSI), which displays the amount of leisure-time physical activity (PA), with high values indicating higher levels of leisure-time PA [53].
- The Alcohol Use Disorders Identification Test Consumption (AUDIT-C) was employed to control for alcohol consumption when testing the hypotheses of the present study [54]. AUDIT-C is a reliable and valid measure for assessing alcohol consumption in German working adults on a 5-point Likert scale (e. g. “How often do you have a drink containing alcohol?”) [55]. AUDIT-C scores range from 0 to 12, with higher scores indicating an increased probability of risky alcohol consumption.
- Furthermore, the participants’ sociodemographic statuses were also assessed: gender, age, relationship status, educational status, type of occupation, medical history, and medication intake. For statistical analysis, educational status was classified into three categories according to the CASMIN Educational Classification for International Research [56]. Participants’ self-reports on prior diseases and medications, which might show an association with indicators of NAFLD or DB, were included as additional control variables. The number of self-reported diseases and medications was counted to obtain an estimate for participants’ medical histories and medications. The following diseases (a) and medications (b) were included based on reviewing the literature:
- a.
- Diabetes mellitus type II, metabolic syndrome, obesity, dyslipidemia [57,58].
- b.
- Amiodarone, Glucocorticoids, Nifedipine/Diltiazem, Tamoxifen/synthetic Estrogens, highly active antiretroviral therapy [59].
2.2.2. Objective Health Measures
To assess the fatty-liver index (FLI), external occupational physicians determined the biological parameters of the participants via a voluntary occupational health check. The FLI was developed over the last few years to screen for NAFLD without the need for a biopsy or imaging measures [60]. This score contains the biomarkers of body mass index (BMI), waist circumference (WC), triglycerides (TG), and γ-glutamyltransferase (GGT) and was confirmed as a valid and economic indicator for NAFLD risk prediction by several studies [61,62]. Participants were encouraged to fast for eight hours before blood sampling for the different laboratory parameters as part of the health check. Blood was drawn from their antecubital vein in the laboratory. The WC was measured while participants were standing in the middle of the highest protruding spot of their iliac crest. The FLI was calculated according to the formula by Bedgoni et al. [5], with higher values of FLI indicating a higher risk of NAFLD [5,61] (cf. Appendix A).
2.3. Data Analysis
Data and statistical analyses were processed with R and RStudio (Version 4.2.1, RStudio, PBC, Boston, MA, USA). Missing data were imputed using multivariate chained equations [63]. Multivariate outliers were excluded with the Mahalanobis D2 measure by following the recommendations of Tabachnick and Fidell as well as Kline [63,64], which also account for univariate outliers. The assumptions of normality, linearity, homoscedasticity, and the independence of residuals were checked and fulfilled before conducting multiple regression and path analysis [65,66]. Means (M), standard deviations (SD), and bivariate correlations (r) where calculated for FL, DB, FLI, HRQOL, and alcohol consumption with a significance level of p < 0.05. In addition, to address the relationship between different nutrients, the 25 food groups of the FFL are displayed in connection with FL, FLI, and HRQOL in the Appendix B. Linear regression for the dependent variables of FL, DB, and FLI and the independent variables of HRQOL, alcohol consumption, gender, age, relationship status, educational status, medical history, and medication intake guided the choice of the included control variables in the path analysis. This approach was conducted similarly in previous path analytic research [42]. A p-value < 0.05 was considered as statistically significant. The path analysis examined the connection of NAFLD with FL and DB in a mediation model. The model fit was measured using the chi-square/df value (X2/df: acceptable, ≤4; good, ≤2); the comparative-fit index (CFI: acceptable, ≥0.95; good, ≥0.97); the root-mean-square error of approximation (RMSEA: adequate, ≤0.08; good, ≤0.05); and the standardized-root-mean-square residual (SRMR: acceptable, ≤0.01; good, ≤0.05) [67,68] for the dependent variables. Furthermore, 95% confidence intervals (95% CI), standard errors (SE), and standardized estimates (β) were calculated for the mediation analysis. Additionally, we measured the adjusted total explained variance by the predictors as R2. Following the recommendations of Cohen [69], the standardized effect sizes of correlation analysis, multiple linear regression, and path analysis greater than ~0.50, ~0.30, and 0.10 were interpreted as strong, moderate, and small, respectively.
3. Results
3.1. Participant Characteristics
The mean age of the 372 participants was 50.8 years (SD 6.3 years); 230 (62%) were male, and 142 (38%) were female. The national background of all the participants was German. Table 1 shows the sociodemographic variables sorted by gender.

Table 1.
Baseline characteristics of the study population.
3.2. Bivariate Correlations
FL showed a moderate positive correlation with DB (r = 0.32, p < 0.001). DB and FLI displayed a negative moderate correlation (r = −0.27, p < 0.001). In addition, a small-to-moderate negative correlation was measured between FL and the FLI (r = −0.16, p < 0.05; Table 2). With respect to the food groups in the DB questionnaire, FL showed a small-to-moderate positive connection with the intake frequency of salad (r = 0.25, p < 0.001), vegetables (r = 0.20, p < 0.001), fruit (r = 0.27, p = 0.003), and oats and granola (r = 0.27, p < 0.001; Appendix B). FL displayed a small-to-moderate negative connection with the intake frequency of processed meat (r = −0.22, p < 0.05), white bread (r = −0.21, p < 0.001), and soda and lemonade (r = −0.31, p < 0.001). Furthermore, we observed a negative small-to-moderate connection between the FLI and the intake frequency of vegetables (r = −0.16, p = 0.001) and fruits (r = −0.30, p < 0.001) as well as oats and granola (r = −0.28, p < 0.001). There was a connection between the FLI and the intake frequency of red meat (r = 0.24, p < 0.001), processed meat (r = 0.28, p < 0.001), and soda and lemonade (r = 0.23, p < 0.001).

Table 2.
Means, SDs, and bivariate correlations for FL, DB, FLI, HRQOL, and alcohol consumption.
3.3. Multiple Linear Regression
Multiple linear regression with the control variables as indicators showed lower FL scores for male working adults compared to females, with a moderate negative effect size (β = −0.49, p < 0.001; Appendix C). FL also displayed a small positive connection to leisure-time PA (β = 0.15, p < 0.01) and the intake of medication (β = 0.12, p = 0.02). Male working adults (β = −0.32, p < 0.01) and blue-collar workers (β = −0.39, p = 0.01) showed lower levels of DB in comparison to female working adults and white-collar workers, respectively. DB displayed a positive connection with age (β = 0.11, p < 0.01), leisure-time PA (β = 0.22, p < 0.01), and HRQOL (β = 0.18, p < 0.001)). Older working adults showed lower FLI scores (β = −0.11, p = 0.03). Male working adults had higher FLI scores in comparison to female working adults, with a moderate effect size (β = 0.35, p < 0.01). HRQOL (β = −0.13, p < 0.01) and leisure-time PA (β = −0.13, p < 0.01) showed a small negative connection with FLI, while alcohol consumption had a positive small-to-moderate connection with the FLI (β = 0.17, p < 0.01).
3.4. Path Analysis
The global fit of the path analysis model was good (Χ2/df = 1.03 CFI = 0.99, RMSEA = 0.01, SRMR = 0.02). The connection between FL and DB showed a significant moderate positive effect size (β = 0.25, p < 0.001), while the path analysis model explained 15% of the adjusted variance for DB. FL and FLI displayed no significant connection in the path analysis (β = −0.05, p = 0.36). A small-to-moderate negative effect size was displayed between DB and FLI (β = −0.14, p = 0.01). The path model explained 13% of the adjusted variance for FLI. A simplified version of the path diagram without control variables is presented in Figure 1.
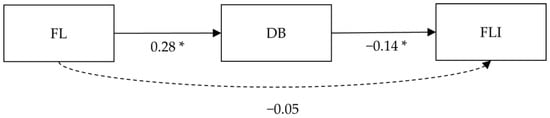
Figure 1.
Simplified version of the path analysis. Note: * p < 0.05; FL = food literacy, DB = dietary behavior, FLI = fatty-liver index, insignificant paths are displayed in dashed arrow lines, control variables are not displayed in this figure.
In addition to the results of the path analysis with direct implications for the hypotheses, a full summary of the path analysis is presented in Table 3. Male participants showed a small negative effect size (β = −0.21, p < 0.001) for FL in comparison with female participants. Medication intake (β = 0.11, p = 0.02) and leisure-time PA (β = 0.16, p < 0.01) were also positively connected with FL with a small effect size. The control variables of HRQOL (β = 0.12, p < 0.001) and leisure-time PA (β = 0.20, p < 0.001) showed a small-to-moderate positive connection with DB, while blue-collar occupations were connected with a small negative effect size (β = −0.14, p < 0.01) in comparison to white-collar occupations. FLI showed a significant connection with leisure-time PA (β = −0.12 p = 0.02) with a negative small effect size, and a positive connection with alcohol consumption (β = 0.19, p < 0.001) with a small-to-moderate effect size. The full model of the path analysis with all control variables can be viewed in Appendix D (Figure A1).

Table 3.
Standardized path coefficients, SEs, and 95% CIs for the path analysis.
4. Discussion
This study focused on German working adults and examined the direct association between FL and DB. It further assumed a negative association between FL and indicators of NAFLD. Lastly, the study investigated the mediating role of DB in the indirect connection between FL and indicators of NAFLD.
4.1. Direct Positive Association between FL and DB
This study’s analyses confirmed the first hypothesis, with respect to the association between FL and DB in German working adults, by indicating a direct positive moderate relationship. These findings were in accordance with previous research, which showed similar effect sizes for the connection between FL and DB in adult samples [26,35,70]. The connection between FL and the different food groups also aligned with previous studies [36,71], such as the connection between FL and lower consumption frequencies for processed meat [71] and sugar-sweetened drinks [72].
In addition to the potential benefits of FL for DB on the personal level as a cognitive factor of the food choice framework, the positive connection between FL and DB in working adults could also imply healthy DB on an organizational level in the connection between FL and the framework’s food-external factors of food information and the food environment [21]. Working adults with high levels of FL might, for example, communicate and apply the recommended level of fruit and vegetable intake to co-workers while selecting and eating lunch at work and thereby also influence the DB of colleagues, which might be particularly likely if this is undertaken by the leadership of the organization [73].
This example underlines the potential value of FL for DB from a personal and environmental perspective in the workplace setting. Rachmah et al. [74] highlighted beneficial outcomes of WHPPs on DB and supported the efficacy of comprehensive WHPPs targeting the environmental and personal components of FL and DB. The importance of environmental factors in regard to DB was also demonstrated by our findings, because this connection was shown to be only moderate by some research, contrary to the food choice framework, even assuming an indirect connection between FL and DB, as environmental aspects such as availability and affordability in the working context might moderate this connection [28].
In summary, our findings and the current state of the research in this field underscore the potential of WHPPs targeting FL to promote healthy DB. However, based on the food choice model, future research should include and quantify contextual factors, such as the social and physical food environment in the workplace, because these factors might determine the availability of healthy food choices. Consequently, this comprehensive approach for WHPPs can be extended to FL by incorporating perspectives and measures of FL on the organizational level, in addition to addressing FL on the personal level, which is currently of major interest in health literacy research [75].
4.2. Direct Connection between FL and FLI
The second hypothesis, which postulated a direct association between FL and the FLI, could not be confirmed based on the findings of the path analysis. Although the calculated bivariate correlation for FL and the FLI showed a small negative connection, indicating a general connection between the two variables, based on the results of the path analysis, we believe that this small negative correlation might have been caused by the effect of the mediating variable of DB [76].
The finding of no direct connection between FL and FLI contradicted previous results pertaining to health literacy and fatty-liver disease [39], as well as the domain-specific health literacy concept focusing on health-enhancing physical activity [42]. This difference with respect to health literacy might have derived from a conceptually closer connection between health literacy and NAFLD in comparison to FL, because factors such as understanding and applying prescription labels or exchanging information with physicians are vital components of this construct [77]. In addition, Cheng et al. [39] used an objective measure of health literacy, contrasting to the self-reported measures of FL that were employed in our study. These differing measures might have accounted for the contradicting results, as self-reported measures can be prone to over-reporting, particularly in male participants [78].
In addition to this, the findings pertaining to the domain-specific health literacy concept focusing on physical activity displayed deviating results in comparison to our study, possibly because this construct targets the promotion of health by physical activity using a personal approach that largely neglects environmental influences [79]. This personal approach concentrates on an individual’s knowledge, abilities, skills, and attitudes towards a physically healthy lifestyle [80]. The conceptualization of FL acknowledges the connection between FL and DB and health in individuals’ food environments [81] but does not directly quantify these environmental factors, which might result in insignificant direct connections with respect to FL and FLI.
This complexity within the potential connection between FL and health might necessitate the inclusion of other factors in addition to FL, such as an individual’s food environment or economic and cultural context parameters, when considering the relationship between FL and NAFLD. As this was, to our knowledge, the first study to address the connection between FL and the FLI, we tried to address the aforementioned complexity of this connection by including DB as a mediator, which will be described in the next subsection.
4.3. Indirect Association between FL and FLI Mediated by DB
This study confirmed the assumptions made in the third hypothesis, which expected an indirect relationship between FL and FLI mediated by DB. DB was a significant mediator between FL and FLI in working adults, displaying a moderate positive connection with FL and a small negative connection with FLI. At the same time, the direct connection between FL and FLI showed no significant effect.
The results of this path analysis indicated an indirect-only mediation [82], i.e., an indirect effect in the absence of a direct effect. The indirect-only mediation found in our study was also in line with prior research investigating the connections separately and showing a positive relationship between FL and DB [22,31,83] and a health-enhancing relationship between healthy DB and the FLI as an indicator of NAFLD [4,15,84]. The contradiction of this indirect-only mediation with respect to the conceptual NAFLD model [8], which postulates a direct influence of health knowledge on NAFLD, might have resulted from the aforementioned conceptual differences between FL and aspects of health literacy [27,42].
However, the indirect connection between FL and NAFLD might be able to increase the effectiveness of dietary interventions to prevent NAFLD, because it could enable working adults to understand dietary advice and increase the overall adherence to dietary programs by improving food-related knowledge and skills and navigating barriers in the food environment [71]. Furthermore, FL might also empower working adults to adapt dietary advice according to the factors of food choice, as studies suggest that the most efficient diet is that which an individual is able to adhere to by maintaining healthier DB [85].
In summary, due to our initial findings on the connection between FL, DB, and NAFLD, future studies should investigate the connections between FL, DB, and health outcomes in longitudinal studies in the workplace. While distinct strategies involving WHPPs have been developed in the recent past to improve health literacy and, consequently, health outcomes, such as NAFLD, a detailed conceptual framework or policy for WHPP targeting FL is still lacking [4]. Furthermore, to our understanding, these WHPPs pertaining to FL should incorporate the complexity of the food environment of working adults and should address the cognitive as well as external factors of the food choice framework.
4.4. Strengths and Limitations
In comparison to previous research, a major strength of this study was the statistical approach including the mediation analysis, a method that has not been conducted as part of previous research on FL, DB, and FLI. This approach delivered initial insight into potential longitudinal connections. Furthermore, another strength of this study was the use of validated tools for assessing the subjective measures of FL, DB, HRQOL, leisure-time PA, and alcohol consumption, along with the examination of the FLI as an objective health outcome to achieve a comprehensive understanding of NAFLD considering a multitude of connected factors.
In addition to these strengths, this study had several limitations. One apparent limitation was the cross-sectional design, which did not allow us to draw causal conclusions [86,87]. Longitudinal studies with control groups are needed to further explore the relationships between FL, DB, and the FLI as a valid indicator of NAFLD to potentially infer causal connections between these concepts. Moreover, this study was conducted in a large German technology company, with the majority of the sample being male, in a relationship, and highly educated. Due to the varying lifestyle factors and cultural backgrounds in comparison to other samples and the specific characteristics of the sample collected in our study, our findings may not be transferable to other populations, countries, or companies [87]. In addition, the FFL by Winkler and Döring [45] does not include portion size in the assessment of DB. This increased the inaccuracy of the DB examination, because portion sizes largely influence DB in addition to the frequency of food consumption, and thereby exacerbated the risk of information bias [46]. Future studies should employ a detailed assessment of DB combining portion size and consumption frequency, such as the food frequency questionnaire developed by Haftenberger et al. [46], which uses pictures to clarify the estimation of portion size and DB. Lastly, this study employed a narrow understanding of the connection between FL, DB, and the FLI, and other relevant factors of the food choice framework, such as the social and physical food environment, were neglected. This understanding derived from the definition used in the conceptualization of the FL measure, which places the focus on the individual [26]. Other FL definitions, however, emphasize that food-related attitudes, knowledge, and skills not only influence the individual, but are interwoven with the entire food system [81].
5. Conclusions
This study filled a gap in the literature, as it provided a preliminary understanding of the complex connections between FL, DB, and the FLI in German working adults. The study showed a positive relationship between FL and DB, as well as a mediating role of DB in the relationship between FL and the FLI. The findings may guide the development of further cross-sectional studies including all factors of the food choice framework and longitudinal studies regarding the proficiency of FL and its effect on DB for preventing NAFLD in working adults. Furthermore, based on our findings, practitioners might be encouraged and informed to develop comprehensive programs to improve DB and NAFLD prevention by incorporating environmental and personal intervention components addressing FL. Therefore, FL might play an important role for public health policy makers, as well as for occupational health managers in companies aiming to lighten the burden of NAFLD. However, owing to the novelty of FL and the promising initial results in terms of health promotion, this construct should be paid more attention by both researchers and practitioners in the future.
Author Contributions
Conceptualization, S.B., F.M., K.P. and N.S.; methodology, S.B., F.M. and M.S.; software, S.B. and N.S.; validation, S.B. and N.S.; formal analysis, S.B. and N.S.; investigation, S.B. and N.S.; resources, S.B., K.P. and F.M.; data curation, S.B. and N.S.; writing—original draft preparation, S.B. and N.S.; writing—review and editing, S.B., N.S., M.S., K.P. and F.M.; visualization, S.B. and N.S.; supervision, S.B. and F.M.; project administration, S.B.; funding acquisition, K.P. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Siemens AG, grant no. 1601789.
Institutional Review Board Statement
The study was conducted according to the guidelines of the 505 Declaration of Helsinki and approved by the Ethics Committee of the Institute of Medicine of the 506 Technical University of Munich (645/20, November 2020).
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study.
Data Availability Statement
The datasets generated and analyzed during the study are not publicly available owing to patient confidentiality, but they will be made available in a highly anonymized form from the corresponding author on reasonable request.
Conflicts of Interest
N.S. and M.S. declare no financial or non-financial conflicts of interest, because no commercial support, research grants, honoria, patents, or employment were received from Siemens AG before, during, or after the work on this manuscript. S.B., K.P. and F.M. declare the potential for the following conflicts of interest. S.B. and F.M. received funding from a research grant from Siemens AG (1601789), and K.P. is employed at Siemens AG. To the understanding of the authors, neither the manuscript and research grant, nor the employment at the company, present a conflict of interest, because the study, research grant, and employment had largely differing aims and did not overlap in the conducted tasks. This research grant was provided to S.B. and F.M. because Siemens AG committed itself to independently evaluating its occupational health management programs and trusted the expertise of S.B. and F.M. to deliver this independent evaluation. This manuscript, however, was targeted at extending previous basic research on NAFLD and FL, which is not related to the outcome of the evaluation of the company’s occupational health management programs. For the evaluation of the occupational health management programs of Siemens AG, S.B. and F.M. could determine and conduct the study design, data collection, and data analysis without the influence of K.P. or Siemens AG independently before and during the evaluation period. For example, data was collected, stored, and analyzed independently without the influence of K.P. or Siemens AG. We therefore argue that the conflicts of interest of S.B., K.P. and F.M. were within the guidelines for good scientific practice. During the writing of the manuscript, K.P. acted as a substantial source of information for the study design and the sample and was occupied with revising and writing the manuscript. There were no financial or non-financial conflicts of interest from commercial sources, honoria, positions on advisory boards, patents, or employment other than the potential conflict of interest stated above.
Appendix A
FLI = ey/1+ ey ∗ 100
with y = 0.953 ∗ loge(triglycerides) + 0.139 ∗ BMI + 0.718 ∗ loge(GGT) + 0.053 ∗ waistcircumference − 15.745
FLI = e(0.953 ∗ loge(triglycerides) + 0.139 ∗ BMI + 0.718 ∗ loge(GGT) + 0.053 ∗ waistcircumference − 15.745)/1 + e(0.953 ∗ loge(triglycerides) + 0.139 ∗ BMI + 0.718 ∗ loge(GGT) + 0.053 ∗ waistcircumference − 15.745) ∗ 100
with y = 0.953 ∗ loge(triglycerides) + 0.139 ∗ BMI + 0.718 ∗ loge(GGT) + 0.053 ∗ waistcircumference − 15.745
FLI = e(0.953 ∗ loge(triglycerides) + 0.139 ∗ BMI + 0.718 ∗ loge(GGT) + 0.053 ∗ waistcircumference − 15.745)/1 + e(0.953 ∗ loge(triglycerides) + 0.139 ∗ BMI + 0.718 ∗ loge(GGT) + 0.053 ∗ waistcircumference − 15.745) ∗ 100
Appendix B

Table A1.
Means, SDs, and bivariate correlations for the food groups listed and FL, FLI, HRQOL, and alcohol consumption.
Table A1.
Means, SDs, and bivariate correlations for the food groups listed and FL, FLI, HRQOL, and alcohol consumption.
| M | SD | FL | FLI | HRQOL | AUDIT-C | ||
|---|---|---|---|---|---|---|---|
| 1. | Red meat | 4.66 | 1.01 | −0.14 * | 0.24 * | 0.07 | 0.16 * |
| 2. | Poultry | 3.75 | 1.08 | 0.05 | 0.17 * | 0.04 | 0.03 |
| 3. | Processed meat | 4.48 | 1.29 | −0.22 * | 0.28 * | 0.03 | 0.22 * |
| 4. | Fish | 3.37 | 1.03 | 0.17 * | −0.06 | 0.11 * | 0.01 |
| 5. | Potatoes | 4.04 | 1.03 | −0.05 | 0.06 | 0.05 | 0.12 * |
| 6. | Baked goods | 4.13 | 0.95 | −0.09 | 0.01 | 0.04 | 0.06 |
| 7. | Rice | 3.45 | 1.04 | 0.07 | −0.01 | 0.08 | −0.01 |
| 8. | Salad | 4.86 | 1.10 | 0.25 * | −0.15 * | 0.10 | −0.06 |
| 9. | Vegetables | 4.67 | 0.98 | 0.20 * | −0.16 * | 0.18 * | −0.09 |
| 10. | Fruits | 4.82 | 1.35 | 0.27 * | −0.30 * | 0.06 | −0.13 * |
| 11. | Chocolate | 4.05 | 1.39 | 0.01 | −0.07 | −0.03 | −0.10 |
| 12. | Cake and pastry | 3.84 | 1.18 | 0.03 | −0.11 * | −0.01 | −0.16 * |
| 13. | Candy | 3.41 | 1.55 | −0.13 * | 0.08 | 0.05 | 0.01 |
| 14. | Salty snacks | 3.26 | 1.27 | −0.03 | 0.06 | −0.10 | 0.09 |
| 15. | White bread | 4.20 | 1.50 | −0.21 * | 0.11 * | −0.01 | 0.13 * |
| 16. | Whole-grain bread | 4.50 | 1.35 | 0.18 * | −0.10 | 0.05 | −0.02 |
| 17. | Oats and granola | 3.40 | 1.80 | 0.27 * | −0.28 * | 0.12 * | −0.19 * |
| 18. | Curd | 4.26 | 1.50 | 0.18 * | −0.14 * | 0.06 | −0.09 |
| 19. | Cheese | 4.75 | 1.22 | 0.05 | −0.09 | 0.03 | −0.07 |
| 20. | Eggs | 3.84 | 1.07 | 0.11 * | −0.00 | 0.07 | 0.03 |
| 21. | Milk | 4.26 | 1.81 | 0.03 | −0.06 | 0.09 | −0.08 |
| 22. | Juice | 3.44 | 1.63 | −0.10 * | 0.03 | 0.00 | −0.01 |
| 23. | Soda and lemonade | 2.52 | 1.53 | −0.31 * | 0.23 * | −0.01 | 0.09 |
| 24. | Water | 6.30 | 1.13 | 0.14 * | −0.05 | 0.08 | −0.08 |
| 25. | Diet drink | 1.93 | 1.62 | 0.02 | 0.23 * | −0.05 | −0.02 |
Note: N = 372, * p < 0.05; M = Mean; SD = standard deviation; FL = food literacy; DB = dietary behavior; FLI = fatty-liver index; HRQOL = health-related quality of life; AUDIT-C = Alcohol Use Disorders Identification Test Consumption.
Appendix C

Table A2.
Multiple linear regression for the control variables, FL, DB, and the FLI.
Table A2.
Multiple linear regression for the control variables, FL, DB, and the FLI.
| FL | DB | FLI | ||
|---|---|---|---|---|
| Age | ||||
| β 95% CI | −0.02 (−0.12, 0.08) | 0.11 (0.02, 0.21) | −0.11 (−0.21, −0.01) | |
| Gender (ref. = female) | ||||
| male | β 95% CI | −0.49 (−0.72, −0.25) | −0.32 (−0.55, −0.10) | 0.36 (0.12, 0.59) |
| Education (ref. = low) | ||||
| medium | β 95% CI | 0.31 (−0.15, 0.77) | 0.01 (−0.46, 0.47) | −0.15 (−0.59, 0.30) |
| high | β 95% CI | 0.31 (−0.19, 0.81) | 0.03 (−0.51, 0.46) | −0.28 (−0.77, 0.20) |
| Relationship (ref. = no relationship) | ||||
| relationship | β 95% CI | 0.07 (−0.20, 0.33) | −0.12 (−0.33, 0.12) | 0.12 (−0.13, 0.37) |
| Type of occupation (ref = white-collar workers) | ||||
| blue-collar workers | β 95% CI | −0.07 (−0.39, 0.24) | −0.39 (−0.69, −0.01) | 0.03 (−0.28, 0.33) |
| HRQOL | ||||
| β 95% CI | 0.05 (−0.06, 0.15) | 0.18 (0.08, 0.28) | −0.13 (−0.23, −0.03) | |
| LSI | ||||
| β 95% CI | 0.15 (0.05, 0.25) | 0.22 (0.12, 0.32) | −0.13 (−0.23, −0.03) | |
| AUDIT-C | ||||
| β 95% CI | −0.03 (−0.13, 0.08) | −0.07 (−0.17, 0.03) | 0.7 (0.06, 0.28) | |
| Medication | ||||
| β 95% CI | 0.12 (0.02, 0.22) | 0.06 (−0.04, 0.16) | −0.07 (−0.16, 0.03) | |
| Diseases | ||||
| β 95% CI | −0.02 (−0.12, 0.08) | −0.02 (−0.11, 0.08) | 0.06 (−0.04, 0.16) |
Note: CI = confidence interval; FL = food literacy; DB = dietary behavior; HRQOL = health-related quality of life; FLI = fatty-liver index; LSI = Leisure Score Index; AUDIT-C= Alcohol Use Disorders Identification Test Consumption.
Appendix D
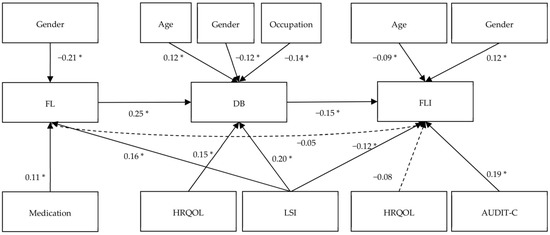
Figure A1.
Complete path analysis with standardized path coefficients for the variables. Note: * p < 0.05, N = 372, FL = food literacy, DB = dietary behavior, HRQOL = health-related quality of life, FLI = fatty-liver index, LSI = Leisure Score Index, AUDIT-C = Alcohol Use Disorders Identification Test Consumption. Reference group for gender is female and for occupation white-collar workers, insignificant connections displayed via dashed arrows.
References
- Rinella, M.E.; Sanyal, A.J. NAFLD in 2014: Genetics, diagnostics and therapeutic advances in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 65–66. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hassani Zadeh, S.; Mansoori, A.; Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1470–1478. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M. Die nicht-alkoholische Fettleber-Erkrankung. MMW Fortschr. Med. 2020, 162, 56–62. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Colombo, M.; Cortez-Pinto, H.; Huang, T.T.K.; Miller, V.; Ninburg, M.; Schattenberg, J.M.; Seim, L.; Wong, V.W.S.; Zelber-Sagi, S. NAFLD—Sounding the alarm on a silent epidemic. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 377–379. [Google Scholar] [CrossRef]
- Thakur, J.S.; Bains, P.; Kar, S.S.; Wadhwa, S.; Moirangthem, P.; Kumar, R.; Wadwalker, S.; Sharma, Y. Integrated healthy workplace model: An experience from North Indian industry. Indian J. Occup. Environ. Med. 2012, 16, 108–113. [Google Scholar] [CrossRef]
- Täuber, S.; Mulder, L.B.; Flint, S.W. The Impact of Workplace Health Promotion Programs Emphasizing Individual Responsibility on Weight Stigma and Discrimination. Front. Psychol. 2018, 9, 2206. [Google Scholar] [CrossRef]
- Tam, G.; Yeung, M.P.S. A systematic review of the long-term effectiveness of work-based lifestyle interventions to tackle overweight and obesity. Prev. Med. 2018, 107, 54–60. [Google Scholar] [CrossRef]
- Li, C.; Xing, J.J.; Shan, A.Q.; Leng, L.; Liu, J.C.; Yue, S.; Yu, H.; Chen, X.; Tian, F.S.; Tang, N.J. Increased risk of nonalcoholic fatty liver disease with occupational stress in Chinese policemen: A 4-year cohort study. Medicine 2016, 95, e5359. [Google Scholar] [CrossRef]
- Song, E.; Kim, J.A.; Roh, E.; Yu, J.H.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Long Working Hours and Risk of Nonalcoholic Fatty Liver Disease: Korea National Health and Nutrition Examination Survey VII. Front. Endocrinol. 2021, 12, 647459. [Google Scholar] [CrossRef]
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013.
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.F.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144. [Google Scholar] [CrossRef]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.W. Global variability in fruit and vegetable consumption. Am. J. Prev. Med. 2009, 36, 402–409.E5. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Schienkiewitz, A.; Haftenberger, M.; Lampert, T.; Ziese, T.; Scheidt-Nave, C. Übergewicht und Adipositas in Deutschland. Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 786–794. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Truthmann, J.; Rabenberg, M.; Heidemann, C.; Haftenberger, M.; Schienkiewitz, A.; Richter, A. Obst- und Gemüsekonsum in Deutschland. Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 779–785. [Google Scholar] [CrossRef]
- Houttu, V.; Csader, S.; Nieuwdorp, M.; Holleboom, A.G.; Schwab, U. Dietary Interventions in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 716783. [Google Scholar] [CrossRef]
- Schliemann, D.; Woodside, J.V. The effectiveness of dietary workplace interventions: A systematic review of systematic reviews. Public Health Nutr. 2019, 22, 942–955. [Google Scholar] [CrossRef]
- Chen, P.-J.; Antonelli, M. Conceptual Models of Food Choice: Influential Factors Related to Foods, Individual Differences, and Society. Foods 2020, 9, 1898. [Google Scholar] [CrossRef]
- Spronk, I.; Kullen, C.; Burdon, C.; O’Connor, H. Relationship between nutrition knowledge and dietary intake. Br. J. Nutr. 2014, 111, 1713–1726. [Google Scholar] [CrossRef]
- Vamos, S.D.; Wacker, C.C.; Welter, V.D.E.; Schlüter, K. Health Literacy and Food Literacy for K-12 Schools in the COVID-19 Pandemic. J. Sch. Health 2021, 91, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Velardo, S. The Nuances of Health Literacy, Nutrition Literacy, and Food Literacy. J. Nutr. Educ. Behav. 2015, 47, 385–389.E1. [Google Scholar] [CrossRef]
- CDC Food Literacy. Available online: https://www.cdc.gov/healthliteracy/researchevaluate/food-literacy.html (accessed on 20 May 2022).
- Gréa Krause, C.; Beer-Borst, S.; Sommerhalder, K.; Hayoz, S.; Abel, T. A short food literacy questionnaire (SFLQ) for adults: Findings from a Swiss validation study. Appetite 2018, 120, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Sommerhalder, K.; Beer-Borst, S.; Abel, T. Just a subtle difference? Findings from a systematic review on definitions of nutrition literacy and food literacy. Health Promot. Int. 2016, 33, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Truman, E.; Lane, D.; Elliott, C. Defining food literacy: A scoping review. Appetite 2017, 116, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Nutbeam, D. The evolving concept of health literacy. Soc. Sci. Med. 2008, 67, 2072–2078. [Google Scholar] [CrossRef]
- Azevedo Perry, E.; Thomas, H.; Samra, H.R.; Edmonstone, S.; Davidson, L.; Faulkner, A.; Petermann, L.; Manafò, E.; Kirkpatrick, S.I. Identifying attributes of food literacy: A scoping review. Public Health Nutr. 2017, 20, 2406–2415. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, T.; Jung, H. The Relationships between Food Literacy, Health Promotion Literacy and Healthy Eating Habits among Young Adults in South Korea. Foods 2022, 11, 2467. [Google Scholar] [CrossRef]
- Cullen, T.; Hatch, J.; Martin, W.; Higgins, J.W.; Sheppard, R. Food Literacy: Definition and Framework for Action. Can. J. Diet Pract. Res. 2015, 76, 140–145. [Google Scholar] [CrossRef]
- Sponselee, H.C.S.; Kroeze, W.; Poelman, M.P.; Renders, C.M.; Ball, K.; Steenhuis, I.H.M. Food and health promotion literacy among employees with a low and medium level of education in the Netherlands. BMC Public Health 2021, 21, 1273. [Google Scholar] [CrossRef]
- Sørensen, K.; Van den Broucke, S.; Fullam, J.; Doyle, G.; Pelikan, J.; Slonska, Z.; Brand, H.; (HLS-EU) Consortium Health Literacy Project European. Health literacy and public health: A systematic review and integration of definitions and models. BMC Public Health 2012, 12, 80. [Google Scholar] [CrossRef]
- Vettori, V.; Lorini, C.; Milani, C.; Bonaccorsi, G. Towards the Implementation of a Conceptual Framework of Food and Nutrition Literacy: Providing Healthy Eating for the Population. Int. J. Environ. Res. Public Health 2019, 16, 5041. [Google Scholar] [CrossRef] [PubMed]
- Begley, A.; Paynter, E.; Butcher, L.M.; Dhaliwal, S.S. Effectiveness of an Adult Food Literacy Program. Nutrients 2019, 11, 797. [Google Scholar] [CrossRef]
- Meyn, S.; Blaschke, S.; Mess, F. Food Literacy and Dietary Intake in German Office Workers: A Longitudinal Intervention Study. Int. J. Environ. Res. Public Health 2022, 19, 16534. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R. Sustainability of Well-being through Literacy. The Effects of Food Literacy on Sustainability of Well-being. Agric. Agric. Sci. Procedia 2016, 8, 99–106. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Shu, J.-H.; Hsu, H.-C.; Liang, Y.; Chou, R.-H.; Hsu, P.-F.; Wang, Y.-J.; Ding, Y.-Z.; Liou, T.-L.; Wang, Y.-W.; et al. High health literacy is associated with less obesity and lower Framingham risk score: Sub-study of the VGH-HEALTHCARE trial. PLoS ONE 2018, 13, e0194813. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Blaha, M.J.; Martin, S.S.; Reed, R.M.; Jones, S.R.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. Nonalcoholic fatty liver disease and serum lipoproteins: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2013, 227, 429–436. [Google Scholar] [CrossRef]
- Mearns, G.J.; Chepulis, L.; Britnell, S.; Skinner, K. Health and Nutritional Literacy of New Zealand Nursing Students. J. Nurs. Educ. 2017, 56, 43–48. [Google Scholar] [CrossRef]
- Blaschke, S.; Carl, J.; Ellinger, J.; Birner, U.; Mess, F. The Role of Physical Activity-Related Health Competence and Leisure-Time Physical Activity for Physical Health and Metabolic Syndrome: A Structural Equation Modeling Approach for German Office Workers. Int. J. Environ. Res. Public Health 2021, 18, 10153. [Google Scholar] [CrossRef]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef]
- Strauss, M.; Foshag, P.; Leischik, R. Prospective Evaluation of Cardiovascular, Cardiorespiratory, and Metabolic Risk of German Office Workers in Comparison to International Data. Int. J. Environ. Res. Public Health 2020, 17, 1590. [Google Scholar] [CrossRef]
- Winkler, G.; Döring, A. Validation of a short qualitative food frequency list used in several German large scale surveys. Z. Ernährungswissenschaft 1998, 37, 234–241. [Google Scholar] [CrossRef]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B.M. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef]
- Winkler, G.; Döring, A. Kurzmethoden zur Charakterisierung des Ernährungsmusters: Einsatz und Auswertung eines Food-Frequency-Fragebogens. Ernährungs-Umsch. 1995, 42, 289–291. [Google Scholar]
- Drixler, M.S.K.; Morfeld, M.; Glaesmer, H.; Brähler, E.; Wirtz, M.A. Validierung der Messung gesundheitsbezogener Lebensqualität mittels des Short-Form-Health-Survey-12 (SF-12 Version 2.0) in einer deutschen Normstichprobe. Z. Psychosom. Med. Psychother. 2020, 66, 272–286. [Google Scholar] [CrossRef]
- Huo, T.; Guo, Y.; Shenkman, E.; Muller, K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: A report from the wellness incentive and navigation (WIN) study. Health Qual. Life Outcomes 2018, 16, 34. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Carl, J.; Grüne, E.; Popp, J.; Pfeifer, K. Physical Activity Promotion for Apprentices in Nursing Care and Automotive Mechatronics-Competence Counts More than Volume. Int. J. Env. Res. Public Health 2020, 17, 793. [Google Scholar] [CrossRef]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- Amireault, S.; Godin, G. The Godin-Shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; DeBenedetti, A.F.; Volk, R.J.; Williams, E.C.; Frank, D.; Kivlahan, D.R. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol. Clin. Exp. Res. 2007, 31, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, H.J.; Hapke, U.; Meyer, C.; John, U. Screening for alcohol use disorders and at-risk drinking in the general population: Psychometric performance of three questionnaires. Alcohol Alcohol. 2002, 37, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Brauns, H.; Scherer, S.; Steinmann, S. The CASMIN Educational Classification in International Comparative Research; Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Mundi, M.S.; Velapati, S.; Patel, J.; Kellogg, T.A.; Abu Dayyeh, B.K.; Hurt, R.T. Evolution of NAFLD and Its Management. Nutr. Clin. Pract. 2020, 35, 72–84. [Google Scholar] [CrossRef]
- Herold, G. Innere Medizin; Gerd Herold: Köln, Germany, 2016. [Google Scholar]
- Lind, L.; Johansson, L.; Ahlström, H.; Eriksson, J.W.; Larsson, A.; Risérus, U.; Kullberg, J.; Oscarsson, J. Comparison of four non-alcoholic fatty liver disease detection scores in a Caucasian population. World J. Hepatol. 2020, 12, 149–159. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.L.; Hansen, B.E.; Hofman, A.; Stricker, B.H.; Janssen, H.L.A. External Validation of the Fatty Liver Index for Identifying Nonalcoholic Fatty Liver Disease in a Population-based Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1201–1204. [Google Scholar] [CrossRef]
- Meffert, P.J.; Baumeister, S.E.; Lerch, M.M.; Mayerle, J.; Kratzer, W.; Völzke, H. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am. J. Gastroenterol. 2014, 109, 1404–1414. [Google Scholar] [CrossRef]
- Buuren, S.V. Flexible Imputation of Missing Data, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2018. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson Eduation Inc.: New Jersey, NJ, USA, 2013. [Google Scholar]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 4th ed.; Guilford Publications: New York, NY, USA, 2015. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. Linear Regression. In An Introduction to Statistical Learning: With Applications in R; James, G., Witten, D., Hastie, T., Tibshirani, R., Eds.; Springer: New York, NY, USA, 2013; pp. 59–126. [Google Scholar]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods Psychol. Res. Online 2003, 8, 23–74. [Google Scholar]
- Hu, L.-T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Luta, X.; Hayoz, S.; Gréa Krause, C.; Sommerhalder, K.; Roos, E.; Strazzullo, P.; Beer-Borst, S. The relationship of health/food literacy and salt awareness to daily sodium and potassium intake among a workplace population in Switzerland. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 270–277. [Google Scholar] [CrossRef]
- Boslooper-Meulenbelt, K.; Boonstra, M.D.; van Vliet, I.M.Y.; Gomes-Neto, A.W.; Osté, M.C.J.; Poelman, M.P.; Bakker, S.J.L.; de Winter, A.F.; Navis, G.J. Food Literacy is Associated with Adherence to a Mediterranean-Style Diet in Kidney Transplant Recipients. J. Ren. Nutr. 2021, 31, 628–636. [Google Scholar] [CrossRef]
- Begley, A.; Butcher, L.M.; Bobongie, V.; Dhaliwal, S.S. Identifying Participants Who Would Benefit the Most from an Adult Food-literacy Program. Int. J. Environ. Res. Public Health 2019, 16, 1272. [Google Scholar] [CrossRef]
- Brach, C. The Journey to Become a Health Literate Organization: A Snapshot of Health System Improvement. Stud. Health Technol. Inf. 2017, 240, 203–237. [Google Scholar]
- Rachmah, Q.; Martiana, T.; Mulyono; Paskarini, I.; Dwiyanti, E.; Widajati, N.; Ernawati, M.; Ardyanto, Y.D.; Tualeka, A.R.; Haqi, D.N.; et al. The Effectiveness of Nutrition and Health Intervention in Workplace Setting: A Systematic Review. J. Public Health Res. 2022, 11, 2312. [Google Scholar] [CrossRef]
- Wong, B.K. Building a Health Literate Workplace. Workplace Health Saf. 2012, 60, 363–369. [Google Scholar] [CrossRef]
- Land, K.C. Principles of Path Analysis. Sociol. Methodol. 1969, 1, 3–37. [Google Scholar] [CrossRef]
- Schönfeld, M.S.; Pfisterer-Heise, S.; Bergelt, C. Self-reported health literacy and medication adherence in older adults: A systematic review. BMJ Open 2021, 11, e056307. [Google Scholar] [CrossRef]
- Lee, S.Y.; Tsai, T.I.; Tsai, Y.W. Accuracy in self-reported health literacy screening: A difference between men and women in Taiwan. BMJ Open 2013, 3, e002928. [Google Scholar] [CrossRef]
- Carl, J.; Grüne, E.; Pfeifer, K. What About the Environment? How the Physical Activity–Related Health Competence Model Can Benefit From Health Literacy Research. Front. Public Health 2021, 9, 635443. [Google Scholar] [CrossRef]
- Carl, J.; Sudeck, G.; Pfeifer, K. Competencies for a Healthy Physically Active Lifestyle-Reflections on the Model of Physical Activity-Related Health Competence. J. Phys. Act. Health 2020, 17, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Truman, E.; Elliott, C. Barriers to Food Literacy: A Conceptual Model to Explore Factors Inhibiting Proficiency. J. Nutr. Educ. Behav. 2019, 51, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Danks, N.P.; Ray, S. Mediation Analysis. In Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R: A Workbook; Hair, J.F., Jr., Hult, G.T.M., Ringle, C.M., Sarstedt, M., Danks, N.P., Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 139–153. [Google Scholar]
- Micha, R.; Shulkin, M.L.; Peñalvo, J.L.; Khatibzadeh, S.; Singh, G.M.; Rao, M.; Fahimi, S.; Powles, J.; Mozaffarian, D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 2017, 12, e0175149. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.L.; Kelly, J.T.; Macdonald, G.A.; Hickman, I.J. ‘Focus on diet quality’: A qualitative study of clinicians’ perspectives of use of the Mediterranean dietary pattern for non-alcoholic fatty liver disease. Br. J. Nutr. 2022, 128, 1220–1230. [Google Scholar] [CrossRef]
- Raynor, H.A.; Champagne, C.M. Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. J. Acad. Nutr. Diet. 2016, 116, 129–147. [Google Scholar] [CrossRef]
- Prietl, I.; Nöhammer, E. Gesundheitskompetenz im Betrieb im Kontext der betrieblichen Gesundheitsförderung. Prävention Gesundh. 2021, 16, 328–333. [Google Scholar] [CrossRef]
- Kreienbrock, L.; Pigeot, I.; Ahrens, W. Epidemiologische Studien. In Epidemiologische Methoden; Kreienbrock, L., Pigeot, I., Ahrens, W., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2012; pp. 53–119. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).