Associations between Conventional and Emerging Indicators of Dietary Carbohydrate Quality and New-Onset Type 2 Diabetes Mellitus in Chinese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Intake Data Collection and Assessment
2.3. Calculation of Dietary GI, CF and CQI
2.4. Ascertainment of T2DM
2.5. Assessment of Covariates
2.6. Statistical Analysis
3. Results
3.1. Sociodemographic, Anthropometric and Lifestyle Characteristics of Study Participants at Baseline
3.2. Associations between Dietary GI, CF and CQI Values and T2DM Risk
3.3. Associations between Dietary GI, CF and CQI Values and T2DM Risk on the Basis of Potential Effect Modifiers
3.4. The Dose–Response Relationship between Dietary GI, CF and CQI Values and the Risk of T2DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 10th Edition. Available online: https://www.diabetesatlas.org (accessed on 20 December 2021).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; van Dam, R.M.; Liu, S. Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mejia; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Stevens, J.; Ahn, K.; Juhaeri; Houston, D.; Steffan, L.; Couper, D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: The ARIC study. Diabetes Care 2002, 25, 1715–1721. [Google Scholar] [CrossRef]
- Mosdøl, A.; Witte, D.R.; Frost, G.; Marmot, M.G.; Brunner, E.J. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am. J. Clin. Nutr. 2007, 86, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; van der Schouw, Y.T.; van der, A.D.; Buckland, G.; Kuijsten, A.; Schulze, M.B.; Amiano, P.; Ardanaz, E.; Balkau, B.; et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J. Nutr. 2013, 143, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Nanri, A.; Kurotani, K.; Goto, A.; Kato, M.; Mizoue, T.; Noda, M.; Inoue, M.; Tsugane, S. Dietary glycemic index, glycemic load and incidence of type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Nutr. J. 2013, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Flood, V.M.; Rochtchina, E.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, dietary fiber, and risk of type 2 diabetes in a cohort of older Australians. Diabetes Care 2007, 30, 2811–2813. [Google Scholar] [CrossRef]
- Matthan, N.R.; Ausman, L.M.; Meng, H.; Tighiouart, H.; Lichtenstein, A.H. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am. J. Clin. Nutr. 2016, 104, 1004–1013. [Google Scholar] [CrossRef]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Vega-López, S.; Ausman, L.M.; Griffith, J.L.; Lichtenstein, A.H. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 2007, 30, 1412–1417. [Google Scholar] [CrossRef]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; et al. Dietary glycemic index and risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 2012, 61, 47–55. [Google Scholar] [CrossRef]
- Mekary, R.A.; Rimm, E.B.; Giovannucci, E.; Stampfer, M.J.; Willett, W.C.; Ludwig, D.S.; Hu, F.B. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am. J. Clin. Nutr. 2011, 94, 1525–1532. [Google Scholar] [CrossRef]
- Sluijs, I.; van der Schouw, Y.T.; van der, A.D.; Spijkerman, A.M.; Hu, F.B.; Grobbee, D.E.; Beulens, J.W. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) study. Am. J. Clin. Nutr. 2010, 92, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Liu, S.; Gao, Y.T.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef]
- AlEssa, H.B.; Ley, S.H.; Rosner, B.; Malik, V.S.; Willett, W.C.; Campos, H.; Hu, F.B. High Fiber and Low Starch Intakes Are Associated with Circulating Intermediate Biomarkers of Type 2 Diabetes among Women. J. Nutr. 2016, 146, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Sánchez-Taínta, A.; Santiago, S.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Martínez, J.A.; Martínez-González, M. Association between dietary carbohydrate intake quality and micronutrient intake adequacy in a Mediterranean cohort: The SUN (Seguimiento Universidad de Navarra) Project. Br. J. Nutr. 2014, 111, 2000–2009. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Fernandez-Lazaro, C.I.; Toledo, E.; Díaz-López, A.; Corella, D.; Goday, A.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Carbohydrate quality changes and concurrent changes in cardiovascular risk factors: A longitudinal analysis in the PREDIMED-Plus randomized trial. Am. J. Clin. Nutr. 2020, 111, 291–306. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.H.; Lim, H. Association between dietary carbohydrate quality and the prevalence of obesity and hypertension. J. Hum. Nutr. Diet. 2018, 31, 587–596. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.; Zhao, W. Status and trends in consumption of grains and dietary fiber among Chinese adults (1982–2015). Nutr. Rev. 2020, 78, 43–53. [Google Scholar] [CrossRef]
- Zhai, F.Y.; Du, S.F.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Popkin, B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes. Rev. 2014, 15 (Suppl. S1), 16–26. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yang, X.; Hemler, E.C.; Fang, Y.; Zhao, L.; Zhang, J.; Yang, Z.; Wang, Z.; He, L.; et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: A cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019, 7, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Augustin, L.S.A.; Brighenti, F.; Delport, E.; Henry, C.J.; Sievenpiper, J.L.; Usic, K.; Yuexin, Y.; Zurbau, A.; Wolever, T.M.S.; et al. Dietary Glycaemic Index Labelling: A Global Perspective. Nutrients 2021, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- China Health and Nutrition Survey. Available online: https://www.cpc.unc.edu/projects/china (accessed on 17 July 2020).
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989-2011. Obes. Rev. 2014, 15 (Suppl. S1), 2–7. [Google Scholar] [CrossRef] [PubMed]
- Chinese Center For Disease Control And Prevention. 2015 China Health and Nutritional Survey Project Launched. Available online: http://www.chinacdc.cn/zxdt/201511/t20151123_122181.html (accessed on 3 August 2021).
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991-2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef]
- Zhao, J.; Zuo, L.; Sun, J.; Su, C.; Wang, H. Trends and Urban-Rural Disparities of Energy Intake and Macronutrient Composition among Chinese Children: Findings from the China Health and Nutrition Survey (1991 to 2015). Nutrients 2021, 13, 1933. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Adherence to dietary guidelines and mortality: A report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014, 100, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhao, J.; Wu, Y.; Wang, H.; Wang, Z.; Wang, Y.; Zhang, B. Temporal Trends in Dietary Macronutrient Intakes among Adults in Rural China from 1991 to 2011: Findings from the CHNS. Nutrients 2017, 9, 227. [Google Scholar] [CrossRef]
- Zhai, F.; Guo, X.; Popkin, B. Evaluation of the 24-Hour Individual Recall Method in China. Food Nutr. Bull. 1996, 17, 1–7. [Google Scholar] [CrossRef]
- Wang, G. Food Composition Table (National Representative Values); People’s Medical Publishing House: Beijing, China, 1991. [Google Scholar]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition Table 2002; Peking University Medical Press: Beijing, China, 2002. [Google Scholar]
- Yang, Y.; He, M.; Pan, X. China Food Composition Table 2004; Peking University Medical Press: Beijing, China, 2004. [Google Scholar]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition Table, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Dodd, H.; Williams, S.; Brown, R.; Venn, B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am. J. Clin. Nutr. 2011, 94, 992–996. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Dahlquist, G.G.; Gyürüs, E.; Green, A.; Soltész, G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Mao, L.; Wu, F.; Wang, J.; Jiao, J.; Zhang, Y. Cooking Oil Consumption Is Positively Associated with Risk of Type 2 Diabetes in a Chinese Nationwide Cohort Study. J. Nutr. 2020, 150, 1799–1807. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, P.; Mao, L.; Chen, X.; Wang, J.; Cheng, L.; Ding, G.; Jiao, J. Current level of fish and omega-3 fatty acid intakes and risk of Type 2 diabetes in China. J. Nutr. Biochem. 2019, 74, 108249. [Google Scholar] [CrossRef]
- He, J.; Fang, A.; Yu, S.; Shen, X.; Li, K. Dietary Nonheme, Heme, and Total Iron Intake and the Risk of Diabetes in Adults: Results From the China Health and Nutrition Survey. Diabetes Care 2020, 43, 776–784. [Google Scholar] [CrossRef]
- World Health Qrganization. Obesity. Available online: https://www.who.int/news-room/facts-in-pictures/detail/6-facts-on-obesity (accessed on 5 August 2021).
- Du, T.; Sun, X.; Huo, R.; Yu, X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: The China Health and Nutrition Survey 2009. Int. J. Obes. (Lond.) 2014, 38, 840–847. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Wu, J.; Wang, R.; Mao, D.; Chen, J.; Li, W.; Yang, Y.; Piao, J.; Yang, L.; et al. Prevalence and Risk Factors for Anemia in Non-pregnant Childbearing Women from the Chinese Fifth National Health and Nutrition Survey. Int. J. Environ. Res. Public Health 2019, 16, 1290. [Google Scholar] [CrossRef]
- Ng, S.W.; Norton, E.C.; Popkin, B.M. Why have physical activity levels declined among Chinese adults? Findings from the 1991-2006 China Health and Nutrition Surveys. Soc. Sci. Med. 2009, 68, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, F.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar] [PubMed]
- Schulz, R.; Slavin, J. Perspective: Defining Carbohydrate Quality for Human Health and Environmental Sustainability. Adv. Nutr. 2021, 12, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 274s–280s. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Ocana, A.M.; Vuksan, V.; Cunnane, S.C.; Jenkins, M.; Wong, G.S.; Singer, W.; Bloom, S.R.; Blendis, L.M.; et al. Metabolic effects of reducing rate of glucose ingestion by single bolus versus continuous sipping. Diabetes 1990, 39, 775–781. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.; Miller, E.R., 3rd; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The OmniCarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef]
- Sloth, B.; Krog-Mikkelsen, I.; Flint, A.; Tetens, I.; Björck, I.; Vinoy, S.; Elmståhl, H.; Astrup, A.; Lang, V.; Raben, A. No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am. J. Clin. Nutr. 2004, 80, 337–347. [Google Scholar] [CrossRef]
- Bulló, M.; Papandreou, C.; Ruiz-Canela, M.; Guasch-Ferré, M.; Li, J.; Hernández-Alonso, P.; Toledo, E.; Liang, L.; Razquin, C.; Corella, D.; et al. Plasma Metabolomic Profiles of Glycemic Index, Glycemic Load, and Carbohydrate Quality Index in the PREDIMED Study. J. Nutr. 2021, 151, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Esko, T.; Hirschhorn, J.N.; Feldman, H.A.; Hsu, Y.H.; Deik, A.A.; Clish, C.B.; Ebbeling, C.B.; Ludwig, D.S. Metabolomic profiles as reliable biomarkers of dietary composition. Am. J. Clin. Nutr. 2017, 105, 547–554. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Hernández-Alonso, P.; Guasch-Ferré, M.; Ruiz-Canela, M.; Li, J.; Wittenbecher, C.; Razquin, C.; Toledo, E.; Dennis, C.; Corella, D.; et al. Dairy consumption, plasma metabolites, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2021, 114, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; Chilloux, J.; Nielsen, T.; Vieira-Silva, S.; Falony, G.; Andrikopoulos, P.; Olanipekun, M.; Hoyles, L.; Djouadi, F.; Neves, A.L.; et al. Human and preclinical studies of the host-gut microbiome co-metabolite hippurate as a marker and mediator of metabolic health. Gut 2021, 70, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Venn, B.J.; Perry, T.; Brown, R.; Wallace, A.; Mann, J.I.; Green, T.J. Another approach to estimating the reliability of glycaemic index. Br. J. Nutr. 2008, 100, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 105, 842–853. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 106, 1246–1256. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Z.; Liu, M.; Zhang, Y.; Li, H.; He, P.; Li, Q.; Liu, C.; Qin, X. Dietary carbohydrate intake and new-onset diabetes: A nationwide cohort study in China. Metabolism 2021, 123, 154865. [Google Scholar] [CrossRef]

| Variables | Total | Quintiles of Dietary GI Value | Quintiles of Dietary CF Value | ||||
|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | ||
| n | 14,590 | 2918 | 2918 | 2918 | 2918 | 2918 | 2918 |
| Age, years | 45 ± 15 | 46 ± 17 | 45 ± 14 | 45 ± 15 | 46 ± 14 | 44 ± 14 | 46 ± 17 |
| Male, n (%) | 7402 (50.7) | 1384 (47.4) | 1510 (51.7) | 1554 (53.3) | 1416 (48.5) | 1468 (50.3) | 1516 (52.0) |
| BMI, kg/m2 | 23.2 ± 3.3 | 23.0 ± 3.4 | 23.2 ± 3.2 | 23.4 ± 3.3 | 23.8 ± 3.5 | 23.4 ± 3.2 | 22.4 ± 3.2 |
| Baseline hypertension, n (%) | 3095 (21.2) | 647 (22.2) | 597 (20.5) | 621 (21.3) | 699 (24.0) | 627 (21.5) | 572 (19.6) |
| Education level, n (%) | |||||||
| Primary | 6860 (47.0) | 1182 (40.5) | 1417 (48.6) | 1420 (48.7) | 1004 (34.4) | 1499 (51.4) | 1568 (53.7) |
| Middle | 4145 (28.4) | 788 (27.0) | 825 (28.3) | 945 (32.4) | 842 (28.9) | 808 (27.7) | 810 (27.8) |
| High | 3585 (24.6) | 948 (32.5) | 676 (23.2) | 553 (19.0) | 1072 (36.7) | 611 (20.9) | 540 (18.5) |
| Urbanization index, n (%) | |||||||
| Low | 4846 (33.2) | 629 (21.6) | 915 (31.4) | 1433 (49.1) | 582 (19.9) | 1171 (40.1) | 1088 (37.3) |
| Moderate | 4881 (33.5) | 918 (31.5) | 999 (34.2) | 939 (32.2) | 856 (29.3) | 959 (32.9) | 1007 (34.5) |
| High | 4863 (33.3) | 1371 (47.0) | 1004 (34.4) | 546 (18.7) | 1480 (50.7) | 788 (27.0) | 823 (28.2) |
| Region | |||||||
| Northern | 6066 (41.6) | 777 (26.6) | 1125 (38.6) | 1823 (62.5) | 1361 (46.6) | 1518 (52.0) | 621 (21.3) |
| Southern | 8524 (58.4) | 2141 (73.4) | 1793 (61.4) | 1095 (37.5) | 1557 (53.4) | 1400 (48.0) | 2297 (78.7) |
| Smoking status, n (%) | |||||||
| No | 9133 (62.6) | 1973 (67.6) | 1772 (60.7) | 1804 (61.8) | 1984 (68.0) | 1778 (60.9) | 1812 (62.1) |
| Yes | 5457 (37.4) | 945 (32.4) | 1146 (39.3) | 1114 (38.2) | 934 (32.0) | 1140 (39.1) | 1106 (37.9) |
| Alcohol consumption, n (%) | |||||||
| No | 7478 (51.3) | 1670 (57.2) | 1367 (46.8) | 1627 (55.8) | 1509 (51.7) | 1456 (49.9) | 1620 (55.5) |
| Yes | 7112 (48.7) | 1248 (42.8) | 1551 (53.2) | 1291 (44.2) | 1409 (48.3) | 1462 (50.1) | 1298 (44.5) |
| Physical activity status, METs-h/week | 103.9 ± 84.0 | 89.5 ± 79.6 | 102.4 ± 83.0 | 118.4 ± 86.7 | 90.7 ± 76.1 | 114.3 ± 88.1 | 103.9 ± 81.9 |
| Total energy intake, kcal/d 2 | 2106.2 ± 13.4 | 2105.8 ± 17.9 | 2106.8 ± 12.5 | 2105.8 ± 10.5 | 2104.8 ± 19.8 | 2106.3 ± 10.8 | 2107.4 ± 11.0 |
| Total carbohydrate intake, % energy 2 | 55.1 ± 11.0 | 51.4 ± 12.2 | 54.4 ± 9.9 | 60.0 ± 10.2 | 48.8 ± 11.8 | 56.8 ± 10.4 | 58.8 ± 9.4 |
| Total dietary fiber intake, g/d 2 | 11.6 ± 5.5 | 12.5 ± 8.2 | 11.1 ± 4.5 | 11.5 ± 4.2 | 18.2 ± 7.6 | 11.0 ± 2.1 | 6.7 ± 1.5 |
| Fat intake, % energy 2 | 31.9 ± 10.3 | 34.6 ± 11.2 | 32.7 ± 9.5 | 27.6 ± 10.1 | 36.5 ± 11.3 | 30.4 ± 9.9 | 29.2 ± 8.9 |
| Cholesterol intake, mg/d 2 | 153.3 ± 135.5 | 190.3 ± 152.5 | 158.6 ± 135.6 | 109.6 ± 125.7 | 171.6 ± 137.9 | 138.4 ± 133.4 | 155.7 ± 130.1 |
| PUFA to SFA ratio 2 | 1.2 ± 0.7 | 1.2 ± 0.7 | 1.2 ± 0.6 | 1.4 ± 0.7 | 1.3 ± 0.6 | 1.4 ± 0.7 | 1.1 ± 0.6 |
| Protein intake, % energy 2 | 12.2 ± 2.5 | 13.1 ± 3.3 | 12.0 ± 2.2 | 11.9 ± 2.0 | 13.5 ± 3.1 | 12.1 ± 2.0 | 11.4 ± 2.4 |
| Dietary GI 2 | 69.9 (65.2, 73.8) | 60.8 (57.9, 62.5) | 69.9 (69.2, 70.7) | 77.0 (75.8, 78.4) | 66.6 (61.5, 71.0) | 71.8 (67.9, 75.2) | 69.0 (63.6, 73.3) |
| CF 2 | 27.8 (21.5, 35.6) | 25.1 (16.5, 39.3) | 27.9 (21.9, 35.3) | 28.6 (24.4, 35.0) | 16.3 (13.4, 18.3) | 27.8 (26.6, 29.0) | 45.1 (41.2, 51.8) |
| CQI | 8.0 (7.0, 10.0) | 10.0 (9.0, 12.0) | 8.0 (7.0, 10.0) | 7.0 (5.0, 8.0) | 11.0 (9.0, 12.0) | 9.0 (7.0, 10.0) | 7.0 (6.0, 8.0) |
| Variables | HR (95% CI) of Quintiles of Carbohydrate Quality Indicators | P-Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Dietary GI | ||||||
| n | 2918 | 2918 | 2918 | 2918 | 2918 | |
| range | (18.5, 63.9) | (63.9, 68.3) | (68.3, 71.4) | (71.4, 74.8) | (74.8, 88.2) | |
| Median | 60.8 | 66.3 | 69.9 | 73.0 | 77.0 | |
| Cases (incidence rate, ‰ person-year) | 184 (8.95) | 224 (7.39) | 216 (6.35) | 212 (6.47) | 217 (8.37) | |
| Model 1 2 | 1.00 (Ref) | 0.66 (0.54, 0.80) | 0.56 (0.46, 0.68) | 0.58 (0.47, 0.70) | 0.85 (0.70, 1.04) | 0.15 |
| Model 2 2 | 1.00 (Ref) | 0.63 (0.52, 0.78) | 0.54 (0.44, 0.66) | 0.52 (0.42, 0.64) | 0.73 (0.58, 0.91) | 0.0023 |
| Model 3 2 | 1.00 (Ref) | 0.66 (0.54, 0.81) | 0.57 (0.46, 0.70) | 0.54 (0.43, 0.67) | 0.72 (0.57, 0.91) | 0.0024 |
| Model 1 3 | 1.74 (1.42, 2.12) | 1.14 (0.95, 1.38) | 0.97 (0.80, 1.17) | 1.00 (Ref) | 1.48 (1.23, 1.79) | 0.15 |
| Model 2 3 | 1.93 (1.56, 2.39) | 1.22 (1.00, 1.49) | 1.04 (0.86, 1.27) | 1.00 (Ref) | 1.40 (1.15, 1.71) | 0.0023 |
| Model 3 3 | 1.86 (1.49, 2.33) | 1.24 (1.01, 1.51) | 1.06 (0.87, 1.29) | 1.00 (Ref) | 1.34 (1.10, 1.64) | 0.0024 |
| CF | ||||||
| n | 2918 | 2918 | 2918 | 2918 | 2918 | |
| range | (3.1, 20.0) | (20.0, 25.4) | (25.4, 30.4) | (30.4, 38.2) | (38.2, 221.2) | |
| Median | 16.3 | 22.9 | 27.8 | 33.6 | 45.1 | |
| Cases (incidence rate, ‰ person-year) | 171 (7.74) | 206 (6.80) | 230 (7.08) | 213 (6.59) | 233 (8.81) | |
| Model 1 2 | 1.00 (Ref) | 0.70 (0.56, 0.87) | 0.63 (0.48, 0.81) | 0.49 (0.35, 0.70) | 0.59 (0.38, 0.90) | 0.0071 |
| Model 2 2 | 1.00 (Ref) | 0.70 (0.56, 0.88) | 0.69 (0.53, 0.91) | 0.62 (0.44, 0.89) | 0.95 (0.61, 1.48) | 0.48 |
| Model 3 2 | 1.00 (Ref) | 0.73 (0.58, 0.91) | 0.71 (0.54, 0.94) | 0.65 (0.46, 0.94) | 1.02 (0.66, 1.59) | 0.70 |
| Model 1 3 | 2.03 (1.44, 2.86) | 1.41 (1.07, 1.87) | 1.27 (1.02, 1.57) | 1.00 (Ref) | 1.19 (0.96, 1.47) | 0.0071 |
| Model 2 3 | 1.61 (1.13, 2.30) | 1.13 (0.84, 1.51) | 1.11 (0.89, 1.39) | 1.00 (Ref) | 1.53 (1.23, 1.91) | 0.48 |
| Model 3 3 | 1.53 (1.07, 2.19) | 1.11 (0.82, 1.49) | 1.09 (0.87, 1.36) | 1.00 (Ref) | 1.56 (1.26, 1.95) | 0.70 |
| CQI | ||||||
| n | 2683 | 1980 | 2661 | 3772 | 3494 | |
| range | (4.0, 6.0) | (7.0, 7.0) | (8.0, 8.0) | (9.0, 10.0) | (11.0, 20.0) | |
| Median | 5.0 | 7.0 | 8.0 | 9.0 | 12.0 | |
| Cases (incidence rate, %) | 182 (8.28) | 144 (7.03) | 182 (7.04) | 291 (7.03) | 254 (7.08) | |
| Model 1 2 | 1.00 (Ref) | 0.84 (0.67, 1.05) | 0.88 (0.72, 1.08) | 0.91 (0.76, 1.10) | 0.85 (0.70, 1.03) | 0.83 |
| Model 2 2 | 1.00 (Ref) | 0.89 (0.71, 1.12) | 0.85 (0.69, 1.05) | 0.89 (0.74, 1.08) | 0.79 (0.64, 0.96) | 0.35 |
| Model 3 2 | 1.00 (Ref) | 0.90 (0.71, 1.14) | 0.83 (0.63, 1.11) | 0.87 (0.62, 1.24) | 0.77 (0.50, 1.19) | 0.30 |
| Variables | n | Cases (Incidence Rate, ‰ Person-Year) | HR (95% CI) of Quintiles of Carbohydrate Quality Indicators | P- Trend | P- Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||||
| Dietary GI | |||||||||
| Age, y | |||||||||
| <60 | 11,881 | 787 (6.38) | 1.00 (Ref) | 0.70 (0.55, 0.89) | 0.62 (0.48, 0.78) | 0.56 (0.43, 0.72) | 0.74 (0.57, 0.97) | 0.0162 | 0.47 |
| ≥60 | 2709 | 266 (13.13) | 1.00 (Ref) | 0.67 (0.46, 0.97) | 0.42 (0.28, 0.64) | 0.53 (0.35, 0.81) | 0.69 (0.44, 1.08) | 0.0311 | |
| Sex | |||||||||
| Male | 7402 | 517 (6.92) | 1.00 (Ref) | 0.62 (0.45, 0.85) | 0.60 (0.44, 0.82) | 0.54 (0.39, 0.75) | 0.77 (0.55, 1.09) | 0.18 | 0.56 |
| Female | 7188 | 536 (7.77) | 1.00 (Ref) | 0.67 (0.51, 0.90) | 0.48 (0.36, 0.66) | 0.50 (0.37, 0.69) | 0.60 (0.43, 0.84) | 0.0007 | |
| BMI, kg/m2 | |||||||||
| <24.0 | 9313 | 423 (4.51) | 1.00 (Ref) | 0.77 (0.55, 1.07) | 0.66 (0.47, 0.93) | 0.50 (0.34, 0.72) | 0.94 (0.65, 1.36) | 0.22 | 0.0259 |
| ≥24.0 | 5277 | 630 (12.62) | 1.00 (Ref) | 0.56 (0.43, 0.74) | 0.47 (0.36, 0.62) | 0.48 (0.36, 0.64) | 0.52 (0.38, 0.71) | 0.0002 | |
| Baseline hypertension | |||||||||
| No | 11,495 | 683 (5.80) | 1.00 (Ref) | 0.71 (0.55, 0.93) | 0.66 (0.50, 0.86) | 0.53 (0.40, 0.71) | 0.72 (0.53, 0.97) | 0.0116 | 0.52 |
| Yes | 3095 | 370 (14.26) | 1.00 (Ref) | 0.69 (0.47, 1.00) | 0.49 (0.34, 0.72) | 0.56 (0.38, 0.84) | 0.69 (0.46, 1.04) | 0.0453 | |
| Urbanization index, median | |||||||||
| <69.46 | 7293 | 491 (5.86) | 1.00 (Ref) | 0.76 (0.47, 1.24) | 0.56 (0.34, 0.91) | 0.43 (0.26, 0.70) | 0.45 (0.27, 0.76) | 0.0004 | 0.0068 |
| ≥69.46 | 7297 | 562 (9.40) | 1.00 (Ref) | 0.58 (0.38, 0.88) | 0.56 (0.37, 0.86) | 0.51 (0.32, 0.81) | 1.16 (0.69, 1.96) | 0.75 | |
| Education level | |||||||||
| Primary or lower | 6860 | 618 (8.22) | 1.00 (Ref) | 0.85 (0.55, 1.32) | 0.54 (0.35, 0.83) | 0.46 (0.29, 0.73) | 0.54 (0.33, 0.88) | 0.0016 | 0.29 |
| Middle or above | 7730 | 435 (6.35) | 1.00 (Ref) | 0.66 (0.41, 1.05) | 0.70 (0.43, 1.14) | 0.53 (0.32, 0.88) | 1.02 (0.59, 1.76) | 0.72 | |
| Dietary PUFA: SFA, median | |||||||||
| <1.15 | 7295 | 479 (6.75) | 1.00 (Ref) | 0.66 (0.49, 0.88) | 0.62 (0.46, 0.84) | 0.64 (0.47, 0.89) | 1.06 (0.74, 1.51) | 0.74 | 0.0069 |
| ≥1.15 | 7295 | 574 (7.90) | 1.00 (Ref) | 0.73 (0.53, 0.99) | 0.58 (0.42, 0.80) | 0.46 (0.33, 0.63) | 0.55 (0.40, 0.76) | <0.0001 | |
| CF | |||||||||
| Age, y | |||||||||
| <60 | 11,881 | 787 (6.38) | 1.00 (Ref) | 0.84 (0.64, 1.09) | 0.78 (0.56, 1.08) | 0.74 (0.48, 1.14) | 1.31 (0.77, 2.22) | 0.64 | 0.33 |
| ≥60 | 2709 | 266 (13.13) | 1.00 (Ref) | 0.47 (0.31, 0.73) | 0.46 (0.28, 0.77) | 0.41 (0.21, 0.79) | 0.42 (0.19, 0.96) | 0.0247 | |
| Sex | |||||||||
| Male | 7402 | 517 (6.92) | 1.00 (Ref) | 0.71 (0.51, 0.99) | 0.62 (0.41, 0.94) | 0.63 (0.37, 1.09) | 0.85 (0.43, 1.67) | 0.48 | 0.68 |
| Female | 7188 | 536 (7.77) | 1.00 (Ref) | 0.74 (0.53, 1.01) | 0.82 (0.56, 1.20) | 0.68 (0.41, 1.12) | 1.19 (0.65, 2.19) | 0.84 | |
| BMI, kg/m2 | |||||||||
| <24.0 | 9313 | 423 (4.51) | 1.00 (Ref) | 0.79 (0.54, 1.15) | 0.52 (0.33, 0.82) | 0.58 (0.32, 1.03) | 0.60 (0.29, 1.23) | 0.11 | 0.0081 |
| ≥24.0 | 5277 | 630 (12.62) | 1.00 (Ref) | 0.66 (0.50, 0.88) | 0.79 (0.56, 1.13) | 0.66 (0.41, 1.06) | 1.41 (0.80, 2.49) | 0.52 | |
| Baseline hypertension | |||||||||
| No | 11,495 | 683 (5.80) | 1.00 (Ref) | 0.75 (0.57, 1.00) | 0.68 (0.48, 0.98) | 0.62 (0.38, 0.99) | 0.88 (0.48, 1.61) | 0.41 | 0.48 |
| Yes | 3095 | 370 (14.26) | 1.00 (Ref) | 0.71 (0.48, 1.07) | 0.73 (0.46, 1.17) | 0.60 (0.32, 1.11) | 1.08 (0.52, 2.23) | 0.90 | |
| Urbanization index, median | |||||||||
| <69.46 | 7293 | 491 (5.86) | 1.00 (Ref) | 0.69 (0.48, 1.00) | 0.60 (0.37, 0.97) | 0.57 (0.30, 1.09) | 0.97 (0.43, 2.18) | 0.88 | 0.44 |
| ≥69.46 | 7297 | 562 (9.40) | 1.00 (Ref) | 0.69 (0.51, 0.94) | 0.79 (0.55, 1.13) | 0.70 (0.44, 1.12) | 0.86 (0.49, 1.52) | 0.51 | |
| Education level | |||||||||
| Primary or lower | 6860 | 618 (8.22) | 1.00 (Ref) | 0.63 (0.46, 0.86) | 0.60 (0.41, 0.89) | 0.52 (0.31, 0.86) | 0.72 (0.38, 1.35) | 0.27 | 0.32 |
| Middle or above | 7730 | 435 (6.35) | 1.00 (Ref) | 0.81 (0.58, 1.15) | 0.88 (0.58, 1.33) | 0.82 (0.48, 1.41) | 1.35 (0.70, 2.60) | 0.63 | |
| Dietary PUFA: SFA, median | |||||||||
| <1.15 | 7295 | 479 (6.75) | 1.00 (Ref) | 0.84 (0.59, 1.20) | 0.80 (0.52, 1.23) | 0.69 (0.40, 1.18) | 0.93 (0.48, 1.81) | 0.64 | 0.40 |
| ≥1.15 | 7295 | 574 (7.90) | 1.00 (Ref) | 0.63 (0.47, 0.86) | 0.65 (0.44, 0.96) | 0.57 (0.33, 0.96) | 1.08 (0.57, 2.06) | 0.75 | |
| CQI | |||||||||
| Age, y | |||||||||
| <60 | 11,881 | 787 (6.38) | 1.00 (Ref) | 0.66 (0.51, 0.86) | 0.71 (0.56, 0.91) | 0.69 (0.55, 0.86) | 0.63 (0.50, 0.80) | 0.0015 | 0.0324 |
| ≥60 | 2709 | 266 (13.13) | 1.00 (Ref) | 1.48 (0.96, 2.26) | 0.97 (0.63, 1.50) | 1.23 (0.83, 1.80) | 1.12 (0.75, 1.66) | 0.82 | |
| Sex | |||||||||
| Male | 7402 | 517 (6.92) | 1.00 (Ref) | 0.71 (0.50, 1.00) | 0.68 (0.49, 0.94) | 0.74 (0.55, 0.99) | 0.64 (0.47, 0.86) | 0.0214 | 0.12 |
| Female | 7188 | 536 (7.77) | 1.00 (Ref) | 1.14 (0.83, 1.57) | 1.04 (0.76, 1.42) | 0.99 (0.75, 1.32) | 0.91 (0.68, 1.21) | 0.29 | |
| BMI, kg/m2 | |||||||||
| <24.0 | 9313 | 423 (4.51) | 1.00 (Ref) | 0.99 (0.70, 1.40) | 0.90 (0.64, 1.26) | 1.04 (0.77, 1.42) | 0.65 (0.46, 0.91) | 0.0400 | 0.07 |
| ≥24.0 | 5277 | 630 (12.62) | 1.00 (Ref) | 0.87 (0.64, 1.18) | 0.78 (0.58, 1.04) | 0.75 (0.58, 0.98) | 0.85 (0.65, 1.10) | 0.20 | |
| Baseline hypertension | |||||||||
| No | 11,495 | 683 (5.80) | 1.00 (Ref) | 0.83 (0.62, 1.11) | 0.91 (0.69, 1.19) | 0.91 (0.70, 1.17) | 0.74 (0.57, 0.96) | 0.08 | 0.47 |
| Yes | 3095 | 370 (14.26) | 1.00 (Ref) | 1.06 (0.71, 1.58) | 0.71 (0.48, 1.05) | 0.87 (0.61, 1.23) | 0.82 (0.58, 1.17) | 0.22 | |
| Urbanization index, median | |||||||||
| <69.46 | 7293 | 491 (5.86) | 1.00 (Ref) | 0.77 (0.54, 1.09) | 0.71 (0.52, 0.99) | 0.81 (0.61, 1.09) | 0.67 (0.49, 0.90) | 0.0356 | 0.94 |
| ≥69.46 | 7297 | 562 (9.40) | 1.00 (Ref) | 0.89 (0.65, 1.22) | 0.84 (0.63, 1.14) | 0.81 (0.61, 1.07) | 0.77 (0.58, 1.03) | 0.07 | |
| Education level | |||||||||
| Primary or lower | 6860 | 618 (8.22) | 1.00 (Ref) | 1.14 (0.84, 1.54) | 1.01 (0.76, 1.36) | 1.08 (0.83, 1.42) | 0.94 (0.72, 1.24) | 0.055 | 0.0069 |
| Middle or above | 7730 | 435 (6.35) | 1.00 (Ref) | 0.61 (0.42, 0.88) | 0.57 (0.40, 0.79) | 0.56 (0.41, 0.76) | 0.46 (0.34, 0.64) | <0.0001 | |
| Dietary PUFA: SFA, median | |||||||||
| <1.15 | 7295 | 479 (6.75) | 1.00 (Ref) | 0.98 (0.71, 1.35) | 0.96 (0.71, 1.30) | 0.81 (0.60, 1.08) | 0.69 (0.51, 0.94) | 0.0083 | 0.38 |
| ≥1.15 | 7295 | 574 (7.90) | 1.00 (Ref) | 0.87 (0.61, 1.23) | 0.79 (0.57, 1.10) | 0.96 (0.72, 1.28) | 0.82 (0.61, 1.10) | 0.43 | |
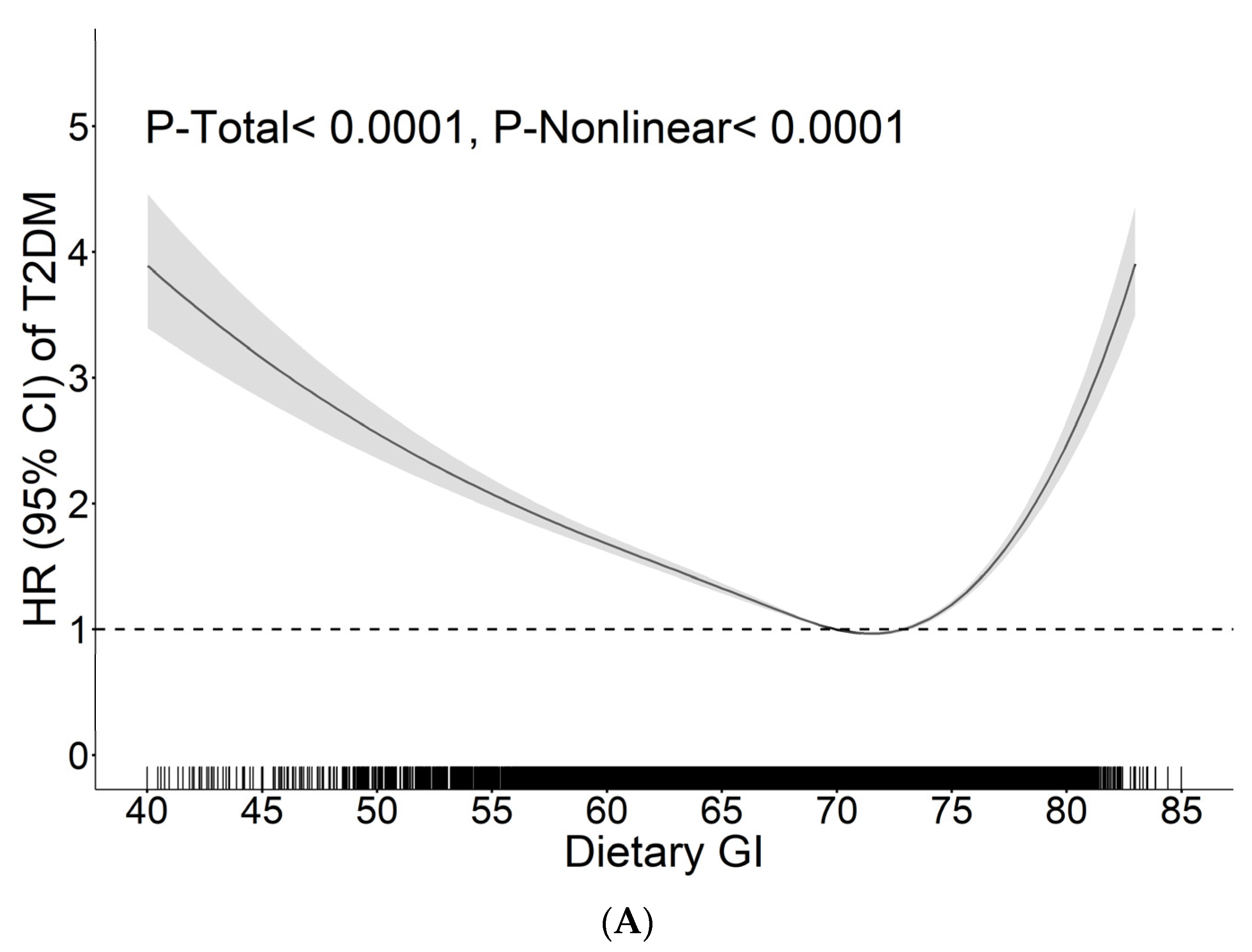
| Carbohydrate Quality Indicators | Inflection Point (95% CI) | Group | HR (95% CI) | P | P-log Likelihood Ratio |
|---|---|---|---|---|---|
| Dietary GI | 72.85 (71.40, 74.05) | <72.85 | 0.95 (0.93, 0.96) | <0.0001 | <0.0001 |
| ≥72.85 | 1.11 (1.07, 1.16) | <0.0001 | |||
| CF | 20.55 (17.92, 21.91) | <20.55 | 0.94 (0.91, 0.96) | <0.0001 | <0.0001 |
| ≥20.55 | 1.03 (1.02, 1.04) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Wu, M.; Liu, K.; Wang, Y.; Kang, T.; Meng, S.; Meng, H. Associations between Conventional and Emerging Indicators of Dietary Carbohydrate Quality and New-Onset Type 2 Diabetes Mellitus in Chinese Adults. Nutrients 2023, 15, 647. https://doi.org/10.3390/nu15030647
Cui Z, Wu M, Liu K, Wang Y, Kang T, Meng S, Meng H. Associations between Conventional and Emerging Indicators of Dietary Carbohydrate Quality and New-Onset Type 2 Diabetes Mellitus in Chinese Adults. Nutrients. 2023; 15(3):647. https://doi.org/10.3390/nu15030647
Chicago/Turabian StyleCui, Zhixin, Man Wu, Ke Liu, Yin Wang, Tong Kang, Shuangli Meng, and Huicui Meng. 2023. "Associations between Conventional and Emerging Indicators of Dietary Carbohydrate Quality and New-Onset Type 2 Diabetes Mellitus in Chinese Adults" Nutrients 15, no. 3: 647. https://doi.org/10.3390/nu15030647
APA StyleCui, Z., Wu, M., Liu, K., Wang, Y., Kang, T., Meng, S., & Meng, H. (2023). Associations between Conventional and Emerging Indicators of Dietary Carbohydrate Quality and New-Onset Type 2 Diabetes Mellitus in Chinese Adults. Nutrients, 15(3), 647. https://doi.org/10.3390/nu15030647






