Assessment of Food Intake Assisted by Photography in Older People Living in a Nursing Home: Maintenance over Time and Performance for Diagnosis of Malnutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Assessment of Food Intake by the SEFI® Assisted by Photography (SEFI®-AP)
2.4. Assessment of Nutritional Status
2.5. Other Data Collection
2.6. Study Endpoints
2.7. Ethical Considerations
2.8. Statistical Analyzes
3. Results
3.1. Patient Recruitment
3.2. Characteristics of the Study Population
3.3. Maintenance over One Month of One-Day Semi-Quantitative Assessment of Food Intake
3.4. Performance of Day 3 SEFI®-AP to Identify Decreased Food Intake during the Following Month
3.5. Malnutrition Prevalence According to Different GLIM Criteria
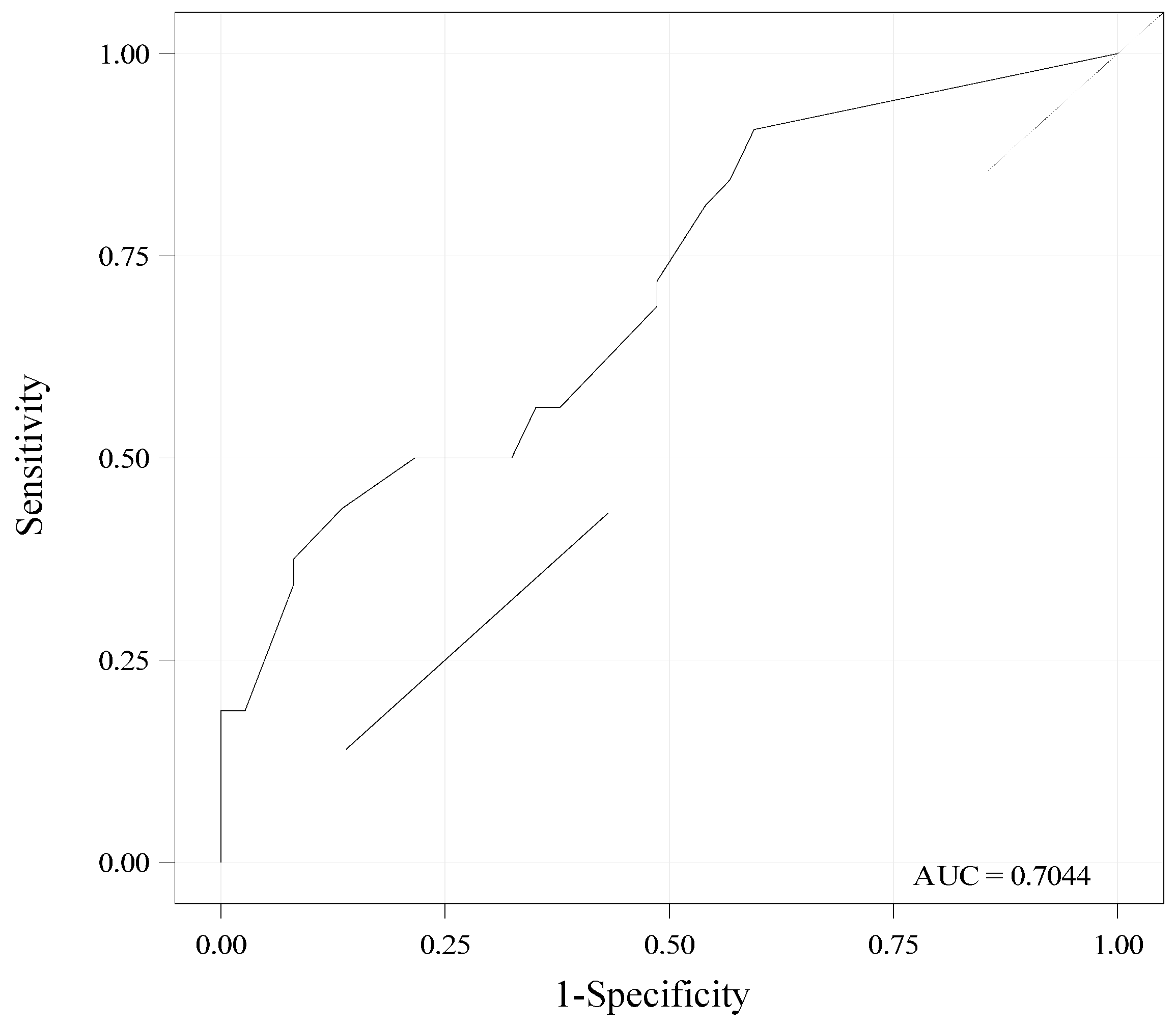
3.6. Performance of Day 3 SEFI®-AP for Diagnosis of Malnutrition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, C.L.; Lee, A.S.W.; Tamura, B.K. Malnutrition in the Nursing Home. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Streicher, M.; Smoliner, C.; Kolb, C.; Hiesmayr, M.; Thiem, U.; Sieber, C.C.; Volkert, D. The Impact of Weight Loss and Low BMI on Mortality of Nursing Home Residents—Results from the NutritionDay in Nursing Homes. Clin. Nutr. 2016, 35, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Meijers, J.M.M.; Tan, F.; Schols, J.M.G.A.; Halfens, R.J.G. Nutritional Care; Do Process and Structure Indicators Influence Malnutrition Prevalence over Time? Clin. Nutr. 2014, 33, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Seguy, D.; Hubert, H.; Robert, J.; Meunier, J.P.; Guérin, O.; Raynaud-Simon, A. Compliance to Oral Nutritional Supplementation Decreases the Risk of Hospitalisation in Malnourished Older Adults without Extra Health Care Cost: Prospective Observational Cohort Study. Clin. Nutr. 2020, 39, 1900–1907. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN Guideline on Clinical Nutrition and Hydration in Geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Liu, P.; Hao, Q.; Hai, S.; Wang, H.; Cao, L.; Dong, B. Sarcopenia as a Predictor of All-Cause Mortality among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Maturitas 2017, 103, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.; Dhillo, W.S.; Frost, G.; Hickson, M. Gastrointestinal Hormones: The Regulation of Appetite and the Anorexia of Ageing. J. Hum. Nutr. Diet. 2012, 25, 3–15. [Google Scholar] [CrossRef]
- Hiesmayr, M.; Schindler, K.; Pernicka, E.; Schuh, C.; Schoeniger-Hekele, A.; Bauer, P.; Laviano, A.; Lovell, A.; Mouhieddine, M.; Schuetz, T.; et al. Decreased Food Intake Is a Risk Factor for Mortality in Hospitalised Patients: The NutritionDay Survey 2006. Clin. Nutr. 2009, 28, 484–491. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the Elderly at Risk for Malnutrition: The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Han, H.; Coulon, S.M.; Allen, H.R.; Champagne, C.M.; Anton, S.D. A Novel Method to Remotely Measure Food Intake of Free-Living Individuals in Real Time: The Remote Food Photography Method. Br. J. Nutr. 2008, 101, 446–456. [Google Scholar] [CrossRef]
- Thibault, R.; Goujon, N.; Le Gallic, E.; Clairand, R.; Sébille, V.; Vibert, J.; Schneider, S.M.; Darmaun, D. Use of 10-Point Analogue Scales to Estimate Dietary Intake: A Prospective Study in Patients Nutritionally at-Risk. Clin. Nutr. 2009, 28, 134–140. [Google Scholar] [CrossRef]
- Bouëtté, G.; Esvan, M.; Apel, K.; Thibault, R. A Visual Analogue Scale for Food Intake as a Screening Test for Malnutrition in the Primary Care Setting: Prospective Non-Interventional Study. Clin. Nutr. 2021, 40, 174–180. [Google Scholar] [CrossRef]
- Mesbah, S.; Mesbah, H.; Haumont, L.A.; Thibault, R. The Evaluation of Consumed Food Portions as a Screening Test for Malnutrition in the Older People Living in a Nursing Home: A Cross-Sectional Pilot Study. Clin. Nutr. Open Sci. 2022; in press. [Google Scholar] [CrossRef]
- Simmons, S.F.; Reuben, D. Nutritional Intake Monitoring for Nursing Home Residents: A Comparison of Staff Documentation, Direct Observation, and Photography Methods. J. Am. Geriatr. Soc. 2000, 48, 209–213. [Google Scholar] [CrossRef]
- Pouyet, V.; Cuvelier, G.; Benattar, L.; Giboreau, A. A Photographic Method to Measure Food Item Intake. Validation in Geriatric Institutions. Appetite 2015, 84, 11–19. [Google Scholar] [CrossRef]
- Hopman-Rock, M.; van Hirtum, H.; de Vreede, P.; Freiberger, E. Activities of daily living in older community-dwelling persons: A systematic review of psychometric properties of instruments. Aging Clin. Exp. Res. 2019, 31, 917–925. [Google Scholar] [CrossRef]
- Brisson, L.; Rousseau, C.; Thirion, R.; Baillard-Cosme, B.; Charras, K.; Somme, D.; Thibault, R. Preliminary study of the semi-quantitative evaluation of the food intake assisted by photography: Reliability and impact on clinical outcomes in nursing home residents. Poster presentation at the 2022 annual congress of the European Society for Clinical Nutrition and Metabolism (ESPEN) 2022. in press.
- Chumlea, W.C.; Roche, A.F.; Steinbaugh, M.L. Estimating Stature from Knee Height for Persons 60 to 90 Years of Age. J. Am. Geriatr. Soc. 1985, 33, 116–120. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for Assessment of the Muscle Mass Phenotypic Criterion for the Global Leadership Initiative on Malnutrition (GLIM) Diagnosis of Malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E.; et al. Assessing Appendicular Skeletal Muscle Mass with Bioelectrical Impedance Analysis in Free-Living Caucasian Older Adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, S.; Wang, J.; Pierson, R.N.; Heymsfield, S.B. Whole-Body Skeletal Muscle Mass: Development and Validation of Total-Body Potassium Prediction Models. Am. J. Clin. Nutr. 2003, 77, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of Skeletal Muscle Mass by Bioelectrical Impedance Analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, V.L.; Michel, J.P.; Slosman, D.O.; Pichard, C. Total Body Mass, Fat Mass, Fat-Free Mass, and Skeletal Muscle in Older People: Cross-Sectional Differences in 60-Year-Old Persons. J. Am. Geriatr. Soc. 2001, 49, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Van Wymelbeke-Delannoy, V.; Juhel, C.; Bole, H.; Sow, A.-K.; Guyot, C.; Belbaghdadi, F.; Brousse, O.; Paindavoine, M. A Cross-Sectional Reproducibility Study of a Standard Camera Sensor Using Artificial Intelligence to Assess Food Items: The FoodIntech Project. Nutrients 2022, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Abbasoglu, O.; Ioannou, E.; Meija, L.; Ottens-Oussoren, K.; Pichard, C.; Rothenberg, E.; Rubin, D.; Siljamäki-Ojansuu, U.; Vaillant, M.F.; et al. ESPEN guideline on hospital nutrition. Clin. Nutr. 2021, 40, 5684–5709. [Google Scholar] [CrossRef]
- Magaziner, J.; German, P.; Zimmerman, S.I.; Hebel, J.R.; Burton, L.; Gruber-Baldini, A.L.; May, C.; Kittner, S. The Prevalence of Dementia in a Statewide Sample of New Nursing Home Admissions Aged 65 and Older: Diagnosis by Expert Panel. Gerontologist 2000, 40, 663–672. [Google Scholar] [CrossRef]
- Volkert, D.; Chourdakis, M.; Faxen-Irving, G.; Frühwald, T.; Landi, F.; Suominen, M.H.; Vandewoude, M.; Wirth, R.; Schneider, S.M. ESPEN Guidelines on Nutrition in Dementia. Clin. Nutr. 2015, 34, 1052–1073. [Google Scholar] [CrossRef]



| Methods for Assessing Muscle Mass | Equations | Thresholds for Reduced Muscle Mass | |
|---|---|---|---|
| Men | Women | ||
| Calf circumference (cm) | - | <31 | <31 |
| ASMM (kg) (Sergi equation) [22] | = 3.964 + (0.227 × RI) + (0.095 × weight(kg)) + (1.384 × gender) + (0.064 × Xc) | <20 | <15 |
| ASMM index (kg/m²) (Sergi equation) [22] | = ASMM of Sergi (kg)/height (m)² | <7 | <5.5 |
| Skeletal Muscle Mass (SMM) (kg) (Wang equation) [22] | = (0.0093 × TBK) − (1.31 × gender) + (0.59 × black) + (0.024 × age) − 3.21 | <20 | <15 |
| SMM index (Wang equation) (kg/m²) [23] | = SMM Wang(kg)/height (m) ² | <7 | <5.7 |
| SMM (kg) (Janssen equation) [24] | = ((height(cm)²/R × 0.401) + (gender × 3.825) + (age × −0.071)) + 5.102 | <20 | <15 |
| SMM index (kg/m²) (Janssen equation) [24] | = SMM of Janssen(kg)/height (m)² | <7 | <5.7 |
| FFM index (kg/m²) [25] | = FFM (kg)/height (m)² | <17 | <15 |
| Variables | Total Population (n = 70) | day 3 SEFI®-AP <7 (n = 27) | ≥7 (n = 43) | p |
|---|---|---|---|---|
| Demographics | ||||
| Gender | 0.49 | |||
| Women | 54 (77.1%) | 22 (81.5%) | 32 (74.4%) | |
| Men | 16 (22.9%) | 5 (18.5%) | 11 (25.6%) | |
| Age (years) | 85.1 ± 6.4 | 86.4 ± 5.1 | 84.3 ± 7.0 | 0.19 |
| Comorbidities | ||||
| Dementia | 61 (87.1%) | 22 (81.5%) | 39 (90.7%) | 0.29 |
| Cancer | 7 (10.0%) | 5 (18.5%) | 2 (4.7%) | 0.1 |
| Organ failure | 18 (25.7%) | 4 (14.8%) | 14 (32.6%) | 0.1 |
| Cardiovascular disease | 53 (75.7%) | 20 (74.1%) | 33 (76.7%) | 0.8 |
| Diabetes | 9 (12.9%) | 4 (14.8%) | 5 (11.6%) | 0.73 |
| Depressive syndrome | 11 (15.7%) | 5 (18.5%) | 6 (14.0%) | 0.74 |
| Diet characteristics | ||||
| Assistance for food intake | 26 (37.1%) | 9 (33.3%) | 17 (39.5%) | 0.60 |
| HPHC diet | 33 (47.1%) | 15 (55.6%) | 18 (41.9%) | 0.26 |
| ONS | 33 (47.1%) | 14 (51.9%) | 19 (44.2%) | 0.53 |
| Treatment | ||||
| Micronutrients | 62 (88.6%) | 25 (92.6%) | 37 (86.0%) | 0.473 |
| Glucose SC infusion | 24 (34.3%) | 7 (25.9%) | 17 (39.5%) | 0.24 |
| NaCl SC infusion | 23 (32.9%) | 6 (22.2%) | 17 (39.5%) | 0.13 |
| ≥5 drugs | 59 (84.3%) | 23 (85.2%) | 36 (83.7%) | 1.00 |
| Nutritional evaluation | ||||
| BMI(kg/m²) | 26.1 ± 5.5 | 24.2 ± 5.0 | 27.4 ± 5.4 | 0.02 |
| 22 ≤ BMI < 25 (normal weight) | 11 (15.9%) | 2 (7.4%) | 9 (21.4%) | 0.18 |
| 25 ≤ BMI < 30 (overweight) | 26 (37.7%) | 14 (51.9%) | 12 (28.6%) | 0.05 |
| BMI ≥ 30 (obesity) | 16 (23.2%) | 2 (7.4%) | 14 (33.3%) | 0.01 |
| BMI < 22 (moderate malnutrition) | 16 (23.2%) | 9 (33.3%) | 7 (16.7%) | 0.11 |
| BMI < 20 (severe malnutrition) | 9 (13.0%) | 8 (29.6%) | 1 (2.4%) | 0.001 |
| Calf circumference (cm) | 33.1 ± 5.0 | 31.1 ± 4.3 | 34.5 ± 5.1 | 0.005 |
| Bioimpedance analysis | ||||
| FM (% of weight) | 36.57 ± 9.53 | 35.73 ± 9.13 | 37.59 ± 9.91 | 0.3781 |
| FM index (kg/m²) | 9.82 ± 3.83 | 8.72 ± 3.25 | 10.68 ± 4.05 | 0.0630 |
| FFM (% of weight) | 63.43 ± 9.53 | 64.27 ± 9.13 | 62.41 ± 9.91 | 0.3781 |
| FFM index (kg/m²) | 16.26 ± 2.97 | 14.96 ± 2.36 | 16.97 ± 3.06 | 0.0013 |
| SMM index (Wang equation) (kg/m²) | 6.79 ± 1.44 | 6.14 ± 0.95 | 7.13 ± 1.53 | 0.0057 |
| ASMM index (Sergi equation) (kg/m²) | 6.51 ± 1.08 | 5.96 ± 0.77 | 6.86 ± 1.10 | 0.0005 |
| SMM index (Janssen equation) (kg/m²) | 8.08 ± 1.60 | 7.39 ± 1.11 | 8.51 ± 1.72 | 0.0063 |
| Total body water (% of weight) | 45.58 ± 6.15 | 46.16 ± 5.97 | 45.32 ± 6.51 | 0.6068 |
| Phase angle (degree) | 3.94 ± 0.61 | 3.81 ± 0.64 | 4.04 ± 0.58 | 0.2143 |
| Follow-Up Period | SEFI®-AP (n = 70) Lunch | Mean of Lunch and Dinner |
|---|---|---|
| D3 | 6.8 ± 3.5 (0; 4; 8; 10; 10) | 6.9 ± 2.8 (0.5; 5; 7.5; 10; 10) |
| D1 to D5 | 6.8 ± 3.1 (1; 4.5; 8; 10; 10) | 7 ± 2.4 (1.5; 5; 7; 9.5; 10) |
| D1 to D10 | 6.7 ± 3.0 (1; 5; 7; 9; 10) | 6.9 ± 2.4 (1; 5; 7; 9.3; 10) |
| D1 to D15 | 6.5 ± 2.9 (1; 5; 7; 9; 10) | 6.9 ± 2.4 (1; 5; 7; 9.5; 10) |
| D1 to D20 | 6.4 ± 2.8 (1; 5; 6; 9; 10) | 6.8 ± 2.4 (1; 4.9; 6.6; 9.3; 10) |
| SEFI®-AP | TP | FP | FN | TN | Sensitivity [95% CI] | Specificity [95% CI] | PPV [95% CI] | NPV [95% CI] |
|---|---|---|---|---|---|---|---|---|
| D4-D10 (L) | 21 | 27 | 6 | 16 | 78% [62–94] | 37% [23–52] | 44% [30–58] | 73% [54–91] |
| D4-D10 (LD) | 24 | 32 | 3 | 11 | 89% [77–100] | 26% [13–39] | 43% [30–56] | 79% [57–100] |
| D4-D15 (L) | 17 | 25 | 10 | 18 | 63% [45–81] | 42% [27–57] | 41% [26–55] | 64% [47–82] |
| D4-D15 (LD) | 20 | 30 | 7 | 13 | 74% [58–91] | 30% [17–44] | 40% [26–54] | 65% [44–86] |
| D4-D20 (L) | 17 | 25 | 10 | 18 | 63% [45–81] | 42% [27–57] | 40% [26–55] | 64% [47–82] |
| D4-D20 (LD) | 21 | 30 | 6 | 13 | 78% [62–94] | 30% [17–44] | 41% [28–55] | 68% [48–89] |
| GLIM Malnutrition Criteria | Total Population (n = 69) | SEFI®-AP at Day 3 < 7 (n = 27) | ≥7 (n = 42) | p |
|---|---|---|---|---|
| BMI < 22 | 16 (23.2%) | 9 (33.3%) | 7 (16.7%) | 0.1094 |
| BMI < 20 | 9 (13.0%) | 8 (29.6%) | 1 (2.4%) | 0.0017 |
| Weight loss at 1 month ≥ 5% | 2 (3.2%) | 0 (0.0%) | 2 (5.3%) | 0.5136 |
| Weight loss at 1 month ≥ 10% | 1 (1.6%) | 0 (0.0%) | 1 (2.6%) | 1.0000 |
| Weight loss at 6 months ≥ 10% | 4 (6.1%) | 1 (4.0%) | 3 (7.3%) | 1.0000 |
| Weight loss at 6 months ≥ 15% | 0 | 0 | 0 | - |
| Phenotypic criterion of reduced muscle mass: Low FFMI | 33 (47.8%) | 15 (55.6%) | 18 (42.9%) | 0.3027 |
| Or low ASMM index (Sergi equation) | 25 (36.2%) | 11 (40.7%) | 14 (33.3%) | 0.5321 |
| Or low calf circumference | 32 (46.4%) | 16 (59.3%) | 16 (38.1%) | 0.0853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billeret, A.; Rousseau, C.; Thirion, R.; Baillard-Cosme, B.; Charras, K.; Somme, D.; Thibault, R. Assessment of Food Intake Assisted by Photography in Older People Living in a Nursing Home: Maintenance over Time and Performance for Diagnosis of Malnutrition. Nutrients 2023, 15, 646. https://doi.org/10.3390/nu15030646
Billeret A, Rousseau C, Thirion R, Baillard-Cosme B, Charras K, Somme D, Thibault R. Assessment of Food Intake Assisted by Photography in Older People Living in a Nursing Home: Maintenance over Time and Performance for Diagnosis of Malnutrition. Nutrients. 2023; 15(3):646. https://doi.org/10.3390/nu15030646
Chicago/Turabian StyleBilleret, Anne, Chloé Rousseau, Rémy Thirion, Béatrice Baillard-Cosme, Kevin Charras, Dominique Somme, and Ronan Thibault. 2023. "Assessment of Food Intake Assisted by Photography in Older People Living in a Nursing Home: Maintenance over Time and Performance for Diagnosis of Malnutrition" Nutrients 15, no. 3: 646. https://doi.org/10.3390/nu15030646
APA StyleBilleret, A., Rousseau, C., Thirion, R., Baillard-Cosme, B., Charras, K., Somme, D., & Thibault, R. (2023). Assessment of Food Intake Assisted by Photography in Older People Living in a Nursing Home: Maintenance over Time and Performance for Diagnosis of Malnutrition. Nutrients, 15(3), 646. https://doi.org/10.3390/nu15030646





