Combining Phenylalanine and Leucine Levels Predicts 30-Day Mortality in Critically Ill Patients Better than Traditional Risk Factors with Multicenter Validation
Abstract
1. Introduction
2. Materials and Methods
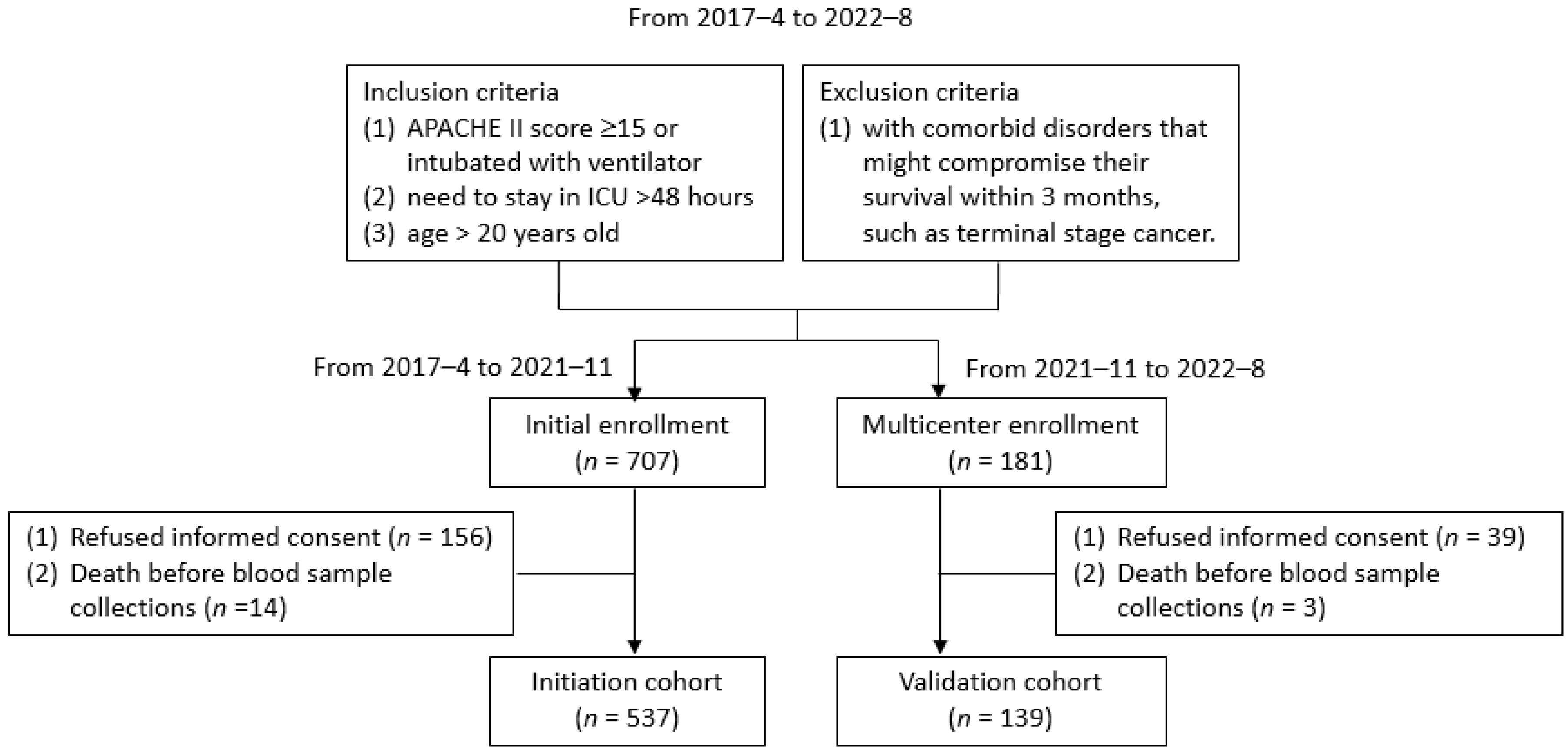
2.1. Patient Enrollment
2.2. Scoring Systems
2.3. Blood Sampling and Examination
2.4. Phenylalanine and Leucine Measurement
2.5. Follow-Up Program
2.6. Development of the Phenylalanine-Leucine Amino Acid (PLA) Score
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Laboratory Data
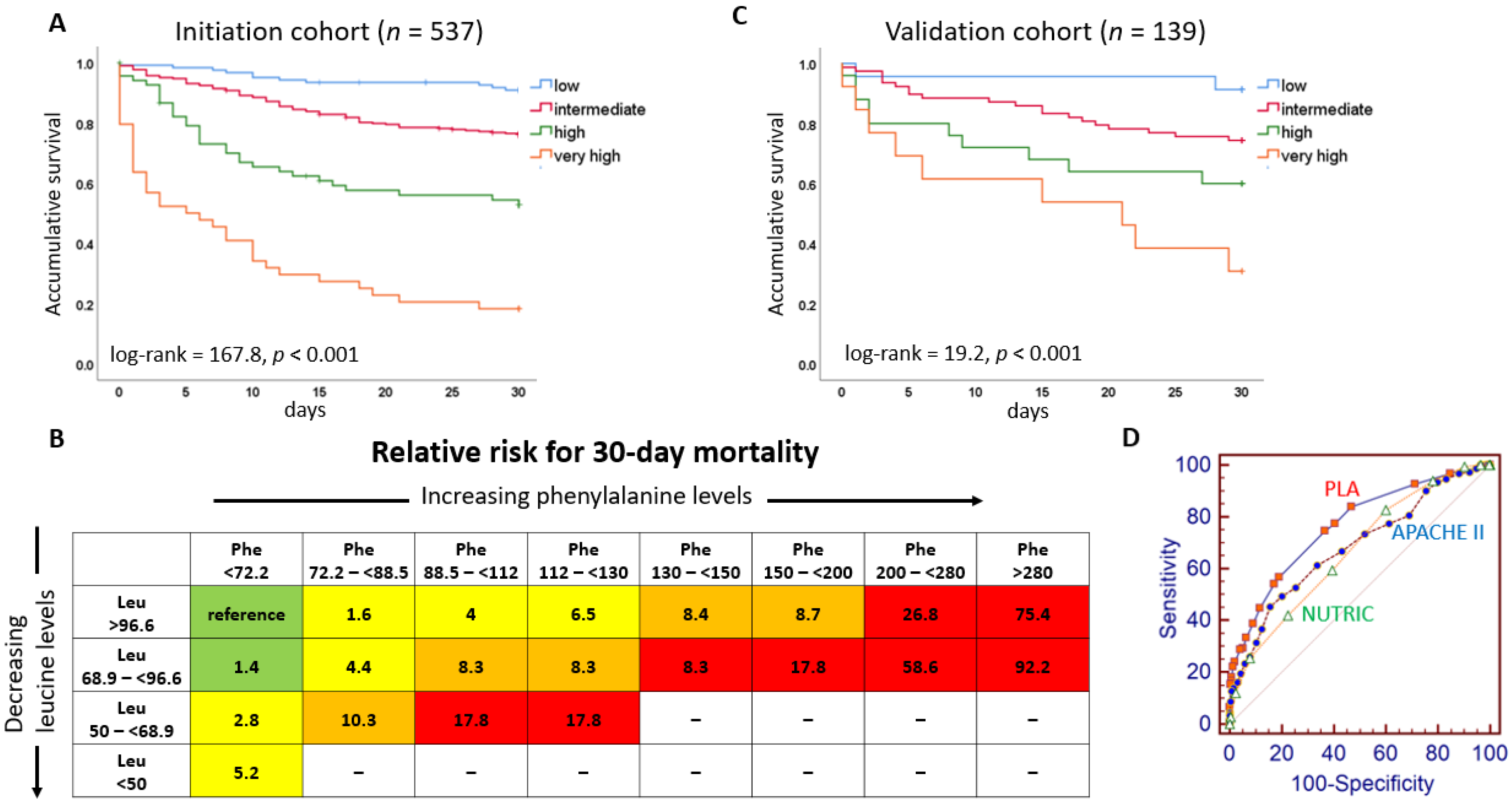
3.2. The Relationship between Mortality and Phenylalanine and Leucine Levels
3.3. The Building of PLA Score
3.4. The Prognostic Value of PLA Scores, Other Scores, and Biomarkers
4. Discussion
4.1. The Meaning of Elevated Phenylalanine Levels
4.2. The Meaning of Leucine Levels
4.3. Comparisons between PLA Score and Traditional Biomarkers
4.4. Clinical Implications of the PLA Score
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, L.; Chronaki, C.E.; Park, S.; Novack, V.; Baumfeld, Y.; Scott, D.; McLennan, S.; Talmor, D.; Celi, L. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive Care Med. 2012, 38, 1654–1661. [Google Scholar] [CrossRef]
- Capuzzo, M.; Volta, C.; Tassinati, T.; Moreno, R.; Valentin, A.; Guidet, B.; Iapichino, G.; Martin, C.; Perneger, T.; Combescure, C.; et al. Working Group on Health Economics of the European Society of Intensive Care Medicine. Hospital mortality of adults admitted to Intensive Care Units in hospitals with and without Intermediate Care Units: A multicentre European cohort study. Crit. Care 2014, 18, 551. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. Tailoring nutrition therapy to illness and recovery. Crit. Care 2017, 21, 316. [Google Scholar] [CrossRef]
- van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit. Care. 2019, 23, 368. [Google Scholar] [CrossRef]
- Huang, S.S.; Lin, J.Y.; Chen, W.S.; Liu, M.H.; Cheng, C.W.; Cheng, M.L.; Wang, C.H. Phenylalanine- and leucine-defined metabolic types identify high mortality risk in patients with severe infection. Int. J. Infect. Dis. 2019, 85, 143–149. [Google Scholar] [CrossRef]
- Luporini, R.L.; Pott-Junior, H.; Di Medeiros Leal, M.C.B.; Castro, A.; Ferreira, A.G.; Cominetti, M.R.; de Freitas Anibal, F. Phenylalanine and COVID-19: Tracking disease severity markers. Int. Immunopharmacol. 2021, 101, 108313. [Google Scholar] [CrossRef] [PubMed]
- Atila, A.; Alay, H.; Yaman, M.E.; Akman, T.C.; Cadirci, E.; Bayrak, B.; Celik, S.; Atila, N.E.; Yaganoglu, A.M.; Kadioglu, Y.; et al. The serum amino acid profile in COVID-19. Amino Acids 2021, 53, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Delles, C.; Rankin, N.J.; Boachie, C.; McConnachie, A.; Ford, I.; Kangas, A.; Soininen, P.; Trompet, S.; Mooijaart, S.P.; Jukema, J.W.; et al. Nuclear magnetic resonance-based metabolomics identifies phenylalanine as a novel predictor of incident heart failure hospitalisation: Results from PROSPER and FINRISK 1997. Eur. J. Heart Fail. 2018, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Wang, C.H.; Cheng, C.W.; Liu, M.H.; Chu, C.M.; Wu, H.P.; Huang, P.C.; Lin, Y.T.; Ko, T.; Chen, W.H.; et al. Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail. 2020, 7, 2884–2893. [Google Scholar] [CrossRef]
- Wang, C.H.; Chen, W.S.; Liu, M.H.; Lee, C.Y.; Wang, M.Y.; Liang, C.Y.; Chu, C.M.; Wu, H.P.; Chen, W.H. Stress hyperphenylalaninemia is associated with mortality in cardiac ICU: Clinical factors, genetic variants, and pteridines. Crit. Care Med. 2022, 50, 1577–1587. [Google Scholar] [CrossRef]
- Cheng, C.W.; Liu, M.H.; Tang, H.Y.; Cheng, M.L.; Wang, C.H. Factors associated with elevated plasma phenylalanine in patients with heart failure. Amino Acids 2021, 53, 149–157. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The genetic landscape and epidemiology of phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Wang, C.H.; Chen, W.S.; Chu, C.M.; Wu, H.P.; Liu, M.H.; Lin, Y.T.; Kao, K.C.; Liang, C.Y.; Chen, W.H.; et al. U-shape relationship between plasma leucine level and mortality in the intensive care unit. Dis. Markers 2022, 2022, 7389258. [Google Scholar] [CrossRef]
- Wang, C.H.; Cheng, M.L.; Liu, M.H. Simplified plasma essential amino acid-based profiling provides metabolic information and prognostic value additive to traditional risk factors in heart failure. Amino Acids 2018, 50, 1739–1748. [Google Scholar] [CrossRef]
- Han, K.S.; Kim, S.J.; Lee, E.J.; Shin, J.H.; Lee, J.S.; Lee, S.W. Development and validation of new poisoning mortality score system for patients with acute poisoning at the emergency department. Crit. Care 2021, 25, 29. [Google Scholar] [CrossRef]
- Gatti, G.; Perrotti, A.; Obadia, J.F.; Duval, X.; Iung, B.; Alla, F.; Chirouze, C.; Selton-Suty, C.; Hoen, B.; Sinagra, G.; et al. Simple scoring system to predict in-hospital mortality after surgery for infective endocarditis. J. Am. Heart Assoc. 2017, 6, e004806. [Google Scholar] [CrossRef]
- Berger, M.M.; Pantet, O.; Schneider, A.; Ben-Hamouda, N. Micronutrient deficiencies in medical and surgical inpatients. J. Clin. Med. 2019, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal 2014, 20, 3040–3077. [Google Scholar] [CrossRef]
- Nishijima, Y.; Sridhar, A.; Bonilla, I.; Velayutham, M.; Khan, M.; Terentyeva, R.; Li, C.; Kuppusamy, P.; Elton, T.S.; Terentyev, D.; et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc. Res. 2011, 91, 71–79. [Google Scholar] [CrossRef]
- Gudivada, K.K.; Kumar, A.; Shariff, M.; Sampath, S.; Varma, M.M.; Sivakoti, S.; Krishna, B. Antioxidant micronutrient supplementation in critically ill adults: A systematic review with meta-analysis and trial sequential analysis. Clin. Nutr. 2021, 40, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pan, T.; Qi, X.; Tan, R.; Wang, X.; Liu, Z.; Tao, Z.; Qu, H.; Zhang, Y.; Chen, H.; et al. Increased mortality of acute respiratory distress syndrome was associated with high levels of plasma phenylalanine. Respir. Res. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Czibik, G.; Mezdari, Z.; Murat Altintas, D.; Bréhat, J.; Pini, M.; d’Humières, T.; Delmont, T.; Radu, C.; Breau, M.; Liang, H.; et al. Dysregulated phenylalanine catabolism plays a key role in the trajectory of cardiac aging. Circulation 2021, 144, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Hadjiconstantinou, M.; Neff, N.H. Enhancing aromatic L-amino acid decarboxylase activity: Implications for L-DOPA treatment in Parkinson’s disease. CNS Neurosci. Ther. 2008, 14, 340–351. [Google Scholar] [CrossRef]
- Williams, R.A.; Mamotte, C.D.; Burnett, J.R. Phenylketonuria: An inborn error of phenylalanine metabolism. Clin. Biochem. Rev. 2008, 29, 31–41. [Google Scholar]
- Fedotcheva, N.I.; Kazakov, R.E.; Kondrashova, M.N.; Beloborodova, N.V. Toxic effects of microbial phenolic acids on the functions of mitochondria. Toxicol. Lett. 2008, 180, 182–188. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Seehus, C.; Weidinger, A.; Talbot, S.; Reissig, S.; Seifert, M.; Pierson, Y.; McNeill, E.; Longhi, M.S.; Turnes, B.L.; et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 2018, 563, 564–568. [Google Scholar] [CrossRef]
- Oshima, T.; Deutz, N.E.; Doig, G.; Wischmeyer, P.E.; Pichard, C. Protein-energy nutrition in the ICU is the power couple: A hypothesis forming analysis. Clin. Nutr. 2016, 35, 968–974. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Wang, C.H.; Shiao, M.S.; Liu, M.H.; Huang, Y.Y.; Huang, C.Y.; Mao, C.T.; Lin, J.F.; Ho, H.Y.; Yang, N.I. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J. Am. Coll. Cardiol. 2015, 65, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Ferrie, S.; Tsang, E. Monitoring nutrition in critical illness: What can we use? Nutr. Clin. Pract. 2018, 33, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.P.; Linnemeier, G.C.; Thomas, A.J.; Manahan, K.J. Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol. Oncol. 2007, 106, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.T.; Rosa, A.R.; Romani, R.F.; Gurski, R.R.; Schirmer, C.C.; Kruel, C.D. Serum transferrin and serum prealbumin as markers of response to nutritional support in patient. Nutr. Hosp. 2009, 24, 241–242. [Google Scholar] [PubMed]
| All | Survival | Death | ||
|---|---|---|---|---|
| n = 537 | n = 387 | n = 150 | p Value | |
| Age (years) | 71.5 ± 13.6 | 71.4 ± 14.0 | 72.0 ± 12.8 | 0.659 |
| Male (%) | 323 (60.1) | 222 (57.4) | 101 (67.3) | 0.034 |
| APACHE II score | 18.8 ± 5.8 | 17.7 ± 5.2 | 21.8 ± 6.3 | <0.001 |
| SOFA score | 7.1 ± 3.3 | 6.4 ± 3.0 | 9.0 ± 3.5 | <0.001 |
| NUTRIC score | 5.3 ± 1.9 | 5.0 ± 1.9 | 6.2 ± 1.8 | <0.001 |
| Co-morbidity | ||||
| Diabetes mellitus (%) | 256 (47.7) | 185 (47.8) | 71 (47.3) | 0.922 |
| Hypertension (%) | 350 (65.2) | 249 (64.3) | 101 (67.3) | 0.514 |
| Coronary artery disease (%) | 231 (43.0) | 172 (44.4) | 59 (39.3) | 0.283 |
| Atrial fibrillation (%) | 80 (14.9) | 50 (12.9) | 30 (20.0) | 0.039 |
| Chronic kidney disease (%) * | 156 (29.1) | 114 (29.5) | 42 (28.0) | 0.739 |
| Ventilator use (%) | 415 (77.3) | 282 (72.9) | 133 (88.7) | <0.001 |
| Inotropic agent use (%) | 209 (38.9) | 124 (32.0) | 85 (56.7) | <0.001 |
| Days in ICU (days) | 11.2 ± 7.4 | 11.2 ± 7.4 | 11.2 ± 7.2 | 0.957 |
| Laboratory data | ||||
| Hemoglobin (g/dL) | 10.8 ± 2.8 | 10.9 ± 2.8 | 10.6 ± 2.8 | 0.247 |
| eGFR (mL/min/1.73 m2) | 45.4 ± 43.6 | 47.9 ± 45.7 | 39.0 ± 37.1 | 0.020 |
| C-reactive protein (mg/L) | 46.8 (11.1–104) | 36.7 (8.1–93.0) | 61.7 (24.7–146) | <0.001 |
| Cholesterol (mg/dL) | 132.0 ± 52.9 | 138.9 ± 54.8 | 114.4 ± 43.2 | <0.001 |
| Triglyceride (mg/dL) | 109 (81.5–152) | 108 (79–150) | 115 (84–158) | 0.270 |
| Albumin (g/dL) | 3.19 ± 0.62 | 3.28 ± 0.59 | 2.95 ± 0.65 | <0.001 |
| Pre-Albumin (mg/dL) | 15.1 ± 8.2 | 16.3 ± 8.7 | 12.0 ± 5.7 | <0.001 |
| Transferrin (mg/dL) | 154.2 ± 49.9 | 162.3 ± 49.1 | 133.1 ± 45.9 | <0.001 |
| B * | Points = B/0.485 ** | HR (95% CI) | p Value | |
|---|---|---|---|---|
| Phenylalanine (μM) | ||||
| <72.2 | Reference | 0 | 1 | |
| 72.2–<88.5 | 0.843 | 1.7 | 2.32 (1.26–4.27) | 0.007 |
| 88.5–<112 | 1.556 | 3.2 | 4.74 (2.65–8.48) | <0.001 |
| 112–<130 | 1.793 | 3.7 | 6.01 (3.02–11.94) | <0.001 |
| 130–<150 | 2.096 | 4.3 | 8.13 (3.73–17.74) | <0.001 |
| 150–<200 | 2.403 | 5 | 11.06 (5.05–24.21) | <0.001 |
| 200–<280 | 3.418 | 7 | 30.50 (14.07–66.15) | <0.001 |
| ≥280 | 4.501 | 9.3 | 90.13 (38.62–210.37) | <0.001 |
| Leucine (μM) | ||||
| ≥96.6 | Reference | 0 | 1 | |
| 68.9–<96.6 | 0.485 | 1 | 1.62 (1.08–2.44) | 0.020 |
| 50.0–<68.9 | 1.359 | 2.8 | 3.89 (2.14–7.09) | <0.001 |
| <50 | 1.633 | 3.4 | 5.12 (2.12–12.36) | <0.001 |
| PLA Score | p for Trend | ||||
|---|---|---|---|---|---|
| 0–1 | 1.1–4 | 4.1–5 | >5 | ||
| Variable | n = 122 | n = 303 | n = 68 | n = 44 | |
| Age (years) | 69.8 ± 14.9 | 72.0 ± 13.1 | 73.0 ± 13.4 | 71.0 ± 14.1 | 0.529 |
| Male (%) | 64 (52.5) | 192 (63.4) | 38 (55.9) | 29 (65.9) | 0.217 |
| APACHE II score | 17.5 ± 5.6 | 18.3 ± 5.5 | 21.2 ± 5.5 | 22.6 ± 6.7 | <0.001 |
| SOFA score | 5.5 ± 2.9 | 7.1 ± 3.0 | 8.5 ± 3.7 | 10.0 ± 3.5 | <0.001 |
| NUTRIC score | 4.7 ± 2.0 | 5.3 ± 1.8 | 5.9 ± 2.1 | 6.5 ± 1.7 | <0.001 |
| Co-morbidity | |||||
| Diabetes mellitus (%) | 61 (50.0) | 143 (47.2) | 33 (48.5) | 19 (43.2) | 0.515 |
| Hypertension (%) | 71 (58.2) | 210 (69.3) | 42 (61.8) | 27 (61.4) | 0.755 |
| Coronary artery disease (%) | 52 (42.6) | 137 (45.2) | 25 (36.8) | 17 (38.6) | 0.420 |
| Atrial fibrillation | 15 (12.3) | 39 (12.9) | 14 (20.6) | 12 (27.3) | 0.008 |
| Chronic kidney disease (%) * | 34 (27.9) | 91 (30.0) | 16 (23.5) | 15 (34.1) | 0.807 |
| Ventilator use (%) | 80 (65.6) | 240 (79.2) | 57 (83.8) | 38 (86.4) | 0.001 |
| Inotropic agent use (%) | 31 (25.4) | 112 (37.0) | 36 (52.9) | 30 (68.2) | <0.001 |
| Days in ICU (day) | 10.2 ± 6.7 | 11.8 ± 7.6 | 12.1 ± 7.1 | 8.9 ± 7.1 | 0.379 |
| Laboratory data | |||||
| Hemoglobin (g/dL) | 10.8 ± 2.4 | 10.9 ± 2.9 | 10.8 ± 2.8 | 9.7 ± 3.1 | 0.025 |
| eGFR (ml/min/1.73 m2) | 59.6 ± 54.8 | 43.4 ± 40.6 | 38.8 ± 34.5 | 29.8 ± 29.6 | <0.001 |
| C-reactive protein (mg/L) | 34 (7–67) | 47 (12–100) | 73 (18–153) | 51 (16–148) | 0.010 |
| Cholesterol (mg/dL) | 144.5 ± 66.8 | 134.6 ± 47.0 | 121.0 ± 43.6 | 94.7 ± 40.8 | <0.001 |
| Triglyceride (mg/dL) | 112 (77–153) | 112 (84–153) | 115 (82–156) | 87 (68–121) | 0.104 |
| Albumin (g/dL) | 3.26 ± 0.56 | 3.25 ± 0.61 | 2.90 ± 0.63 | 2.97 ± 0.75 | <0.001 |
| Pre-Albumin (mg/dL) | 17.1 ± 6.9 | 15.4 ± 6.9 | 11.7 ± 6.2 | 10.2 ± 5.8 | <0.001 |
| Transferrin (mg/dL) | 160.7 ± 44.4 | 159.5 ± 50.0 | 138.1 ± 49.0 | 125.9 ± 48.4 | <0.001 |
| Univariate | Multivariable (Model 1) * | Multivariable (Model 2) † | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| PLA score | 1.62 (1.51–1.75) | <0.001 | 1.46 (1.33–1.60) | <0.001 | 1.48 (1.35–1.62) | <0.001 |
| APACHE II score | 1.12 (1.09–1.15) | <0.001 | 1.07 (1.03–1.11) | <0.001 | ||
| SOFA score | 1.20 (1.15–1.26) | <0.001 | 1.04 (0.98–1.11) | 0.061 | ||
| NUTRIC score | 1.34 (1.23–1.46) | <0.001 | 1.18 (1.07–1.30) | 0.001 | ||
| Age (years) | 1.00 (0.99–1.01) | 0.728 | ||||
| Sex (male) | 1.43 (1.02–2.01) | 0.040 | 1.37 (0.96–1.96) | 0.086 | 1.33 (0.93–1.90) | 0.118 |
| Atrial fibrillation | 1.50 (1.01–2.24) | 0.046 | 1.06 (0.69–1.62) | 0.793 | 0.95 (0.61–1.47) | 0.816 |
| C-reactive protein (log) | 1.78 (1.36–2.32) | <0.001 | 1.29 (0.96–1.72) | 0.087 | 1.29 (0.96–1.74) | 0.086 |
| eGFR (mL/min/1.73 m2) | 0.99 (0.99–1.00) | 0.034 | 1.00 (0.99–1.01) | 0.718 | 0.99 (0.99–1.00) | 0.804 |
| Cholesterol (mg/dL) | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.99–1.01) | 0.551 | 0.99 (0.99–1.00) | 0.261 |
| Albumin (g/dL) | 0.48 (0.37–0.62) | <0.001 | 0.88 (0.64–1.20) | 0.411 | 0.85 (0.62–1.15) | 0.284 |
| Pre-Albumin (mg/dL) | 0.92 (0.89–0.94) | <0.001 | 0.99 (0.96–1.02) | 0.626 | 0.99 (0.98–1.02) | 0.937 |
| Transferrin (mg/dL) | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.99–1.01) | 0.277 | 0.99 (0.99–1.00) | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, Y.-L.; Wang, C.-H.; Chen, W.-S.; Wu, H.-P.; Liu, M.-H.; Lin, H.-C.; Chang, J.-J.; Tsai, M.-S.; Chen, T.-Y.; Cheng, C.-I.; et al. Combining Phenylalanine and Leucine Levels Predicts 30-Day Mortality in Critically Ill Patients Better than Traditional Risk Factors with Multicenter Validation. Nutrients 2023, 15, 649. https://doi.org/10.3390/nu15030649
Tsou Y-L, Wang C-H, Chen W-S, Wu H-P, Liu M-H, Lin H-C, Chang J-J, Tsai M-S, Chen T-Y, Cheng C-I, et al. Combining Phenylalanine and Leucine Levels Predicts 30-Day Mortality in Critically Ill Patients Better than Traditional Risk Factors with Multicenter Validation. Nutrients. 2023; 15(3):649. https://doi.org/10.3390/nu15030649
Chicago/Turabian StyleTsou, Yi-Liang, Chao-Hung Wang, Wei-Siang Chen, Huang-Ping Wu, Min-Hui Liu, Hsuan-Ching Lin, Jung-Jung Chang, Meng-Shu Tsai, Tien-Yu Chen, Cheng-I Cheng, and et al. 2023. "Combining Phenylalanine and Leucine Levels Predicts 30-Day Mortality in Critically Ill Patients Better than Traditional Risk Factors with Multicenter Validation" Nutrients 15, no. 3: 649. https://doi.org/10.3390/nu15030649
APA StyleTsou, Y.-L., Wang, C.-H., Chen, W.-S., Wu, H.-P., Liu, M.-H., Lin, H.-C., Chang, J.-J., Tsai, M.-S., Chen, T.-Y., Cheng, C.-I., Yeh, J.-K., & Hsieh, I.-C. (2023). Combining Phenylalanine and Leucine Levels Predicts 30-Day Mortality in Critically Ill Patients Better than Traditional Risk Factors with Multicenter Validation. Nutrients, 15(3), 649. https://doi.org/10.3390/nu15030649





