The Role of a Colon-in-Continuity in Short Bowel Syndrome
Abstract
1. Introduction
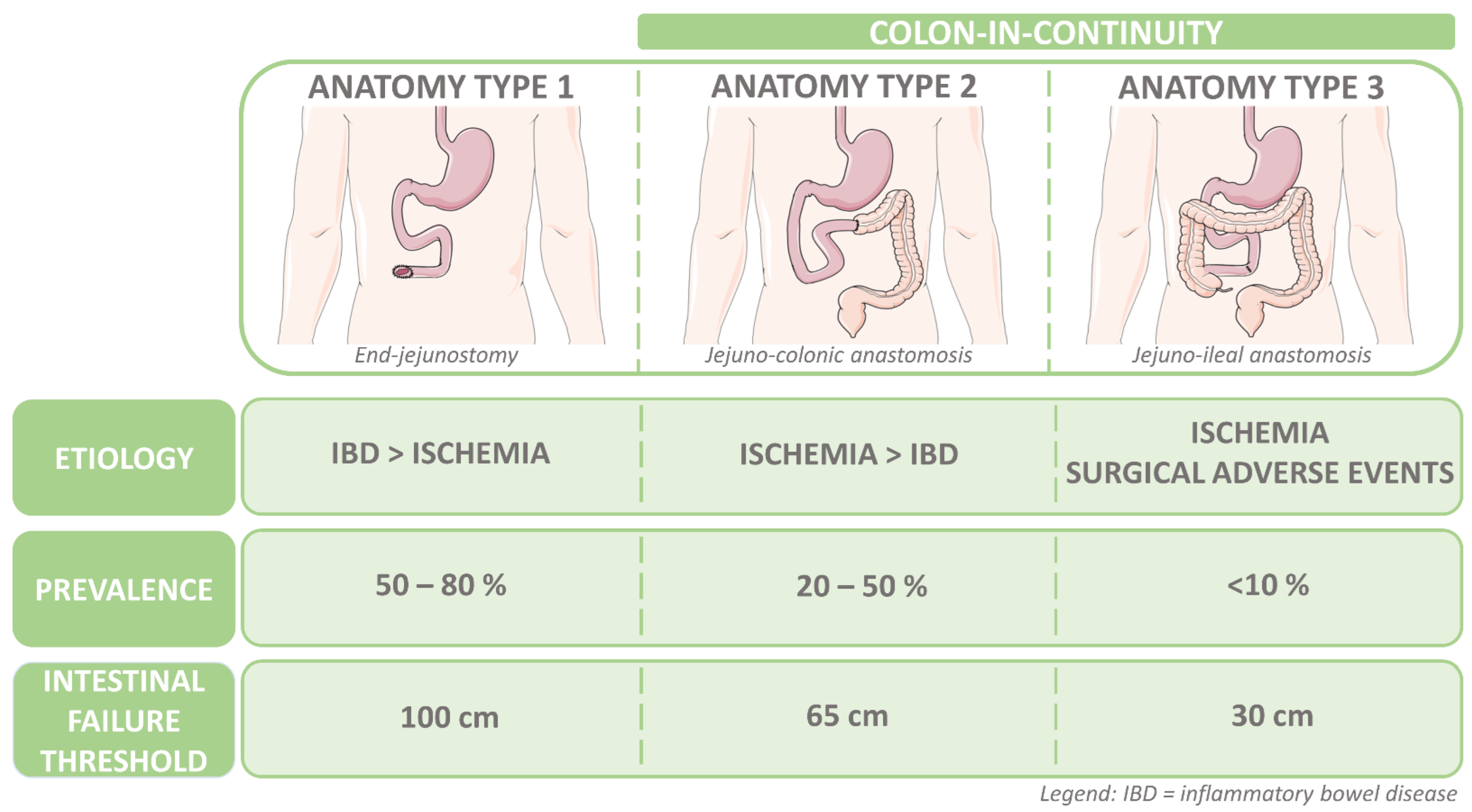
1.1. Short Bowel Syndrome and Intestinal Failure with a Colon-in-Continuity
1.2. The Functional Characteristics of a Colon in Health
2. The Role of the Colon in Intestinal Adaptation
2.1. Intestinal Adaptation
2.2. The Role of Food Intake and Hyperphagia
2.3. Why Anatomy Matters
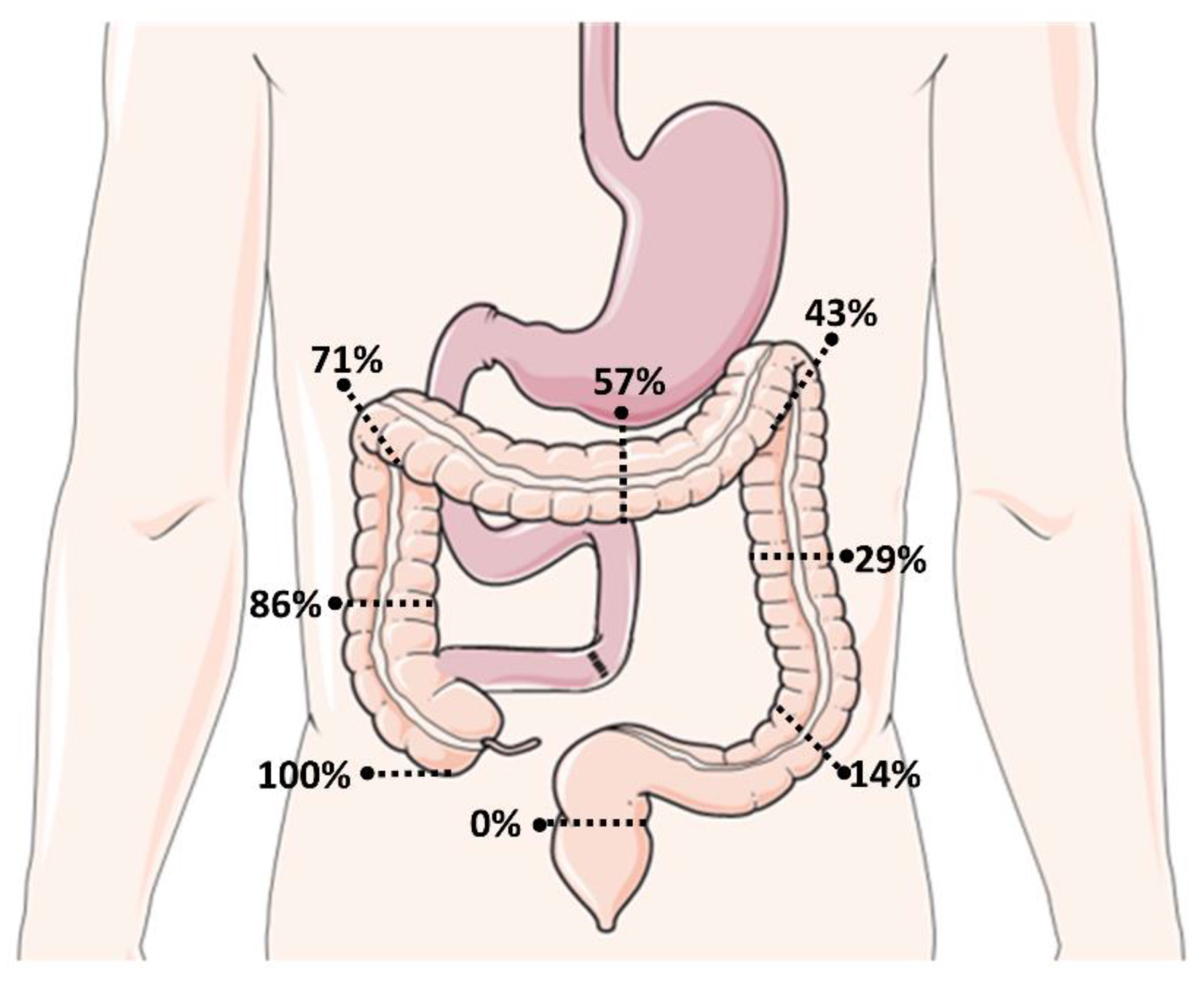
2.4. Energetic Recovery and Restoration of the Fluid and Electrolyte Balance
2.5. The Colonic Microbiota in SBS
3. GLP-2 Analogs as a Disease Modifying Therapy for SBS-IF and the Effect in SBS-IF-CiC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations: Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Verbiest, A.; Wauters, L.; Vanuytsel, T. Enterohormone therapy for short bowel syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Spectrum of short bowel syndrome in adults: Intestinal insufficiency to intestinal failure. JPEN J. Parenter. Enteral Nutr. 2014, 38 (Suppl. 1), 8S–13S. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Steiger, E.; Joly, F.; Jeppesen, P.B.; Wanten, G.; Sasdelli, A.S.; Chambrier, C.; Aimasso, U.; Mundi, M.S.; Szczepanek, K.; et al. Characteristics of adult patients with chronic intestinal failure due to short bowel syndrome: An international multicenter survey. Clin. Nutr. ESPEN 2021, 45, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; James, W.P.; Wiggins, H.S. Role of the colon in ileal-resection diarrhoea. Lancet 1973, 1, 344–347. [Google Scholar] [CrossRef]
- Jeppesen, P.B. Short bowel syndrome-characterisation of an orphan condition with many phenotypes. Expert Opin. Orphan Drugs 2013, 1, 515–525. [Google Scholar] [CrossRef]
- Van Nueten, J.M.; Janssen, P.A.; Fontaine, J. Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 3: In vitro studies on the peristaltic reflex and other experiments on isolated tissues. Arzneimittel-Forschung 1974, 24, 1641–1645. [Google Scholar]
- Mark, E.B.; Klinge, M.W.; Grønlund, D.; Poulsen, J.L.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system: Effect of opioids. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2020, 32, e13753. [Google Scholar] [CrossRef]
- Prahm, A.P.; Brandt, C.F.; Askov-Hansen, C.; Mortensen, P.B.; Jeppesen, P.B. The use of metabolic balance studies in the objective discrimination between intestinal insufficiency and intestinal failure. Am. J. Clin. Nutr. 2017, 106, 831–838. [Google Scholar] [CrossRef]
- Lambe, C.; Goulet, O.; Norsa, L. Colon importance in short bowel syndrome. Aging 2019, 11, 9961–9962. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Messing, B.; Corcos, O.; Panis, Y.; Joly, F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin. Nutr. 2013, 32, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Messing, B.; Crenn, P.; Beau, P.; Boutron-Ruault, M.C.; Rambaud, C.; Matuchansky, C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999, 117, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Billiauws, L.; Thomas, M.; Le Beyec-Le Bihan, J.; Joly, F. Intestinal adaptation in short bowel syndrome. What is new? Nutr. Hosp. 2018, 35, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Eckburg, P.B.; Bik, E.M.; Relman, D.A. Assembly of the human intestinal microbiota. Trends Ecol. Evol. 2006, 21, 517–523. [Google Scholar] [CrossRef]
- DiBaise, J.K.; Young, R.J.; Vanderhoof, J.A. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin. Gastroenterol. Hepatol. 2006, 4, 11–20. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Geboes, K.P.; De Hertogh, G.; De Preter, V.; Luypaerts, A.; Bammens, B.; Evenepoel, P.; Ghoos, Y.; Geboes, K.; Rutgeerts, P.; Verbeke, K. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br. J. Nutr. 2006, 96, 1078–1086. [Google Scholar] [CrossRef]
- Chacko, A.; Cummings, J.H. Nitrogen losses from the human small bowel: Obligatory losses and the effect of physical form of food. Gut 1988, 29, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Geibel, J.P. Secretion and absorption by colonic crypts. Annu. Rev. Physiol. 2005, 67, 471–490. [Google Scholar] [CrossRef]
- Binder, H. Intestinal Fluid and Electrolyte Movement; Boron, W., Boulpaep, E., Eds.; Medical Physiology A Cellular and Molecular Approach; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Phillips, S.F. Functions of the large bowel: An overview. Scand. J. Gastroenterol. Suppl. 1984, 93, 1–12. [Google Scholar] [PubMed]
- Sandle, G.I. Salt and water absorption in the human colon: A modern appraisal. Gut 1998, 43, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Dray, X.; Corcos, O.; Barbot, L.; Kapel, N.; Messing, B. Tube Feeding Improves Intestinal Absorption in Short Bowel Syndrome Patients. Gastroenterology 2009, 136, 824–831. [Google Scholar] [CrossRef]
- Le Beyec, J.; Billiauws, L.; Bado, A.; Joly, F.; Le Gall, M. Short Bowel Syndrome: A Paradigm for Intestinal Adaptation to Nutrition? Annu. Rev. Nutr. 2020, 40, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P.; Morin, M.C.; Joly, F.; Penven, S.; Thuillier, F.; Messing, B. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut 2004, 53, 1279–1286. [Google Scholar] [CrossRef]
- Messing, B.; Pigot, F.; Rongier, M.; Morin, M.C.; Ndeïndoum, U.; Rambaud, J.C. Intestinal absorption of free oral hyperalimentation in the very short bowel syndrome. Gastroenterology 1991, 100, 1502–1508. [Google Scholar] [CrossRef]
- Maljaars, P.W.J.; Peters, H.P.F.; Mela, D.J.; Masclee, A.A.M. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef]
- Fich, A.; Steadman, C.J.; Phillips, S.F.; Camilleri, M.; Brown, M.L.; Haddad, A.C.; Thomforde, G.M. Ileocolonic transit does not change after right hemicolectomy. Gastroenterology 1992, 103, 794–799. [Google Scholar] [CrossRef]
- Nightingale, J.M.D.; Kamm, M.A.; Van Der Sijp, J.R.M.; Morris, G.P.; Walker, E.R.; Mather, S.J.; Britton, K.E.; Lennard-Jones, J.E. Disturbed gastric emptying in the short bowel syndrome. Evidence for a “colonic brake.”. Gut 1993, 34, 1171–1176. [Google Scholar] [CrossRef]
- Nightingale, J.M.D.; Kamm, M.A.; Van Der Sijp, J.R.M.; Ghatei, M.A.; Bloom, S.R.; Lennard-Jones, J.E. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the “colonic brake” to gastric emptying. Gut 1996, 39, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.E.; Brubaker, P.L. Glucagon-like peptide 1 secretion by the L-cell: The view from within. Diabetes 2006, 55 (Suppl. 2), S70–S77. [Google Scholar] [CrossRef]
- Orskov, C.; Holst, J.J.; Knuhtsen, S.; Baldissera, F.G.; Poulsen, S.S.; Nielsen, O. V Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 1986, 119, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L. The glucagon-like peptides: Pleiotropic regulators of nutrient homeostasis. Ann. N. Y. Acad. Sci. 2006, 1070, 10–26. [Google Scholar] [CrossRef]
- Xiao, Q.; Boushey, R.P.; Drucker, D.J.; Brubaker, P.L. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 1999, 117, 99–105. [Google Scholar] [CrossRef]
- Fehmann, H.C.; Habener, J.F. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology 1992, 130, 159–166. [Google Scholar] [CrossRef]
- Orskov, C.; Holst, J.J.; Nielsen, O. V Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 1988, 123, 2009–2013. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Göke, B.; Drewe, J.; Hildebrand, P.; Ketterer, S.; Handschin, D.; Winterhalder, R.; Conen, D.; Beglinger, C. Glucagon-like peptide-1: A potent regulator of food intake in humans. Gut 1999, 44, 81–86. [Google Scholar] [CrossRef]
- Janssen, P.; Rotondo, A.; Mulé, F.; Tack, J. Review article: A comparison of glucagon-like peptides 1 and 2. Aliment. Pharmacol. Ther. 2013, 37, 18–36. [Google Scholar] [CrossRef]
- Drucker, D.J.; Ehrlich, P.; Asa, S.L.; Brubaker, P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA 1996, 93, 7911–7916. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hill, M.; Asa, S.L.; Brubaker, P.L.; Drucker, D.J. Intestinal growth-promoting properties of glucacon-like peptide-2 in mice. Am. J. Physiol. 1997, 273, 77–84. [Google Scholar]
- Jeppesen, P.B.; Hartmann, B.; Thulesen, J.; Graff, J.; Lohmann, J.; Hansen, B.S.; Toften, F.; Poulsen, S.S.; Madsen Lysgaard, J.; Holst, J.J.; et al. Glucagon-like Peptide 2 Improves Nutrient Absorption and Nutritional Status in Short-Bowel Patients with No Colon. Gastroenterology 2001, 120, 806–815. [Google Scholar] [CrossRef]
- Nagell, C.F.; Wettergren, A.; Pedersen, J.F.; Mortensen, D.; Holst, J.J. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand. J. Gastroenterol. 2004, 39, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Hartmann, B.; Thulesen, J.; Hansen, B.S.; Holst, J.J.; Poulsen, S.S.; Mortensen, P.B. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut 2000, 47, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Dahly, E.M.; Gillingham, M.B.; Guo, Z.; Murali, S.G.; Nelson, D.W.; Holst, J.J.; Ney, D.M. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G670-82. [Google Scholar] [CrossRef] [PubMed]
- Ljungmann, K.; Hartmann, B.; Kissmeyer-Nielsen, P.; Flyvbjerg, A.; Holst, J.J.; Laurberg, S. Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G779–G785. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Hartmann, B.; Hansen, B.S.; Thulesen, J.; Holst, J.J.; Mortensen, P.B. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Nutr. Clin. Metab. 2000, 14, 63–64. [Google Scholar] [CrossRef]
- Bond, A.; Huijbers, A.; Pironi, L.; Schneider, S.M.; Wanten, G.; Lal, S. Review article: Diagnosis and management of intestinal failure-associated liver disease in adults. Aliment. Pharmacol. Ther. 2019, 50, 640–653. [Google Scholar] [CrossRef]
- Mutanen, A.; Lohi, J.; Heikkilä, P.; Jalanko, H.; Pakarinen, M.P. Loss of ileum decreases serum fibroblast growth factor 19 in relation to liver inflammation and fibrosis in pediatric onset intestinal failure. J. Hepatol. 2015, 62, 1391–1397. [Google Scholar] [CrossRef]
- Harrison, S.A.; Neff, G.; Guy, C.D.; Bashir, M.R.; Paredes, A.H.; Frias, J.P.; Younes, Z.; Trotter, J.F.; Gunn, N.T.; Moussa, S.E.; et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2021, 160, 219–231.e1. [Google Scholar] [CrossRef] [PubMed]
- Adaba, F.; Rajendran, A.; Patel, A.; Cheung, Y.K.; Grant, K.; Vaizey, C.J.; Gabe, S.M.; Warusavitarne, J.; Nightingale, J.M.D. Mesenteric infarction clinical outcomes after restoration of bowel continuity. Ann. Surg. 2015, 262, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Mayeur, C.; Messing, B.; Lavergne-Slove, A.; Cazals-Hatem, D.; Noordine, M.L.; Cherbuy, C.; Duée, P.H.; Thomas, M. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.R.; Fernández-Estívariz, C.; Gu, L.H.; Bazargan, N.; Umeakunne, K.; Wallace, T.M.; Diaz, E.E.; Rosado, K.E.; Pascal, R.R.; Galloway, J.R.; et al. Distribution of the H+/peptide transporter PepT1 in human intestine: Up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am. J. Clin. Nutr. 2002, 75, 922–930. [Google Scholar] [CrossRef]
- Tutton, P.J.; Barkla, D.H. Further studies on the effect of adenosine cyclic monophosphate derivatives on cell proliferation in the jejunal crypts of rat. Clin. Exp. Pharmacol. Physiol. 1982, 9, 671–674. [Google Scholar] [CrossRef]
- Sakata, T.; Yajima, T. Influence of short chain fatty acids on the epithelial cell division of digestive tract. Q. J. Exp. Physiol. 1984, 69, 639–648. [Google Scholar] [CrossRef]
- Koruda, M.J.; Rolandelli, R.H.; Settle, R.G.; Zimmaro, D.M.; Rombeau, J.L. Effect of parenteral nutrition supplemented with short-chain fatty acids on adaptation to massive small bowel resection. Gastroenterology 1988, 95, 715–720. [Google Scholar] [CrossRef]
- Nordgaard, I.; Hansen, B.S.; Mortensen, P.B. Colon as a digestive organ in patients with short bowel. Lancet 1994, 343, 373–376. [Google Scholar] [CrossRef]
- Goulet, O.; Colomb-Jung, V.; Joly, F. Role of the colon in short bowel syndrome and intestinal transplantation. J. Pediatr. Gastroenterol. Nutr. 2009, 48, S66–S71. [Google Scholar] [CrossRef]
- Nordgaard, I.; Hansen, B.S.; Mortensen, P.B. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am. J. Clin. Nutr. 1996, 64, 222–231. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Mortensen, P.B. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut 1998, 43, 478–483. [Google Scholar] [CrossRef]
- Goulet, O.; Joly, F. Intestinal microbiota in short bowel syndrome. Gastroentérol. Clin. Biol. 2010, 34, S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Briet, F.; Flourié, B.; Achour, L.; Maurel, M.; Rambaud, J.C.; Messing, B. Bacterial adaptation in patients with short bowel and colon in continuity. Gastroenterology 1995, 109, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, F.; Li, Y.; Wang, J.; Li, J. Fecal microbiota signatures of adult patients with different types of short bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, C.; Gillard, L.; Le Beyec, J.; Bado, A.; Joly, F.; Thomas, M. Extensive Intestinal Resection Triggers Behavioral Adaptation, Intestinal Remodeling and Microbiota Transition in Short Bowel Syndrome. Microorganisms 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Mayeur, C.; Bruneau, A.; Noordine, M.L.; Meylheuc, T.; Langella, P.; Messing, B.; Duée, P.H.; Cherbuy, C.; Thomas, M. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 2010, 92, 753–761. [Google Scholar] [CrossRef]
- Gillard, L.; Mayeur, C.; Robert, V.; Pingenot, I.; Le Beyec, J.; Bado, A.; Lepage, P.; Thomas, M.; Joly, F. Microbiota is involved in post-resection adaptation in humans with short bowel syndrome. Front. Physiol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, C.; Gratadoux, J.J.; Bridonneau, C.; Chegdani, F.; Larroque, B.; Kapel, N.; Corcos, O.; Thomas, M.; Joly, F. Faecal D/L Lactate Ratio Is a Metabolic Signature of Microbiota Imbalance in Patients with Short Bowel Syndrome. PLoS ONE 2013, 8, e54335. [Google Scholar] [CrossRef]
- Kowlgi, N.G.; Chhabra, L. D-lactic acidosis: An underrecognized complication of short bowel syndrome. Gastroenterol. Res. Pract. 2015, 2015, 476215. [Google Scholar] [CrossRef]
- Caldarini, M.I.; Pons, S.; D’Agostino, D.; Depaula, J.A.; Greco, G.; Negri, G.; Ascione, A.; Bustos, D. Abnormal fecal flora in a patient with short bowel syndrome: An in vitro study on effect of pH on D-lactic acid production. Dig. Dis. Sci. 1996, 41, 1649–1652. [Google Scholar] [CrossRef]
- Stephen, A.M.; Cummings, J.H. Mechanism of action of dietary fibre in the human colon. Nature 1980, 284, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Fabian, C.; Ahrens, F.; Spengler, M.; Kasper, H. Effect of Starch Malabsorption on Colonic Function and Metabolism in Humans. Gastroenterology 1988, 95, 1549–1955. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap. Adv. Gastroenterol. 2012, 5, 159–171. [Google Scholar] [CrossRef]
- Marier, J.-F.; Beliveau, M.; Mouksassi, M.-S.; Shaw, P.; Cyran, J.; Kesavan, J.; Wallens, J.; Zahir, H.; Wells, D.; Caminis, J. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J. Clin. Pharmacol. 2008, 48, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- NCT03690206 Efficacy And Safety Evaluation of Glepaglutide in Treatment of SBS (EASE SBS 1). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03690206 (accessed on 15 December 2022).
- NCT03905707 Evaluation of Long Term Safety and Efficacy of Glepaglutide in Treatment of SBS (EASE SBS 2). Available online: https://clinicaltrials.gov/ct2/show/NCT03905707 (accessed on 15 December 2022).
- NCT04627025 Trial to Evaluate Efficacy and Safety of Apraglutide in SBS-IF (STARS). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT04627025&cntry=&state=&city=&dist= (accessed on 15 December 2022).
- Jeppesen, P.B.; Sanguinetti, E.L.; Buchman, A.; Howard, L.; Scolapio, J.S.; Ziegler, T.R.; Gregory, J.; Tappenden, K.A.; Holst, J.; Mortensen, P.B. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant qlucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 2005, 54, 1224–1231. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’Keefe, S.J.D.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012, 143, 1473–1481. [Google Scholar] [CrossRef]
- Schwartz, L.K.; O’Keefe, S.J.D.; Fujioka, K.; Gabe, S.M.; Lamprecht, G.; Pape, U.F.; Li, B.; Youssef, N.N.; Jeppesen, P.B. Long-Term Teduglutide for the Treatment of Patients With Intestinal Failure Associated With Short Bowel Syndrome. Clin. Transl. Gastroenterol. 2016, 7, e142. [Google Scholar] [CrossRef]
- Seidner, D.L.; Gabe, S.M.; Lee, H.-M.; Olivier, C.; Jeppesen, P.B. Enteral Autonomy and Days Off Parenteral Support With Teduglutide Treatment for Short Bowel Syndrome in the STEPS Trials. JPEN J. Parenter. Enteral Nutr. 2020, 44, 697–702. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gabe, S.M.; Seidner, D.L.; Lee, H.M.; Olivier, C. Factors Associated With Response to Teduglutide in Patients With Short-Bowel Syndrome and Intestinal Failure. Gastroenterology 2018, 154, 874–885. [Google Scholar] [CrossRef]
- Chen, K.; Joly, F.; Mu, F.; Kelkar, S.S.; Olivier, C.; Xie, J.; Seidner, D.L. Predictors and timing of response to teduglutide in patients with short bowel syndrome dependent on parenteral support. Clin. Nutr. ESPEN 2021, 43, 420–427. [Google Scholar] [CrossRef]
- Lam, K.; Schwartz, L.; Batisti, J.; Iyer, K.R. Single-Center Experience with the Use of Teduglutide in Adult Patients with Short Bowel Syndrome. JPEN J. Parenter. Enteral Nutr. 2018, 42, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Klag, T.; Wendler, J.; Bernhard, S.; Adolph, M.; Kirschniak, A.; Goetz, M.; Malek, N.; Wehkamp, J. GLP-2 analog teduglutide significantly reduces need for parenteral nutrition and stool frequency in a real-life setting. Therap. Adv. Gastroenterol. 2018, 11, 1756284818793343. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Seguy, D.; Nuzzo, A.; Chambrier, C.; Beau, P.; Poullenot, F.; Thibault, R.; Armengol Debeir, L.; Layec, S.; Boehm, V.; et al. Six-month outcomes of teduglutide treatment in adult patients with short bowel syndrome with chronic intestinal failure: A real-world French observational cohort study. Clin. Nutr. 2020, 39, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Puello, F.; Wall, E.; Herlitz, J.; Lozano, E.S.; Semrad, C.; Micic, D. Long-Term Outcomes With Teduglutide From a Single Center. JPEN J. Parenter. Enteral Nutr. 2021, 45, 318–322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbiest, A.; Jeppesen, P.B.; Joly, F.; Vanuytsel, T. The Role of a Colon-in-Continuity in Short Bowel Syndrome. Nutrients 2023, 15, 628. https://doi.org/10.3390/nu15030628
Verbiest A, Jeppesen PB, Joly F, Vanuytsel T. The Role of a Colon-in-Continuity in Short Bowel Syndrome. Nutrients. 2023; 15(3):628. https://doi.org/10.3390/nu15030628
Chicago/Turabian StyleVerbiest, Astrid, Palle Bekker Jeppesen, Francisca Joly, and Tim Vanuytsel. 2023. "The Role of a Colon-in-Continuity in Short Bowel Syndrome" Nutrients 15, no. 3: 628. https://doi.org/10.3390/nu15030628
APA StyleVerbiest, A., Jeppesen, P. B., Joly, F., & Vanuytsel, T. (2023). The Role of a Colon-in-Continuity in Short Bowel Syndrome. Nutrients, 15(3), 628. https://doi.org/10.3390/nu15030628





