Energy Guidance Using Indirect Calorimetry for Intestinal Failure Patients with Home Parenteral Nutrition: The Right Bag Right at the Start
Abstract
1. Introduction
2. Methods
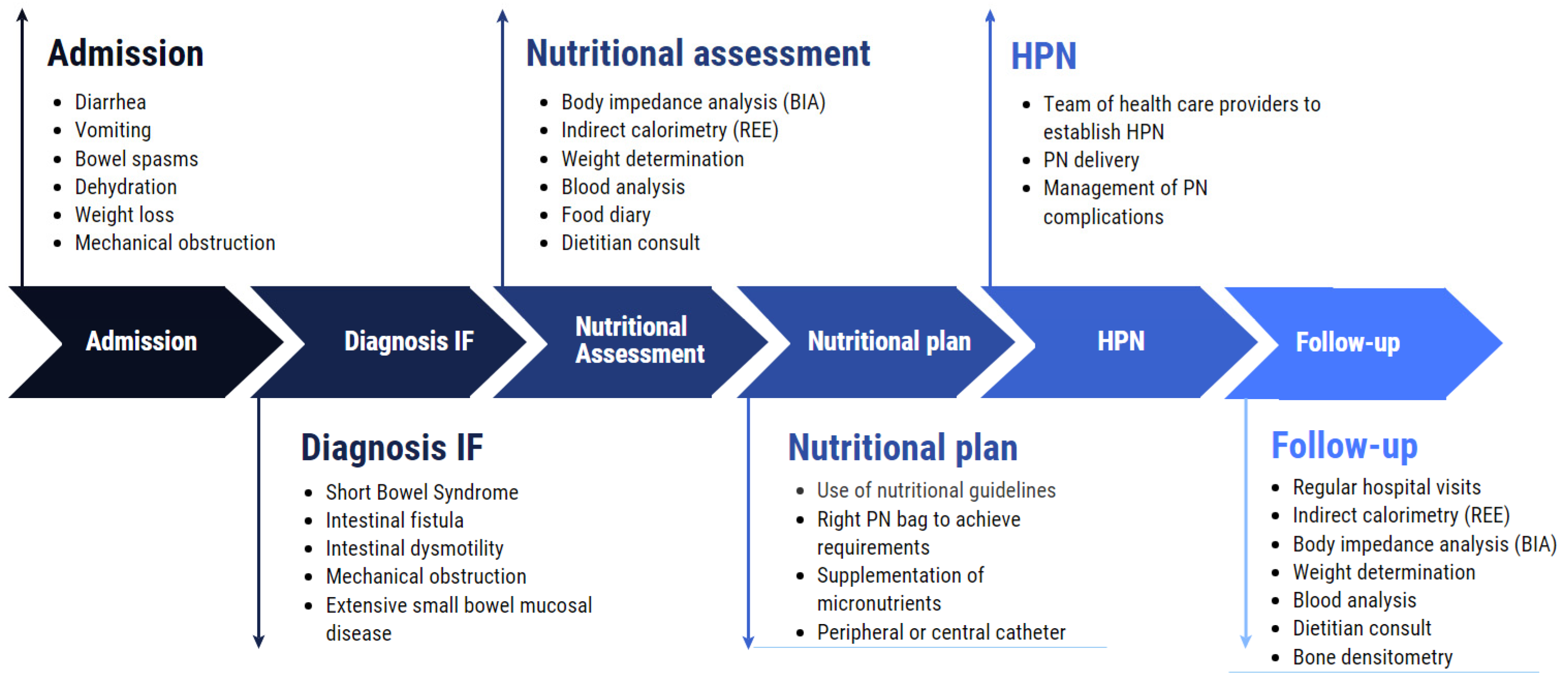
3. Importance and Management of (H)PN in IF
4. Nutritional Assessment Methods
4.1. Predictive Equations, Low Accuracy Alternative?
4.2. Direct Calorimetry
4.3. IC Technology
4.4. IC Added Value
4.5. Disadvantages of IC
5. IC from Bench to Bedside
5.1. Use of IC in HPN Patients
5.2. Protocols
5.3. Implementation Science
5.4. IC Implementation on a Larger Scale
6. Dietary Recommendations
Macro- and Micronutrients
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skallerup, A.; Nygaard, L.; Olesen, S.S.; Vinter-Jensen, L.; Køhler, M.; Rasmussen, H.H. Can We Rely on Predicted Basal Metabolic Rate in Patients with Intestinal Failure on Home Parenteral Nutrition? J. Parenter. Enter. Nutr. 2017, 41, 1139–1145. [Google Scholar] [CrossRef]
- Pironi, L.; Steiger, E.; Joly, F.; Jeppesen, P.B.; Wanten, G.; Sasdelli, A.S.; Chambrier, C.; Aimasso, U.; Mundi, M.S.; Szczepanek, K.; et al. Characteristics of adult patients with chronic intestinal failure due to short bowel syndrome: An international multicenter survey. Clin. Nutr. ESPEN 2021, 45, 433–441. [Google Scholar] [CrossRef]
- Pironi, L.; Steiger, E.; Brandt, C.; Joly, F.; Wanten, G.; Chambrier, C.; Aimasso, U.; Sasdelli, A.S.; Zeraschi, S.; Kelly, D.; et al. Home parenteral nutrition provision modalities for chronic intestinal failure in adult patients: An international survey. Clin. Nutr. 2020, 39, 585–591. [Google Scholar] [CrossRef]
- Mundi, M.S.; Mohamed Elfadil, O.; Hurt, R.T.; Bonnes, S.; Salonen, B.R. Management of long-term home parenteral nutrition: Historical perspective, common complications, and patient education and training. JPEN J. Parenter. Enteral Nutr. 2022, 47, S24–S34. [Google Scholar] [CrossRef]
- Meira, A.P.C.; dos Santos, C.O.; Lucho, C.L.C.; Kasmirscki, C.; Silva, F.M. Refeeding Syndrome in Patients Receiving Parenteral Nutrition Is Not Associated to Mortality or Length of Hospital Stay: A Retrospective Observational Study. Nutr. Clin. Pract. 2021, 36, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Lin, H.C.; Lee, P.C.; Ma, W.Y. Hyperglycemia correlates with outcomes in patients receiving total parenteral nutrition. Am. J. Med. Sci. 2007, 333, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Mühlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN guideline on home parenteral nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef]
- Duan, J.Y.; Zheng, W.H.; Zhou, H.; Xu, Y.; Huang, H. Bin Energy delivery guided by indirect calorimetry in critically ill patients: A systematic review and meta-analysis. Crit. Care 2021, 25, 88. [Google Scholar] [CrossRef]
- De Waele, E.; Jonckheer, J.; Wischmeyer, P.E. Indirect calorimetry in critical illness: A new standard of care? Curr. Opin. Crit. Care 2021, 27, 334–343. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations: Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Messing, B.; Corcos, O.; Panis, Y.; Joly, F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin. Nutr. 2013, 32, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Forbes, A.; Joly, F.; Colomb, V.; Lyszkowska, M.; Van Gossum, A.; Baxter, J.; Thul, P.; Hébuterne, X.; Gambarara, M.; et al. Survival of Patients Identified as Candidates for Intestinal Transplantation: A 3-Year Prospective Follow-Up. Gastroenterology 2008, 135, 61–71. [Google Scholar] [CrossRef]
- Pironi, L.; Paganelli, F.; Labate, A.M.M.; Merli, C.; Guidetti, C.; Spinucci, G.; Miglioli, M. Safety and efficacy of home parenteral nutrition for chronic intestinal failure: A 16-year experience at a single centre. Dig. Liver Dis. 2003, 35, 314–324. [Google Scholar] [CrossRef]
- Pironi, L.; Joly, F.; Forbes, A.; Colomb, V.; Lyszkowska, M.; Baxter, J.; Gabe, S.; Hébuterne, X.; Gambarara, M.; Gottrand, F.; et al. Long-term follow-up of patients on home parenteral nutrition in Europe: Implications for intestinal transplantation. Gut 2011, 60, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Messing, B.; Joly, F. Guidelines for management of home parenteral support in adult chronic intestinal failure patients. Gastroenterology 2006, 130, S43–S51. [Google Scholar] [CrossRef]
- Howard, L. Home parenteral nutrition: Survival, cost, and quality of life. Gastroenterology 2006, 130, S52–S59. [Google Scholar] [CrossRef]
- Wengler, A.; Micklewright, A.; Hébuterne, X.; Bozzetti, F.; Pertkiewicz, M.; Moreno, J.; Pironi, L.; Thul, P.; Van Gossum, A.; Staun, M. Monitoring of patients on home parenteral nutrition (HPN) in Europe: A questionnaire based study on monitoring practice in 42 centres. Clin. Nutr. 2006, 25, 693–700. [Google Scholar] [CrossRef]
- Hallum, N.S.; Baxter, J.P.; O’Reilly, D.S.J.; McKee, R.F. Home parenteral nutrition in Scotland: Frequency of monitoring, adequacy of review and consequence for complication rates. Nutrition 2010, 26, 1139–1145. [Google Scholar] [CrossRef]
- Kovacevich, D.S.; Frederick, A.; Kelly, D.; Nishikawa, R.; Young, L. Standards for specialized nutrition support: Home care patients. Nutr. Clin. Pract. 2005, 20, 579–590. [Google Scholar] [CrossRef]
- Bendavid, I.; Lobo, D.N.; Barazzoni, R.; Cederholm, T.; Coëffier, M.; de van der Schueren, M.; Fontaine, E.; Hiesmayr, M.; Laviano, A.; Pichard, C.; et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin. Nutr. 2021, 40, 690–701. [Google Scholar] [CrossRef]
- Ławiński, M.; Singer, P.; Gradowski, Ł.; Gradowska, A.; Bzikowska, A.; Majewska, K. Predicted versus measured resting energy expenditure in patients requiring home parenteral nutrition. Nutrition 2015, 31, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Edakkanambeth Varayil, J.; Hurt, R.T.; Kelly, D.G. How hyperalimentation may be necessary to reverse severe malnutrition in selected patients receiving home parenteral nutrition. Nutr. Clin. Pract. 2014, 29, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Olguín, G.; Medina-Vera, I.; Serralde-Zúñiga, A.E.; Gulias-Herrero, A.; Sánchez-Rosales, A.I.; Guevara-Cruz, M. Accurate determination of energy requirements in hospitalised patients with parenteral nutrition. J. Hum. Nutr. Diet. 2018, 31, 810–817. [Google Scholar] [CrossRef]
- Jésus, P.; Achamrah, N.; Grigioni, S.; Charles, J.; Rimbert, A.; Folope, V.; Petit, A.; Déchelotte, P.; Coëffier, M. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a Nutrition Unit. Clin. Nutr. 2015, 34, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Matarese, L.E.; Steiger, E.; Seidner, D.L.; Richmond, B. Body composition changes in cachectic patients receiving home parenteral nutrition. J. Parenter. Enter. Nutr. 2002, 26, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Santarpia, L.; Pironi, L.; Thul, P.; Klek, S.; Gavazzi, C.; Tinivella, M.; Joly, F.; Jonkers, C.; Baxter, J.; et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: A multi-centre observational study with prospective follow-up of 414 patients. Ann. Oncol. 2014, 25, 487–493. [Google Scholar] [CrossRef]
- Cotogni, P.; Monge, T.; Passera, R.; Brossa, L.; De Francesco, A. Clinical characteristics and predictive factors of survival of 761 cancer patients on home parenteral nutrition: A prospective, cohort study. Cancer Med. 2020, 9, 4686–4698. [Google Scholar] [CrossRef]
- Sahin, P.; Molnár, A.; Varga, M.; Bíró, I.; Kőmíves, C.; Fejér, C.; Futó, J.; Tomsits, E.; Topa, L. Clinical nutrition therapy in patients with short bowel syndrome in line with principles of personalized medicine. Orv. Hetil. 2014, 155, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Hofsteenge, G.H.; Weijs, P.J.M. Predicting resting energy expenditure in underweight, normal weight, overweight, and obese adult hospital patients. Nutr. Metab. 2016, 13, 85. [Google Scholar] [CrossRef]
- Rousseau, A.-F.; Fadeur, M.; Colson, C.; Misset, B. Measured Energy Expenditure Using Indirect Calorimetry in Post-Intensive Care Unit Hospitalized Survivors: A Comparison with Predictive Equations. Nutrients 2022, 14, 3981. [Google Scholar] [CrossRef]
- Frankenfield, D.C. On heat, respiration, and calorimetry. Nutrition 2010, 26, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Mtaweh, H.; Tuira, L.; Floh, A.A.; Parshuram, C.S. Indirect calorimetry: History, technology, and application. Front. Pediatr. 2018, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.P.; Notley, S.R.; Gagnon, D. Direct calorimetry: A brief historical review of its use in the study of human metabolism and thermoregulation. Eur. J. Appl. Physiol. 2017, 117, 1765–1785. [Google Scholar] [CrossRef]
- Oshima, T.; Berger, M.M.; De Waele, E.; Guttormsen, A.B.; Heidegger, C.P.; Hiesmayr, M.; Singer, P.; Wernerman, J.; Pichard, C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2017, 36, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Delsoglio, M.; De Waele, E.; Berger, M.M.; Pichard, C. Indirect calorimetry: The 6 main issues. Clin. Nutr. 2021, 40, 4–14. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ravussin, E. Indirect calorimetry: An indispensable tool to understand and predict obesity. Eur. J. Clin. Nutr. 2017, 71, 318–322. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Molinger, J.; Haines, K. Point-Counterpoint: Indirect Calorimetry Is Essential for Optimal Nutrition Therapy in the Intensive Care Unit. Nutr. Clin. Pract. 2021, 36, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; De Waele, E.; Sanchez, C.; Ruiz Santana, S.; Montejo, J.C.; Laterre, P.F.; Soroksky, A.; Moscovici, E.; Kagan, I. TICACOS international: A multi-center, randomized, prospective controlled study comparing tight calorie control versus Liberal calorie administration study. Clin. Nutr. 2021, 40, 380–387. [Google Scholar] [CrossRef]
- Allingstrup, M.J.; Kondrup, J.; Wiis, J.; Claudius, C.; Pedersen, U.G.; Hein-Rasmussen, R.; Bjerregaard, M.R.; Steensen, M.; Jensen, T.H.; Lange, T.; et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: The single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017, 43, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect calorimetry in clinical practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.D.; Ramachandran, R.; Venkatesan, P.; Anoop, S.; Joseph, M.; Thomas, N. Indirect calorimetry: From bench to bedside. Indian J. Endocrinol. Metab. 2017, 21, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.M.; Davies, P.S.; Bauer, J.; Battistutta, D. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J. Appl. Physiol. 2004, 97, 130–134. [Google Scholar] [CrossRef]
- Tatucu-Babet, O.A.; Ridley, E.J. Clinimetrics: Indirect calorimetry. J. Physiother. 2019, 65, 240. [Google Scholar] [CrossRef]
- Wright, A.; Shepelev, J.; Kriz, A. PAM5 Indirect Calorimetry Is a Cost-Saving Strategy to Manage Patients in Hospital Intensive Care. Value Heal. 2020, 23, S409. [Google Scholar] [CrossRef]
- Haugen, A.H.; Chan, L.N.; Li, F. Indirect calorimetry: A practical guide for clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef]
- Guenter, P.; Abdelhadi, R.; Anthony, P.; Blackmer, A.; Malone, A.; Mirtallo, J.M.; Phillips, W.; Resnick, H.E. Malnutrition diagnoses and associated outcomes in hospitalized patients: United States, 2018. Nutr. Clin. Pract. 2021, 36, 957–969. [Google Scholar] [CrossRef]
- Proctor, E.K.; Landsverk, J.; Aarons, G.; Chambers, D.; Glisson, C.; Mittman, B. Implementation research in mental health services: An emerging science with conceptual, methodological, and training challenges. Adm. Policy Ment. Health 2009, 36, 24–34. [Google Scholar] [CrossRef]
- Clark, D.; Edwards, E.; Murray, P.; Langevin, H. Implementation Science Methodologies for Complementary and Integrative Health Research. J. Altern. Complement. Med. 2021, 27, S7–S13. [Google Scholar] [CrossRef]
- Baumann, A.A.; Cabassa, L.J. Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv. Res. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.; Cohen, S.S.; Irvin, S.R.; Alberda, C. Achieving Protein Targets in the ICU Using a Specialized High-Protein Enteral Formula: A Quality Improvement Project. Nutr. Clin. Pract. 2020, 35, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Arney, B.D.; Senter, S.A.; Schwartz, A.C.; Meily, T.; Pelekhaty, S. Effect of Registered Dietitian Nutritionist Order-Writing Privileges on Enteral Nutrition Administration in Selected Intensive Care Units. Nutr. Clin. Pract. 2019, 34, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Quach, A.; Bridgeman, D.; Tsow, F.; Forzani, E.; Tao, N.; Tsow, F. Personalized Indirect Calorimeter for Energy Expenditure (EE) Measurement. Glob. J. Obes. Diabetes Metab. Syndr. 2015, 2, 004–008. [Google Scholar] [CrossRef]
- Mora, S.J.; Sprowls, M.; Tipparaju, V.V.; Wheatley-Guy, C.M.; Kulick, D.; Johnson, B.; Xiaojun, X.; Forzani, E. Comparative study of a novel portable indirect calorimeter to a reference breath-by-breath instrument and its use in telemedicine settings. Clin. Nutr. ESPEN 2021, 46, 361–366. [Google Scholar] [CrossRef]
- Wall, E.A. An Overview of Short Bowel Syndrome Management: Adherence, Adaptation, and Practical Recommendations. J. Acad. Nutr. Diet. 2013, 113, 1200–1208. [Google Scholar] [CrossRef]
- Matarese, L.E.; Steiger, E. Dietary and Medical Management of Short Bowel Syndrome In Adult Patients. J. Clin. Gastroenterol. 2006, 40, S85–S93. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Singer, P.; Wierzchowska-McNew, R.A.; Viana, M.V.; Ben-David, I.A.; Pantet, O.; Thaden, J.J.; Ten Have, G.A.M.; Engelen, M.P.K.J.; Berger, M.M. Comprehensive metabolic amino acid flux analysis in critically ill patients. Clin. Nutr. 2021, 40, 2876–2897. [Google Scholar] [CrossRef]
- Bielawska, B.; Allard, J.P. Parenteral nutrition and intestinal failure. Nutrients 2017, 9, 466. [Google Scholar] [CrossRef]
- Cuerda, C.; Pironi, L.; Arends, J.; Bozzetti, F.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin. Nutr. 2021, 40, 5196–5220. [Google Scholar] [CrossRef]
- Lal, S.; Pironi, L.; Wanten, G.; Arends, J.; Bozzetti, F.; Cuerda, C.; Joly, F.; Kelly, D.; Staun, M.; Szczepanek, K.; et al. Clinical approach to the management of Intestinal Failure Associated Liver Disease (IFALD) in adults: A position paper from the Home Artificial Nutrition and Chronic Intestinal Failure Special Interest Group of ESPEN. Clin. Nutr. 2018, 37, 1794–1797. [Google Scholar] [CrossRef]
- Gillanders, L.; Angstmann, K.; Ball, P.; Chapman-Kiddell, C.; Hardy, G.; Hope, J.; Smith, R.; Strauss, B.; Russell, D. AuSPEN clinical practice guideline for home parenteral nutrition patients in Australia and New Zealand. Nutrition 2008, 24, 998–1012. [Google Scholar] [CrossRef]
- Bering, J.; Dibaise, J.K. Home Parenteral and Enteral Nutrition. Nutrients 2022, 14, 2558. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.G.; Nannapaneni, N. An Insulin Protocol for Management of Hyperglycemia in Patients Receiving Parenteral Nutrition Is Superior to Ad Hoc Management. J. Parenter. Enter. Nutr. 2012, 36, 183–188. [Google Scholar] [CrossRef]
- Llop, J.M.; Leiva, E.; Mateu-de Antonio, J.; Berlana, D.; Badia, M.; Casasín, T.; Miana, M.; Pons, M.; Maroto, M.; Chicharro, L.; et al. Study of hyperglycemia in non critically-ill patients receiving parenteral nutrition: Incidence and risk factors. Nutr. Hosp. 2012, 27, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hébuterne, X.; Pertkiewicz, M.; et al. ESPEN Guidelines on Parenteral Nutrition: Home Parenteral Nutrition (HPN) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef]
- Siepler, J. Principles and strategies for monitoring home parenteral nutrition. Nutr. Clin. Pract. 2007, 22, 340–350. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosseel, Z.; Cortoos, P.-J.; De Waele, E. Energy Guidance Using Indirect Calorimetry for Intestinal Failure Patients with Home Parenteral Nutrition: The Right Bag Right at the Start. Nutrients 2023, 15, 1464. https://doi.org/10.3390/nu15061464
Rosseel Z, Cortoos P-J, De Waele E. Energy Guidance Using Indirect Calorimetry for Intestinal Failure Patients with Home Parenteral Nutrition: The Right Bag Right at the Start. Nutrients. 2023; 15(6):1464. https://doi.org/10.3390/nu15061464
Chicago/Turabian StyleRosseel, Zenzi, Pieter-Jan Cortoos, and Elisabeth De Waele. 2023. "Energy Guidance Using Indirect Calorimetry for Intestinal Failure Patients with Home Parenteral Nutrition: The Right Bag Right at the Start" Nutrients 15, no. 6: 1464. https://doi.org/10.3390/nu15061464
APA StyleRosseel, Z., Cortoos, P.-J., & De Waele, E. (2023). Energy Guidance Using Indirect Calorimetry for Intestinal Failure Patients with Home Parenteral Nutrition: The Right Bag Right at the Start. Nutrients, 15(6), 1464. https://doi.org/10.3390/nu15061464






