Abstract
Celiac disease is a rising disorder and is becoming frequently diagnosed in recent years. To date, the only available treatment is the gluten-free diet (GFD). The role of gluten on components of metabolic syndrome and on related inflammatory response is still unclear due to controversial results. In recent years, scientific focus on this topic has been growing up, in particular regarding the role of the GFD on glycometabolic parameters and diabetes. In addition, studies on the remaining components showed discordant results, which was likely due to heterogeneous and large celiac disease populations and to the lack of prospective studies. Furthermore, knowledge about the role of the GFD on inflammatory cytokines and the relationship among vitamin D and celiac disease, metabolic syndrome (MS) and GFD is needed. In this narrative review, we provided evidence regarding the role of the GFD on glycometabolic parameters, cholesterol, triglycerides, waist circumference, blood pressure and inflammatory cascade, also evaluating the role of vitamin D, trying to summarize whether this nutritional pattern may be a value-added for subjects with dysmetabolic conditions. Finally, due to the limited findings and very low-certainty evidence, predominantly based on observational studies, the real effects of a GFD on different components of MS, however, are unclear; nevertheless, an improvement in HDL levels has been reported, although data on glycemic levels are discordant.
1. Introduction
Celiac disease (CD) is a disorder, occurring in genetically susceptible subjects, characterized by an abnormal immune reaction to ingested gluten proteins. Its frequency appears to have significantly increased in recent years. To date, the only available treatment in patients affected by CD is the gluten-free diet (GFD). In recent years, scientific interest in the role of the GFD in components of metabolic syndrome (MS) on glycometabolic parameters and diabetes mellitus (DM) has been growing, but results are still unclear due to controversial results in the literature.
In this narrative review, we report evidence regarding the role of the GFD on glycemia, glycometabolic parameters, cholesterol and triglycerides, waist circumference blood pressure and inflammatory parameters, trying to summarize whether this diet may be an added value for subjects with dysmetabolic conditions.
1.1. Celiac Disease
CD is increasing in incidence perhaps due to the use of more sophisticated biomarkers and diagnostic tools [1,2]; it occurs only in genetically susceptible subjects in association with environmental factors. Definitive diagnosis of CD is possible when duodenal villous atrophy occurs in the presence of circulating antibodies against tissue transglutaminase.
Among the comorbidities associated with CD is DM and in particular type 1 diabetes (T1D) [3] with prevalence ranging from 3% to 16% in children and from 1.4% to 6.8% in adults [4,5,6,7].
Finally, is noteworthy to mention the important role of the inflammatory cascade linked to this disease, as reported later in the text.
1.2. Gluten-Free Diet
The GFD is the currently available treatment for CD and it is and it is unfortunately associated with low intake of dietary fiber, minerals, and vitamins, combined with high intake of calories, carbohydrates, fats, other micronutrients [8] and food with a usually high glycemic index. In particular, the GFD consists of a complete elimination of gluten-containing foods, including gluten proteins in wheat (gliadin), rye (secalins), and barley (hordeins) [9]. In addition, to prevent weight gain, the GFD should be individualized in order to maintain optimal body composition and lead to an eventual reduction in peripheral adipose tissue (when needed), as it is desirable in subjects affected by insulin resistance [10].
According to the Food and Drugs Administration (FDA), a gluten-free product is defined as having <20 ppm (parts per million) of gluten while considering possible contamination during product creation [11,12]. Since 2005, the European Union (EU) has declared that food containing gluten must have listed in the ingredients list, specifying two levels—gluten-free (≤20 ppm/mg/kg) and low gluten (21–100 ppm/mg/kg) [12].
It is important to consider that a GFD inadequately balanced might have serious consequences on the metabolic system and increase the risk of MS [8].
1.3. Metabolic Syndrome
MS is a condition characterized by the concomitant presence of three or more metabolic abnormalities, including waist circumference ≥ 94 cm in males or ≥80 cm in females (for WHO and IDF report for Caucasian population), fasting serum glucose >100 mg/dL, triglycerides >150 mg/dL, HDL cholesterol <40 mg/dL in males and <50 mg/dL in females, systolic blood pressure ≥130 mmHg and diastolic blood pressure ≥ 85 mmHg, according to the IDF and AHA/NHLBI representatives; so, the presence of any three of five risk factors constitutes a diagnosis of MS [13,14,15].
2. Materials and Methods
This narrative review was performed on all available prospective, retrospective and review studies that have been published since 2000 in PubMed. Data were extracted from the text and tables of the manuscripts. The keywords used in this study were: “gluten-free diet and metabolic syndrome”, “gluten-free diet and blood sugar”, “gluten-free diet and cholesterol”, “gluten-free diet and weight circumference”, “gluten-free diet and blood pressure”, “gluten-free diet and cardiovascular disease”, “celiac disease and metabolic syndrome”, “gluten-free diet and inflammation”, “vitamin D and metabolic syndrome and diseases”, “vitamin D and gluten-free diet and metabolic syndrome”, and “vitamin D and gluten-free diet”.
3. Gluten-Free Diet and Components of the Metabolic Syndrome
Due to controversial results and unspecified diet composition in some studies, the role of the GFD on components of MS is still unclear. Therefore, we focused on studies that evaluated the effect of the GFD on all MS components (Table 1) as well as the effect on inflammatory response (Table 2).

Table 1.
Summary of effects of GFD on components of metabolic syndrome in studies on subjects with and without celiac disease.

Table 2.
Studies about the effects of inflammation on subjects treated with GFD.
3.1. Glycemia and Glycemic Parameters
Literature data regarding the effects of the GFD on glycemic parameters are controversial. Studies on subjects with CD and T1D have shown an improvement in fasting blood glucose with a reduction in islet-specific autoantibodies upon administering a GFD [42]. In addition, the relationship among CD and T2D is still not well defined: some studies showed a prevalence comparable with that of healthy subjects, while others showed higher prevalence [8,43,44]. In a prospective open-label randomized 1 year-controlled trial (RCT) on subjects with T1D and subclinical CD treated with a GFD, there was an improvement of glycemic control and a decrease in hypoglycemic episodes compared to subjects in normal diet [16].
In contrast, in 2015, an Italian study showed increased levels of glycemia and altered values of components of MS upon 1 year of evaluation of subjects on a GFD [17,20]. In T1D subjects, asymptomatic but biopsy-confirmed for CD, no HbA1c differences were observed between a GFD and diet with gluten; however, an increase in postprandial glycemia was present in the GFD group [18].
In a recent metanalysis and in a systematic review of the literature, children on a GFD with asymptomatic T1D and CD were evaluated, and no significant effects on HbA1c were observed [19]. Furthermore, in a case-control, observational study, T1D and CD long-term GFD-treated, glycemic control did not worsen [23]. In particular, these studies showed that a GFD has no significant impact on glycemic levels [19,20,21,22]. Moreover, a study performed in 20 hospitals in the Netherlands, designed to evaluate the effect of a GFD on glycemic control in subjects with T1D and CD, demonstrated no changes in terms of reduction in HbA1c [3].
In a large prospective study on children, mostly CD asymptomatic, HbA1c increased by 0.6% after 1 year of GFD treatment [24]; other trials on subjects with CD showed no significant difference in HbA1c [20,25,26,27,28]. In a randomized clinical trial on subjects with MS and CD, treated vs. untreated with a GFD, fasting glycemia decreased significantly in the GFD group [29].
Regarding data on subjects without CD, in an RCT, a GFD appears to improve fasting blood glucose [2]. Interestingly, studies performed in an experimental animal model of T2D mice showed that a GFD improves disease parameters by acting on intestinal barrier function and TLR4 receptor stimulation, where gliadin enhances insulin resistance and β-cell dysfunction [45], thus suggesting a positive role of GFD on T2D mice without CD [46]. In 2017, in a double-blind randomized crossover trial on healthy adults, the postprandial glycemic responses to GFD were compared to wheat pasta. Remarkably, glucose levels were 57% higher compared to traditional wheat pasta [37], suggesting a potential detrimental effect on glucose metabolism. Finally, in 2022, in a systematic review regarding the long-term effect of a gluten-reduced diet or GFD on the prevention of cardiovascular disease (CVD) [36], the authors hypothesized that it is uncertain whether higher gluten intake can increase the risk of developing T2D, as shown in Table 1.
3.2. HDL and Triglycerides
To date, the effects of a GFD in CD subjects regarding lipid metabolism and lipid levels are still unclear.
A 2015 study reported that a GFD reduced HDL cholesterol levels [17], whereas in a study on CD children, it showed significantly higher HDL levels in girls than in boys [30], suggesting a gender difference in response to a GFD. A trial in 2016 showed that a GFD induced significantly lower HDL-C levels in children with T1D and CD compared to the control group: indeed, a GFD induced a significant increase in HDL-C (60.9 ± 13.7 vs. 51.3 ± 13.6 mg/dL, p < 0.0001) [31]. In contrast, a significant reduction in HDL was found in a retrospective cohort with CD [29].
With regard to triglycerides, Tortora et al., in a prospective case-control study on subjects with CD, found a worsening of triglycerides [17,32], while a retrospective study conducted by Ciccone et al. did not show a significant reduction in triglycerides [29]. Finally, another interesting study performed on children receiving a GFD showed higher triglyceride levels in girls than in boys, indicating that the lipid profiles of children with CD may differ across gender [30,37].
Moreover, in regard to subjects with CD treated with a GFD, Kim et al. [38] and Di Giacomo et al. [47] showed an improvement of HDL levels, whereas another RCT study showed that a GFD in subjects without CD could improve triglycerides levels [2] (Table 1).
3.3. Waist Circumference
An important component of MS is an increase in waist circumference (WC). Recently, in a randomized clinical trial, enrolling 50 subjects with MS and CD, and randomly divided into a group receiving a GFD and a group continuing a regular diet, the normocaloric GFD reduced WC compared with the control diet group (p < 0.05) [2]. In contrast, some studies revealed that a GFD worsened WC [17]. In particular, in a population with CD, WC increased upon GFD nutritional intervention [17].
In subjects without CD on a GFD, a study showed an improvement in WC [38]. Interestingly, an improvement in WC was also demonstrated by data obtained from the National Health and Nutrition Examination Survey (NHANES) 2009–2014 in non-CD subjects [33,38] (Table 1).
3.4. Blood Pressure
Blood pressure is an MS component that needs to be evaluated, and it may depend on an unbalanced dietary pattern. Interestingly, high blood pressure was observed in subjects with CD after 1 year of GFD [17]. As mentioned above, in a study performed on children with CD and receiving a GFD or newly diagnosed children, the first group had blood pressure significantly higher in girls than in boys also compared with the group without a GFD [30]. Furthermore, in a prospective study conducted to assess the effects of a GFD on CVD risk among newly diagnosed pediatric CD subjects, no significant data were displayed in regard to changes in blood pressure [34]. In a randomized clinical trial, evaluating subjects with MS and CD, a group treated with GFD and the other with regular diet, no significant differences were reported regarding systolic and diastolic blood pressure values [29]. A systematic review of the literature showed no modifications of blood pressure after adhering to a GFD in CD subjects [35]. Furthermore, in a case-control observational study performed on 34 T1D CD patients in comparison to 66 patients with T1D alone, both groups GFD-treated, no differences were found in systolic blood pressure, while diastolic blood pressure was significantly lower in T1D CD subjects (p = 0.003) [23].
A recent revision of the literature, evaluating in subjects without CD the effects of a gluten-reduced diet or GFD on primary prevention of CVD, showed not significant differences in either systolic or diastolic blood pressure after six months of follow-up [36], as shown in Table 1.
4. Gluten-Free Diet and Inflammation
The presence of MS leads to a chronic low-grade inflammation and to an activation of the immune system, which is a potential significant pathogenetic factor of obesity-related insulin resistance and T2D. Indeed, an increase in macrophages and other immune cells in involved in a pro-inflammatory action via cytokines alterations in main tissues and organs [48].
As known, CD is related to an immune response driven by CD4+ T cells specific for deamidated gluten peptides; in fact, these molecules bind to disease-associated human leukocyte antigen (HLA)–DQ allotypes, where the CD4 T cells interact with B cells and also CD8 T cells [49,50]. This increase in T cell subtypes activates specific signaling including NF-kB, GATA1, JAK or STAT5, which is targeted by diet or drugs. Furthermore, IL-15 also plays a central and targeted role in this process by activating CD8+ T cells via CD4+ T cells [51].
Additionally, other possible pathological pathways could be involved, such as the activation of the innate immune response via the α-gliadin peptide, p31-43 peptides, directly damaging the CD mucosa, which is supported by the activation of neutrophils, eosinophils, complement proteins and mast cells [52,53,54].
It is interesting that an important role in balancing the cascade of inflammation is performed by prebiotics and probiotics, stimulating the metabolic activity of gut microbiota with beneficial effects on the immune response and cytokine production [55,56,57]. Indeed, among probiotics, the supplementation of Bifidobacterium and Lactobacilli showed a decrease in inflammation via a reduction in cytokine and antibody production. Nonetheless, the use of prebiotics and probiotics in subjects with CD is not yet formally allowed in clinical practice since no clinical RCT have been performed yet [58].
Studies on subjects with irritable bowel syndrome (IBS) have indicated that gluten exposure leads to monocyte and cytokine production and intestinal low-grade inflammation, whereas no data on gluten-induced inflammation have been produced as yet [41], as shown in Table 2.
In NCG/WS (non-celiac gluten or wheat sensitivity), a GFD restores the microbiota population and reduces pro-inflammatory species. Innate and adaptive immunity coexist in these conditions: the first one shows an increase in mucosal toll-like receptor 2 (TLR2), granulocyte colony-stimulating factor (GCSF), IL-10, transforming growth factor alpha (TNF-α) and CXCL-10 chemokine from peripheral blood mononuclear cells (PBMCs), while the second one increases interferon (IFN)-gamma mRNA [39]. Finally, in a study using a GFD and dairy-free diet in children with steroid-resistant nephrotic syndrome (SRNS), findings showed a decrease in the inflammatory status of this population [40].
5. The Role of Vitamin D in Metabolic Syndrome, Celiac Disease and in Gluten Free Diet Treatment
Vitamin D is a hormone which plays a pivotal action on calcium and bone metabolism. In the adult population, serum levels of vitamin D in its hydroxylated form (25-hydroxy-vitamin D or calcidiol) are defined as deficient when they are below 20 ng/mL and insufficient when they are between 20 and 30 ng/mL [59]. Since low levels of vitamin D are not uncommon in the general population and its supplementation is becoming increasingly popular, great attention has been paid to this hormone and its receptor, as well as a condition of hypovitaminosis D (HypoD), in regard to extra-skeletal effects as well as being a co-factor in several diseases. In particular, several studies have investigated the relationship between vitamin deficiency and components of MS.
5.1. Vitamin D and MS
Vitamin D deficiency appears to be bidirectional related to obesity: on the one hand, the excess adipose mass might act as a storage site for a lipophilic hormone such as vitamin D [60]; on the other hand, vitamin D plays an important role in adipocyte physiology and glucose metabolism, which is typically dysregulated in obese subjects [61]. Although there has been some heterogeneity in the literature, most studies confirm an inverse correlation between vitamin D levels and the prevalence of MS. HypoD has been associated with increased risk or prevalence of abdominal obesity, hypertension, impaired glucose homeostasis, dyslipidemia, and increased waist circumference [62,63,64,65,66,67,68,69]. Recently, a cross-sectional study on more than 800 participants, aged > 60, showed a reduced risk of MS above specific serum vitamin D cut-off levels, which was different according to sex, 40 ng/mL in men and 20 mg/dl in women, respectively [70]. Additionally, a large survey of nearly 10,000 participants identified HypoD as an additional risk factor to the presence of MS for the development of cardiovascular events and all-cause mortality, which were shown to be inversely related to vitamin D levels [71]. Finally, it appears noteworthy to evaluate the relationship between vitamin D and CD, the prevalence of HypoD among subjects with CD compared to subjects without CD, and the effect of a GFD on levels of the hormone, which appears to play an important role in the development and progression of MS.
5.2. Vitamin D and CD
As previously mentioned, CD is a chronic auto-immune and self-inflammatory condition that damages intestinal tissues with alterations in intestinal villi and crypts and indirectly with altered absorption of various nutrients, leading to malnutrition [72]. Micronutrient deficits are common in individuals with CD, during both childhood and adulthood, although data in the literature are heterogeneous [73]. Notably, among micronutrients, several guidelines recommend the measurement of vitamin D levels at the time of CD diagnosis [74,75]. The link between CD and HypoD is not entirely clear: since vitamin D has an immunomodulatory and controlling action on intestinal permeability, it has been hypothesized that low levels of this hormone may play a role in the pathogenesis and development of CD by counteracting the mechanisms leading to the onset of intestinal autoimmunity [76]. The prevalence of HypoD in patients with CD varies among studies: low levels appear to be significantly related to CD in pediatric populations [77,78], while differences are smaller as compared to healthy controls when considering adult patients [73]. However, CD, as other inflammatory bowel diseases, is also linked to alterations in bone metabolism, so celiac subjects have lower BMD values than non-celiac subjects (up to 70% of cases in some studies) [79].
5.3. Vitamin D and GFD
The effect of a GFD on vitamin D levels in CD subjects has been evaluated in several studies. In regard to pediatric case, it appears that celiac children, observing a GFD, have dietary trends similar to the corresponding healthy controls, with both celiac and non-celiac subjects having vitamin D intake below reference standards [80,81]. However, in two prospective studies on pediatric celiac patients, after one year or six months of GFD, respectively, a significant increase in vitamin D level was observed, which was associated or not with a decline in PTH levels [82,83]. Similar results were obtained in a cohort of the adult population, where patients not adhering to a GFD had lower vitamin D and BMD values at diagnosis, and higher PTH values than subjects adhering to the diet [84]. While lower levels of vitamin D are more common in newly diagnosed CD or untreated subjects, it has been seen that HypoD may also be found in those on a GFD [85,86]. The reason could be due to nutritional deficits caused by malabsorption in celiac patients with bowel damage [87]. According to recent evidence, the deficit would persist regardless of the duration and adherence to the GFD, being mainly related to poor intake of the nutrient itself, which in 95% of cases is not added to gluten-free products [88]. Despite this, it has been suggested that vitamin D supplementation is essential in those subjects undergoing dietary treatment, especially during the first year of the GFD [89].
6. Discussion
The evaluation of the effects of a GFD on each component of the MS is a measure to consider in the prevention of potential increasing risk for cardiovascular diseases in general and, in particular, in specific populations, such as those with DM, overweight or obesity, even evaluating the impact on inflammation. To date, several trials have evaluated the role of a GFD on components of MS, mainly in CD, less in those without CD. and to the best of our knowledge, there are controversial results, with few RCT and intervention trials of longer duration and larger sample size.
6.1. Summarizing Controversial Data on Components of MS
To date, these controversial and inconclusive data might also be derived from a poor detailed description of GFD composition, which could be considered a bias when results of different studies are compared. Summarizing this discrepancy in a few reports: Tortora et al., treating subjects affected by CD with a GFD, showed an elevated risk of MS after one year [17]; whereas De Marchi et al., in a study on young adults with CD, demonstrated that a GFD improved HDL, IMT and endothelium-dependent dilatation [90]. In non-celiac patients, a GFD may slightly improve overall cardiac risk factors; nevertheless, its use in the absence of a gluten-related disease needs larger studies with a better-defined dietary composition of food [8] (Figure 1).
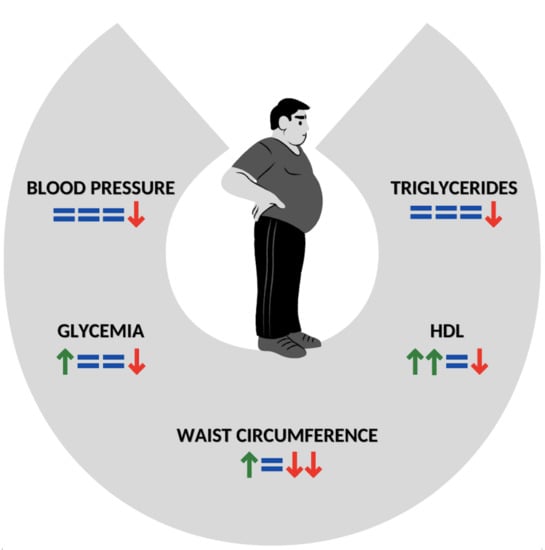
Figure 1.
Impact of GFD on components of MS in subjects with CD. Green arrow: improvement of component; Red arrow: worsening of component; =: not significant data. The number of arrows means in a scale 1–4 the power of data. GFT: Gluten-free diet; MS: metabolic syndrome; CD: celiac disease; HDL: HDL Cholesterol.
6.2. Potential Risks of GFD Using for Non-Celiac Subjects
To date, a GFD has not proved to be beneficial in subjects without CD [8]; therefore, we should not consider a GFD in non-CD patients to promote weight loss or improve triglycerides. Finally, considering the scientific evidence, although discordant, it is fair to conclude that subjects without CD or gluten intolerance, might not choose a GFD in place of traditional foods to obtain metabolic improvement or prevention of MS.
6.3. Future Perspectives
Even if in recent years, the use of the GFD is growing, not only in subjects with CD, the components of MS still remain one of the main topics to focus to improve the reduction in cardiovascular risks. Anyhow, there is a gap of knowledge to fill. In this direction, it could be useful to organize further evaluations with larger RCT of longer duration with a large sample size, possibly including evaluation on glycemic variability, using continuous glucose monitoring systems (CGMs) to better evaluate subjects with prediabetes or diabetes to optimize treatment. Furthermore, a clear and well-characterized dietary composition in all studies is needed. Finally, it could be useful to promote a more complete evaluation of the effects of the GFD on inflammatory pathways, bone metabolism and fractures, microbiome imbalance and sexual dysfunctions.
Hence, the proposed path is to achieve a GFD tailored to the type of subjects with DM, CVD, bone disease, etc.
7. Conclusions
Given the limited findings in the literature on the GFD, predominantly based on observational studies, few definitive conclusions can be drawn on the real effects of this nutritional pattern on different components of MS. With this narrative review of the literature, we report that data on blood glucose and waist circumference are discordant; HDL levels seem to be improved while triglyceride levels are modulated by the use of the GFD. Finally, blood pressure is increased by the GFD in most of the studies evaluated, and vitamin D supplementation should be assessed in CD subjects on the GFD. In conclusion, there are still multiple aspects to explore and to characterize, and further studies are needed to better define the effects of a GFD in subjects without CD, such as a potential role of microbiome axis as well cardiovascular risk factors, diabetes complications and osteometabolic diseases.
Author Contributions
Conceptualization, S.M. and G.D.; methodology, G.D. and S.M.; writing—original draft preparation, G.D., M.C.M. and G.T; writing—review and editing: G.D., L.C. and S.M.; supervision, N.N. and S.M.; funding acquisition, S.M. G.D., M.C.M., G.T. and S.M. are members of the Italian Society of Food Sciences. All authors have read and agreed to the published version of the manuscript.
Funding
Research was in part supported by MIUR grant number 2017HBHA98 to S.M., G.D. and L.C. are supported by a grant “Interferenti endocrini nelle donne affette da patologie metaboliche: analisi di biomarcatori circolanti e valutazione di danno infiammatorio” grant number A0375-2020-36592 “Progetti di Gruppi di Ricerca 2020 POR FESR Lazio 2014-2020 to SM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami, M.; Shakerhosseini, R.; Sedaghat, F.; Hedayati, M.; Eini-Zinab, H.; Hekmatdoost, A. The Effect of Gluten Free Diet on Components of Metabolic Syndrome: A Randomized Clinical Trial. Asian Pac. J. Cancer Prev. 2018, 19, 2979–2984. [Google Scholar] [CrossRef]
- Bakker, S.F.; Tushuizen, M.E.; Von Blomberg, M.E.; Mulder, C.J.; Simsek, S. Type 1 diabetes and celiac disease in adults: Glycemic control and diabetic complications. Acta Diabetol. 2012, 50, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sud, S.; Marcon, M.; Assor, E.; Palmert, M.R.; Daneman, D.; Mahmud, F.H. Celiac Disease and Pediatric Type 1 Diabetes: Diagnostic and Treatment Dilemmas. Int. J. Pediatr. Endocrinol. 2010, 2010, 161285. [Google Scholar] [CrossRef] [PubMed]
- Page, S.R.; Lloyd, C.A.; Hill, P.G.; Peacock, I.; Holmes, G.K.T. The prevalence of coeliac disease in adult diabetes mellitus. QJM 1994, 87, 631–637. [Google Scholar]
- Mahmud, F.H.; De Melo, E.N.; Noordin, K.; Assor, E.; Sahota, K.; Davies-Shaw, J.; Cutz, E.; Somers, G.; Lawson, M.; Mack, D.R.; et al. The Celiac Disease and Diabetes-Dietary Intervention and Evaluation Trial (CD-DIET) protocol: A randomised controlled study to evaluate treatment of asymptomatic coeliac disease in type 1 diabetes. BMJ Open 2015, 5, e008097. [Google Scholar] [CrossRef]
- Mahmud, F.H.; Murray, J.A.; Kudva, Y.C.; Zinsmeister, A.R.; Dierkhising, R.A.; Lahr, B.D.; Dyck, P.J.; Kyle, R.A.; El-Youssef, M.; Burgart, L.J.; et al. Celiac Disease in Type 1 Diabetes Mellitus in a North American Community: Prevalence, Serologic Screening, and Clinical Features. Mayo Clin. Proc. 2005, 80, 1429–1434. [Google Scholar] [CrossRef]
- Dhruva, V.; Lawson, C.; Green, C.; Newberry, C. “The Gluten-Free Diet and Its Relationship with Metabolic Syndrome: Dietary Friend or Foe?”. Curr. Nutr. Rep. 2021, 10, 282–287. [Google Scholar] [CrossRef]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef]
- Soares, F.L.P.; Matoso, R.D.O.; Teixeira, L.G.; Menezes, Z.; Pereira, S.S.; Alves, A.C.; Batista, N.V.; de Faria, A.M.C.; Cara, D.C.; Ferreira, A.V.M.; et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J. Nutr. Biochem. 2013, 24, 1105–1111. [Google Scholar] [CrossRef]
- Jones, A.L. The Gluten-Free Diet: Fad or Necessity? Diabetes Spectr. 2017, 30, 118–123. [Google Scholar] [CrossRef]
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free Diet Indications, Safety, Quality, Labels, and Challenges. Nutrients 2017, 9, 846. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series; 894; WHO Consultation on Obesity: Geneva, Switzerland, 1999; pp. 1–253.
- Kaur, P.; Agarwala, A.; Makharia, G.; Bhatnagar, S.; Tandon, N. Effect of Gluten-FREE Diet on Metabolic Control and Anthropometric Parameters in Type 1 Diabetes with Subclinical Celiac Disease: A Randomized Controlled Trial. Endocr. Pract. 2020, 26, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Tortora, R.; Capone, P.; De Stefano, G.; Imperatore, N.; Gerbino, N.; Donetto, S.; Monaco, V.; Caporaso, N.; Rispo, A. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2015, 41, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, F.H.; Clarke, A.B.; Joachim, K.C.; Assor, E.; McDonald, C.; Saibil, F.; Lochnan, H.A.; Punthakee, Z.; Parikh, A.; Advani, A.; et al. Screening and Treatment Outcomes in Adults and Children with Type 1 Diabetes and Asymptomatic Celiac Disease: The CD-DIET Study. Diabetes Care 2020, 43, 1553–1556. [Google Scholar] [CrossRef]
- Burayzat, S.; Elsahoryi, N.; Freitekh, A.; Alzoubi, O.; Al-Najjar, R.; Tayyem, R. Does a Gluten-Free Diet Affect BMI and Glycosylated Hemoglobin in Children and Adolescents with Type 1 Diabetes and Asymptomatic Celiac Disease? A Meta-Analysis and Systematic Review. Children 2022, 9, 1247. [Google Scholar] [CrossRef]
- Hansen, D.; Brock-Jacobsen, B.; Lund, E.; Bjørn, C.; Hansen, L.P.; Nielsen, C.; Fenger, C.; Lillevang, S.T.; Husby, S. Clinical Benefit of a Gluten-Free Diet in Type 1 Diabetic Children with Screening-Detected Celiac Disease: A population-based screening study with 2 years’ follow-up. Diabetes Care 2006, 29, 2452–2456. [Google Scholar] [CrossRef]
- Wherrett, D.; Huot, C.; Mitchell, B.; Pacaud, D. Type 1 Diabetes in Children and Adolescents. Can. J. Diabetes 2013, 37, S153–S162. [Google Scholar] [CrossRef]
- DeMelo, E.N.; McDonald, C.; Saibil, F.; Marcon, M.A.; Mahmud, F.H. Celiac Disease and Type 1 Diabetes in Adults: Is This a High-Risk Group for Screening? Can. J. Diabetes 2015, 39, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Creanza, A.; Lupoli, R.; Lembo, E.; Tecce, N.; Della Pepa, G.; Lombardi, G.; Riccardi, G.; Di Bonito, P.; Capaldo, B. Glycemic control and microvascular complications in adults with type 1 diabetes and long-lasting treated celiac disease: A case-control study. Diabetes Res. Clin. Pract. 2018, 143, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Puttha, R.; Ghezaiel, S.; Skae, M.; Cooper, C.; Amin, R.; on behalf of the North West England Paediatric Diabetes Network. The effect of biopsy-positive silent coeliac disease and treatment with a gluten-free diet on growth and glycaemic control in children with Type 1 diabetes. Diabet. Med. 2009, 26, 1250–1254. [Google Scholar] [CrossRef]
- Saadah, O.I.; Zacharin, M.; O’Callaghan, A.; Oliver, M.R.; Catto-Smith, A.G. Effect of gluten-free diet and adherence on growth and diabetic control in diabetics with coeliac disease. Arch. Dis. Child. 2004, 89, 871–876. [Google Scholar] [CrossRef]
- Abid, N.; McGlone, O.; Cardwell, C.; McCallion, W.; Carson, D. Clinical and metabolic effects of gluten free diet in children with type 1 diabetes and coeliac disease. Pediatr. Diabetes 2011, 12, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Albisua, I.; Wolf, J.; Neu, A.; Geiger, H.; Wascher, I.; Stern, M. Coeliac disease in children with Type 1 diabetes mellitus: The effect of the gluten-free diet. Diabet. Med. 2005, 22, 1079–1082. [Google Scholar] [CrossRef]
- Taler, I.; Phillip, M.; Lebenthal, Y.; de Vries, L.; Shamir, R.; Shalitin, S. Growth and metabolic control in patients with type 1 diabetes and celiac disease: A longitudinal observational case-control study. Pediatr. Diabetes 2012, 13, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.; Gabrieli, D.; Cardinale, R.; Di Ruscio, M.; Vernia, F.; Stefanelli, G.; Necozione, S.; Melideo, D.; Viscido, A.; Frieri, G.; et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion 2019, 100, 262–268. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Fernicola, P.; Diani, L.; Scrivo, B.; Salfi, N.C.; Pessina, A.C.; Lima, M.; Conti, V.; Pession, A. Gluten-Free Diet and Lipid Profile in Children with Celiac Disease: Comparison with General Population Standards. J. Craniofacial Surg. 2015, 61, 224–229. [Google Scholar] [CrossRef]
- Salardi, S.; Maltoni, G.; Zucchini, S.; Iafusco, D.; Confetto, S.; Zanfardino, A.; Toni, S.; Piccini, B.; Zioutas, M.; Marigliano, M.; et al. Celiac Disease Negatively Influences Lipid Profiles in Young Children with Type 1 Diabetes: Effect of the Gluten-Free Diet. Diabetes Care 2016, 39, e119–e120. [Google Scholar] [CrossRef]
- Tortora, R.; Rispo, A.; Alisi, A.; Imperatore, N.; Crudele, A.; Ferretti, F.; Nobili, V.; Miele, L.; Gerbino, N.; Caporaso, N.; et al. PNPLA3 rs738409 Polymorphism Predicts Development and Severity of Hepatic Steatosis but Not Metabolic Syndrome in Celiac Disease. Nutrients 2018, 10, 1239. [Google Scholar] [CrossRef]
- Emilsson, L.; Semrad, C.E. Obesity, Metabolic Syndrome, and Cardiac Risk Factors: Going Gluten-Free, for Better or worse? Dig. Dis. Sci. 2017, 62, 2215–2216. [Google Scholar] [CrossRef]
- Zifman, E.; Waisbourd-Zinman, O.; Marderfeld, L.; Zevit, N.; Guz-Mark, A.; Silbermintz, A.; Assa, A.; Mozer-Glassberg, Y.; Biran, N.; Reznik, D.; et al. The Effect of Gluten-free Diet on Cardiovascular Risk Factors in Newly Diagnosed Pediatric Celiac Disease Patients. J. Craniofacial Surg. 2019, 68, 684–688. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Brienesse, S.C.; Walker, M.M.; Boyle, A.; Talley, N.J. Effect of the gluten-free diet on cardiovascular risk factors in patients with coeliac disease: A systematic review. J. Gastroenterol. Hepatol. 2018, 33, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, C.; Eisele-Metzger, A.; Meerpohl, J.J.; Lehane, C.; de Gaudry, D.K.; Lohner, S.; Schwingshackl, L. Effects of a gluten-reduced or gluten-free diet for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2022, 2, CD013556. [Google Scholar] [CrossRef]
- Johnston, C.S.; Snyder, D.; Smith, C. Commercially available gluten-free pastas elevate postprandial glycemia in comparison to conventional wheat pasta in healthy adults: A double-blind randomized crossover trial. Food Funct. 2017, 8, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Demyen, M.F.; Mathew, J.; Kothari, N.; Feurdean, M.; Ahlawat, S.K. Obesity, Metabolic Syndrome, and Cardiovascular Risk in Gluten-Free Followers Without Celiac Disease in the United States: Results from the National Health and Nutrition Examination Survey 2009–2014. Dig. Dis. Sci. 2017, 62, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; de Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef]
- Pérez-Sáez, M.J.; Uffing, A.; Leon, J.; Murakami, N.; Watanabe, A.; Borges, T.J.; Sabbisetti, V.S.; Cureton, P.; Kenyon, V.; Keating, L.; et al. Immunological Impact of a Gluten-Free Dairy-Free Diet in Children with Kidney Disease: A Feasibility Study. Front. Immunol. 2021, 12, 624821. [Google Scholar] [CrossRef]
- Palmieri, B.; Vadala', M.; Laurino, C. Gluten-free diet in non-celiac patients: Beliefs, truths, advantages and disadvantages. Minerva Gastroenterol. Dietol. 2019, 65, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ac, C.T.; Montuori, M.; Trovato, C.M.; Mba, F.P.; Filardi, T.; Valitutti, F.; Lenzi, A.; Cucchiara, S.; Morano, S. Gluten-free diet impact on dynamics of pancreatic islet-specific autoimmunity detected at celiac disease diagnosis. Pediatr. Diabetes 2020, 21, 774–780. [Google Scholar] [CrossRef]
- Kylökäs, A.; Kaukinen, K.; Huhtala, H.; Collin, P.; Mäki, M.; Kurppa, K. Type 1 and type 2 diabetes in celiac disease: Prevalence and effect on clinical and histological presentation. BMC Gastroenterol. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Kizilgul, M.; Ozcelik, O.; Beysel, S.; Akinci, H.; Kan, S.; Ucan, B.; Apaydin, M.; Cakal, E. Screening for celiac disease in poorly controlled type 2 diabetes mellitus: Worth it or not? BMC Endocr. Disord. 2017, 17, 62. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Zhang, Y.; Zhang, W.J.; Xu, W.; Qin, Y.; Xu, J.; Zou, D. TLR4 is required for the obesity-induced pancreatic beta cell dysfunction. Acta Biochim. Biophys. Sin. 2013, 45, 1030–1038. [Google Scholar] [CrossRef]
- Di Liberto, D.; Carlisi, D.; D’Anneo, A.; Emanuele, S.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Gluten Free Diet for the Management of Non Celiac Diseases: The Two Sides of the Coin. Healthcare 2020, 8, 400. [Google Scholar] [CrossRef]
- DiGiacomo, D.V.; Tennyson, C.A.; Green, P.H.; Demmer, R.T. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: Results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand. J. Gastroenterol. 2013, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pr. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Goel, G.; Tye-Din, J.A.; Qiao, S.-W.; Russell, A.K.; Mayassi, T.; Ciszewski, C.; Sarna, V.K.; Wang, S.; Goldstein, K.E.; Dzuris, J.L.; et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv. 2019, 5, eaaw7756. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polańczyk, A.; Sałaga, M.; Huk, I. T cell-activated signaling pathways and locally produced cytokines as potential targets in celiac disease. Curr. Drug Targets 2015, 16, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374. [Google Scholar] [CrossRef]
- Barone, M.V.; Troncone, R.; Auricchio, S. Gliadin Peptides as Triggers of the Proliferative and Stress/Innate Immune Response of the Celiac Small Intestinal Mucosa. Int. J. Mol. Sci. 2014, 15, 20518–20537. [Google Scholar] [CrossRef]
- Kim, S.M.; Mayassi, T.; Jabri, B. Innate immunity: Actuating the gears of celiac disease pathogenesis. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 425–435. [Google Scholar] [CrossRef]
- Drabińska, N.; Jarocka-Cyrta, E.; Złotkowska, D.; Abramowicz, P.; Krupa-Kozak, U. Daily oligofructose-enriched inulin intake impacts bone turnover markers but not the cytokine profile in pediatric patients with celiac disease on a gluten-free diet: Results of a randomised, placebo-controlled pilot study. Bone 2019, 122, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Klemenak, M.; Dolinšek, J.; Langerholc, T.; Di Gioia, D.; Mičetić-Turk, D. Administration of Bifidobacterium breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig. Dis. Sci. 2015, 60, 3386–3392. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Jahromi, M.F.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2016, 206, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cirota, G.G.; Rossini, B.; Lungaro, L.; Di Biase, A.R.; Colecchia, A.; Volta, U.; de Giorgio, R.; Festi, D.; Caio, G. Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients 2020, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Bordelon, P.; Ghetu, M.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam Physician 2009, 80, 841–846. [Google Scholar] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A.; on behalf of Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef]
- Migliaccio, S.; Di Nisio, A.; Magno, S.; Romano, F.; Barrea, L.; Colao, A.M.; Muscogiuri, G.; Savastano, S. Vitamin D deficiency: A potential risk factor for cancer in obesity? Int. J. Obes. 2022, 46, 707–717. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.L.; Tikkanen, E.; Eriksson, J.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, E.Y.; Lee, J.H.; Kim, J.E.; Kim, K.J.; Rhee, Y.; Kim, H.C.; Youm, Y.; Kim, C.O. Associations of serum 25-hydroxyvitamin D with metabolic syndrome and its components in elderly men and women: The Korean Urban Rural Elderly cohort study. BMC Geriatr. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Poloni, P.F.; Orsatti, C.L.; Nahas, E.A.P. Vitamin D deficiency is associated with metabolic syndrome in postmenopausal women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Sukik, A.; Alaayesh, H.; Rasoulinejad, H.; Shraim, M. Serum vitamin D concentrations are inversely related to prevalence of metabolic syndrome in Qatari women. Biofactors 2019, 46, 180–186. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Tofano, R.J.; de Campos, A.L.; Rodrigues, A.S.; Quesada, K.; Bechara, M.D.; Goulart, R.D.A.; Oshiiwa, M. Association between vitamin D status and metabolic syndrome risk factors. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 501–507. [Google Scholar] [CrossRef]
- Qi, K.-J.; Zhao, Z.-T.; Zhang, W.; Yang, F. The impacts of vitamin D supplementation in adults with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 1033026. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Yi, Y.; Qu, T.; Gao, S.; Shi, A.; Zhao, Y.; Xu, S.; Yang, L.; Lin, Y.; Liu, Y.; et al. The beneficial cutoffs of vitamin D for metabolic syndrome varies by sex among the elderly Chinese population: A cross-sectional study. Nutr. Res. 2022, 104, 91–100. [Google Scholar] [CrossRef]
- Liu, L.; Cui, S.; Volpe, S.L.; May, N.S.; Sukumar, D.; DiMaria-Ghalili, R.A.; Eisen, H.J. Vitamin d deficiency and metabolic syndrome: The joint effect on cardiovascular and all-cause mortality in the United States adults. World J. Cardiol. 2022, 14, 411–426. [Google Scholar] [CrossRef]
- Barton, S.H.; Kelly, D.G.; Murray, J. Nutritional Deficiencies in Celiac Disease. Gastroenterol. Clin. North Am. 2007, 36, 93–108. [Google Scholar] [CrossRef]
- Bledsoe, A.C.; King, K.S.; Larson, J.J.; Snyder, M.; Absah, I.; Choung, R.S.; Murray, J.A. Micronutrient Deficiencies Are Common in Contemporary Celiac Disease Despite Lack of Overt Malabsorption Symptoms. Mayo Clin. Proc. 2019, 94, 1253–1260. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Camilletti, D.; Polzonetti, V. Possible Role of Vitamin D in Celiac Disease Onset. Nutrients 2020, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Deora, V.; Aylward, N.; Sokoro, A.; El-Matary, W. Serum Vitamins and Minerals at Diagnosis and Follow-up in Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 185–189. [Google Scholar] [CrossRef]
- Aydemir, Y.; Erdogan, B.; Türkeli, A. Vitamin D deficiency negatively affects both the intestinal epithelial integrity and bone metabolism in children with Celiac disease. Clin. Res. Hepatol. Gastroenterol. 2020, 45, 101523. [Google Scholar] [CrossRef]
- Bianchi, M.-L.; Bardella, M.T. Bone in celiac disease. Osteoporos. Int. 2008, 19, 1705–1716. [Google Scholar] [CrossRef]
- Fernández, C.B.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Children and Adolescents with Celiac Disease on a Gluten Free Diet Compared to Non-Celiac Disease Controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef]
- Öhlund, K.; Olsson, C.; Hernell, O.; Öhlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet. 2010, 23, 294–300. [Google Scholar] [CrossRef]
- Quero, J.S.; Jaime, B.E.; Martínez, A.R.; Martín, F.A.; Jiménez, R.G.; Murillo, M.R.; Martín, A.P. Nutritional assessment of gluten-free diet. Is gluten-free diet deficient in some nutrient? An. Pediatr. 2015, 83, 33–39. [Google Scholar] [CrossRef]
- Verma, A.; Lata, K.; Khanna, A.; Singh, R.; Sachdeva, A.; Jindal, P.; Yadav, S. Study of effect of gluten-free diet on vitamin D levels and bone mineral density in celiac disease patients. J. Fam. Med. Prim. Care 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Bilancio, G.; Russo, I.; Iovino, P.; Cavallo, P.; Santonicola, A.; Bucci, C.; Cirillo, M.; Zingone, F. 25-Hydroxyvitamin D, 1,25-Dihydroxyvitamin D, and Peripheral Bone Densitometry in Adults with Celiac Disease. Nutrients 2020, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Churruca, I.; Miranda, J.; Lasa, A.; Bustamante, M.Á.; Larretxi, I.; Simon, E. Analysis of Body Composition and Food Habits of Spanish Celiac Women. Nutrients 2015, 7, 5515–5531. [Google Scholar] [CrossRef]
- González, T.; Larretxi, I.; Vitoria, J.C.; Castaño, L.; Simón, E.; Churruca, I.; Navarro, V.; Lasa, A. Celiac Male’s Gluten-Free Diet Profile: Comparison to that of the Control Population and Celiac Women. Nutrients 2018, 10, 1713. [Google Scholar] [CrossRef]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef]
- Jivraj, A.; Hutchinson, J.M.; Ching, E.; Marwaha, A.; Verdu, E.F.; Armstrong, D.; Pinto-Sanchez, M.I. Micronutrient deficiencies are frequent in adult patients with and without celiac disease on a gluten-free diet, regardless of duration and adherence to the diet. Nutrition 2022, 103, 111809. [Google Scholar] [CrossRef]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, S.; Chiarioni, G.; Prior, M.; Arosio, E. Young adults with coeliac disease may be at increased risk of early atherosclerosis. Aliment. Pharmacol. Ther. 2013, 38, 162–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).